- *Corresponding Author:
- M. A. Azam
Department of Pharmaceutical Chemistry, J. S. S. College of Pharmacy, Ootacamund-643 001, India
E-mail: afzal9azam@yahoo.co.in
| Date of Submission | 2 November 2007 |
| Date of Decision | 25 March 2008 |
| Date of Acceptance | 15 October 2008 |
| Indian J Pharm Sci, 2008, 70 (5): 672-677 |
Abstract
A number of substituted-a,β -unsaturated carbonyl compounds (1a-i) were prepared by Claisen-Schmidt condensation of substituted acetophenone with selected araldehydes, which on cycloaddition with thiourea furnished 4,6-disubstituted pyrimidine-2-thiols (2a-i). Reaction of (2a-i) with ethyl chloroacetate followed by condensation with hydrazine hydrate yielded 2-[(4,6-disubstituted pyrimidine-2-yl) thio] acetohydrazides (4a-c). Condensation of compounds (4a-c) with phenyl isothiocyanate gave 2-{[(4,6-disubstituted pyrimidine-2-yl) thio] acetyl}-N-phenylhydrazinecarbothioamides (5a-c) which on treatment with concentrated sulphuric acid afforded titled compounds 5-{(4,6-disubstituted pyrimidine-2-yl) thio] methyl}-N-phenyl-1,3,4-thiadiazole-2-amines (6a-c). These compounds have been characterized on the basis of elemental analysis, IR, 1 H NMR and MS. Compounds have been evaluated for their anticancer and antioxidant activities. Compounds 2b, 2c and 6b exhibited significant antitumor activity against human breast cancer MCF 7 cell line. However, moderate antioxidant activity was observed with compounds 2c, 2d, 2g and 6b.
Keywords
Thiadiazoles, pyrimidines, chalcones, thiourea and anticancer activity
In recent years pyrimidine derivatives have received significant attention owing to their diverse range of biological properties particularly being antifungal [1], antitubercular [2], antibacterial [3,4], antiviral [5-8], anticancer [9] and antioxidant [10]. 2,5-Disubstituted-1,3,4-thiadiazoles represent one of the most active classes of compounds possessing wide spectrum of biological activities. 2,5- Disubstituted-1,3,4-thiadiazole derivatives exhibit in vitro antimycobacterial [11], antibacterial [12], anticancer [13,14] and antioxidant [15] properties. Considering the above facts, the goal of the present study was to combine disubstituted pyrimidines with 1,3,4-thiadiazole residues in order to develop hybrid molecules with potential of enhanced activity and to test their antioxidant and antitumor activities.
Melting points were taken in open capillary tubes and are uncorrected. The IR spectra (KBr, cm-1) were recorded on a Shimadzu FTIR 800 series spectrophotometer and 1H NMR spectra (CDCl3) on Varian EM 390 MHz spectrometer using TMS as internal standard. Mass spectra were recorded on Shimadzu 2010A LC-MS system. The reactions were monitored by thin layer chromatography using silica gel plates and detected by UV chamber and iodine as visualizing agent. The purity of the compounds was checked on silica gel precoated plates. All the solvents used were purified according to the standard methods [16]. Phenyl isothiocyanate was prepared according to the standard method [17].
For the preparation of 4, 6-disubstituted pyrimidine- 2-thiols (2a-i) a mixture of appropriate chalcones (1a-i, Scheme 1) (0.01 mol) and thiourea (0.01 mol) in ethanol (50 ml) and sodium hydroxide (0.01 mol) dissolved in minimum quantity of water was refluxed on a water bath for 12 h and poured into 250 ml of cold water. The solid that separated in each case was filtered, washed with water and recrystallized from ethyl acetate (Table 1); 2a: IR (KBr, cm-1): 3095 (aromatic C-H str.), 2830 (S-H str.), 1640 (C=N), 1590, 1610 (aromatic C=C str.), 1520 (C-N str.); MS: m/z 264 (M+); Anal. Calcd. for C16H12N2S: C, 72.72; H, 4.54; N, 10.60. Found: C, 72.75; H, 4.58; N, 10.56%; 2b: IR (KBr, cm-1): 3120 (aromatic C-H str.), 2840 (S-H str.), 1651 (C=N), 1582, 1606 (aromatic C=C str.), 1516 (C-N str.), 1265 (C-O-C); 1H NMR (CDCl3): δ 9.72 (s, 1H, SH), 6.81-8.32 (m, 11H, aromatic and heterocyclic), 3.75 (s, 3H, OCH3); MS: m/z 294 (M+); Anal. Calcd. for C17H14N2OS: C, 69.38; H, 4.76; N, 9.52. Found: C, 69.37; H, 4.81; N, 9.58%; 2c: IR (KBr, cm-1): 3330 (OH), 3088 (aromatic C-H str.), 2842 (S-H str.), 1649 (C=N), 1608 (aromatic C=C str.), 1518 (C-N str.); MS: m/z 280 (M+); Anal. Calcd. for C16H12N2 OS : C, 68.57; H, 4.28; N, 10.00. Found: C, 68.61; H, 4.31; N, 9.97%; 2d: IR (KBr, cm-1): 3360 (OH), 3082 (aromatic C-H str.), 2835 (S-H str.), 1635 (C=N), 1580, 1608 (aromatic C=C str.), 1524 (C-N str.), 1280 (C-O-C); MS: m/z 310 (M+); Anal. Calcd. for C17H14N2SO2 : C, 65.80; H, 4.51; N, 9.03. Found: C, 65.68; H, 4.54; N, 9.10%; 2e: IR (KBr, cm-1): 3082 (aromatic C-H str.), 2820 (S-H str.), 1635 (C=N), 1580, 1608 (aromatic C=C str.), 1524 (C-N str.), 1345 (NO2), 1275 (C-O-C); MS: m/z 339 (M+); Anal. Calcd. for C17H13N3O3S : C, 60.17; H, 3.83; N, 12.38. Found: C, 60.12; H, 3.85; N, 12.33%; 2f: IR (KBr, cm-1): 3328 (OH), 3086 (aromatic C-H str.), 2840 (S-H str.), 1644 (C=N), 1580, 1605 (aromatic C=C str.), 1524 (C-N str.); MS: m/z 280 (M+); Anal. Calcd. for C16H12N2 OS : C, 68.57; H, 4.28; N, 10.00. Found: C, 68.63; H, 4.34; N, 9.93%; 2g: IR (KBr, cm-1): 3330 (OH), 3060 (aromatic C-H str.), 3010 (C=C, alkene), 2825 (S-H str.), 1615 (C=N), 1598 (aromatic C=C str.), 1524 (C-N str.); MS: m/z 306 (M+); Anal. Calcd. for C18H14N2OS : C, 70.58; H, 4.57; N, 9.15. Found: C, 70.60; H, 4.53; N, 9.19%; 2h: IR (KBr, cm-1): 3310 (OH), 3072 (aromatic C-H str.), 2833 (S-H str.), 1618 (C=N), 1585 (aromatic C=C str.), 1520 (C-N str.), 1105 (C-O-C); MS: m/z 270 (M+); Anal. Calcd. for C14H10N2O2S: C, 62.22; H, 3.70; N, 10.37. Found: C, 62.28; H, 3.67; N, 10.29%; 2i: IR (KBr, cm-1): 3075 (aromatic C-H str.), 2830 (S-H str.), 1610 (C=N), 1605 (aromatic C=C str.), 1522 (C-N str.), 1352 (NO2); MS: m/z 334 (M+); Anal. Calcd. for C18H12N3O2S : C, 64.67; H, 3.59; N, 12.57. Found: C, 64.60; H, 3.62; N, 12.61%.
| Compd. | R | R' | Mol. Formula | M.P. 0C | %Yielda |
|---|---|---|---|---|---|
| 1a | C6H5 | C6H5 | C15H12O | 57 | 80 |
| 1b | C6H5 | 4-OCH3.C6H4 | C16H14O2 | 72 | 85 |
| 1c | C6H5 | 2-OH.C6H4 | C15H12O2 | 66 | 81 |
| 1d | 2-OH.C6H4 | 4-OCH3.C6H4 | C6H14O3 | 75 | 78 |
| 1e | 4-NO2.C6H4 | 4-OCH3.C6H4 | C16H13O4N | 77 | 65 |
| 1f | 2-OH.C6H4 | C6H5 | C15H12O2 | 60 | 85 |
| 1g | 2-OH.C6H4 | C6H5CH=CH- | C17H14O2 | 69 | 55 |
| 1H | 2-OH.C6H4 | 3-furyl | C13H10O3 | 82 | 67 |
| 1i | 4-NO2.C6H4 | C6H5.CH=CH- | C17H13O3N | 78 | 60 |
| 2a | C6H5 | C6H5 | C16H12N2S | 165 | 81 |
| 2b | C6H5 | 4-OCH3.C6H4 | C17H14N2OS | 80 | 75 |
| 2c | C6H5 | 2-OH.C6H5 | C16H12N2OS | 130 | 56 |
| 2d | 2-OH.C6H4 | 4-OCH3.C6H4 | C17H14N2O2S | 120 | 84 |
| 2e | 4-NO2.C6H4 | 4-OCH3.C6H4 | C17H13N3O3S | 140 | 96 |
| 2f | 4-OH.C6H4 | C6H5 | C16H12N2OS | 175 | 89 |
| 2g | 2-OH.C6H4 | C6H5.CH=CH- | C18H14N2OS | 265 | 77 |
| 2h | 2-OH.C6H4 | 3-Furyl | C14H10N2O2S | 101 | 82 |
| 2i | 4-NO2.C6H4 | C6H5.CH=CH- | C18H12N3O2S | 270 | 63 |
| 3a | C6H5 | C6H5 | C20H18N2O2S | 118 | 86 |
| 3b | C6H5 | 4-OCH3.C6H4 | C21H20N2O3S | 198 | 65 |
| 3c | C6H5 | 2-OH.C6H4 | C20H18N2O3S | 212 | 57 |
| 4a | C6H5 | C6H5 | C18H16N4OS | 202 | 65 |
| 4b | C6H5 | 4-OCH3.C6H4 | C19H18N4O2S | 199 | 55 |
| 4c | C6H5 | 2-OH.C6H4 | C18H16N4O2S | 215 | 61 |
| 5a | C6H5 | C6H5 | C25H21N5OS2 | 186 | 62 |
| 5b | C6H5 | 4-OCH3.C6H4 | C26H23N5O2S2 | 189 | 53 |
| 5c | C6H5 | 2-OH.C6H4 | C25H21N5O2S2 | 175 | 64 |
| 6a | C6H5 | C6H5 | C25H19N5S2 | 198 | 56 |
| 6b | C6H5 | 4-OCH3.C6H4 | C26H21N5OS2 | 210 | 51 |
| 6c | C6H5 | 2-OH.C6H4 | C25H19N5OS2 | 235 | 58 |
aIsolated yield, compounds 1a-i were synthesized by the known procedure [21], dall compounds showed satisfactory elemental analysis
Table 1: Characterization Data of Synthesized Compoundsd
Preparation of ethyl [(4,6-disubstituted pyrimidine- 2-yl) thio] acetates (3a-c) was achieved by mixing equimolar quantities of 4,6-disubstituted pyrimidine- 2-thiols (2a-c) (0.017 mol), ethyl chloroacetate (2.017 g, 0.017 mol) and anhydrous potassium carbonate (1.10 g, 0.01 mol) in dry acetone (15 ml) and refluxing on a water bath for about 13 h. The mixture was then diluted with benzene and washed with water. The organic layer was dried (Na2SO4) and the solvent was removed under reduced pressure. The resulting solid in each case was recrystallized from benzene:petroleum ether (1:1) (Table 1); 3a: IR (KBr, cm-1): 3065 (aromatic C-H str.), 2910, 2886 (aliphatic C-H str.), 1745 (>C=O of ester), 712 (C-S-C); MS m/z: 350 (M+); Anal. Calcd. for C20H18N2O2S: C, 68.57; H, 5.14; N, 8.00. Found: C, 68.61; H, 5.18; N, 7.95%; 3b: IR (KBr, cm-1): 3061 (aromatic C-H str.), 2912, 2875 (aliphatic C-H str.), 1736 (>C=O of ester), 1240 (C-O-C), 710 (C-S-C); 1H NMR (CDCl3): δ 6.82-8.10 (m, 10H, aromatic and heterocyclic), 4.32 (q, 2H, COOCH2CH3), 4.12 (s, 2H, S-CH2-CO), 3.72 (s, 3H, OCH3), 1.05 (t, 3H, -COOCH2CH3); MS m/z: 380 (M+); Anal. Calcd. for C21H20N2O3S: C, 66.31; H, 5.26; N, 7.36. Found: C, 66.25; H, 5.32; N, 7.29%; 3c: IR (KBr, cm-1): 3320 (OH), 3070 (aromatic C-H str.), 2930, 2885 (aliphatic C-H str.), 1742 (>C=O of ester), 715 (C-S-C); MS m/z: 366 (M+); Anal. Calcd. for C20H18N2O3S: C, 65.57; H, 4.91; N, 7.65. Found: C, 65.63; H, 5.14; N, 7.58%.
For preparation of 2-[(4,6-disubstituted pyrimidine- 2-yl) thio] acetohydrazides (4a-c), a solution of the appropriate esters (3a-c) (0.01 mol), hydrazine hydrate (3.5 ml) and ethanol (25 ml) was refluxed on a water bath for about 10 h. The solvent was then removed under reduced pressure and the residue obtained in each case was recrystallized from methanol (Table 1); 4a: IR (KBr, cm-1): 3420, 3375 (NH-NH2) 1660 (>C=O, amido), 1622 (C=N str.), 1605 (aromatic C=C), 715 (C-S-C); MS m/z: 336 (M+); Anal. Calcd. for C18H16N4OS: C, 64.28; H, 4.76; N, 16.66. Found: C, 64.33; H, 4.69; N, 16.71%; 4b: IR (KBr, cm-1): 3438, 3380, 3310 (NH-NH2) 1653 (>C=O, amido), 1642 (C=N str.), 1598 (aromatic C=C), 1250 (C-O-C), 712 (C-S-C); 1H NMR (CDCl3): δ 9.51 (s, 1H, CONH), 6.82-7.91 (m, 10H, aromatic and heterocyclic), 6.49 (bs, 2H, NH2), 4.20 (s, 2H, S-CH2-CO), 3.71 (s, 3H, OCH3); MS m/z: 366 (M+); Anal. Calcd. for C19H18N4O2S: C, 62.29; H, 4.92; N, 15.30. Found: C, 62.31; H, 4.87; N, 15.29%; 4c: IR (KBr, cm-1): 3380 (OH), 3345, 3320 (NH-NH2), 1668 (>C=O, amido), 1622 (C=N str.), 1598 (aromatic C=C), 711 (C-S-C); MS m/z: 352 (M+); Anal. Calcd. for C18H16N4O2S: C, 61.36; H, 4.54; N, 15.90. Found: C, 61.41; H, 4.59; N, 15.83%.
For preparation of 2-[(4, 6-disubstituted pyrimidine- 2-yl) thio] acetyl-N-phenylhydrazine carbothiamide (5a-c), a mixture of the acid hydrazides (4a-c) (0.01 mol) and phenylisothiocyanate (0.0015 mol) in ethanol (10 ml) was refluxed on a water bath for about 8 h. The solution was allowed to reach ambient temperature and the resulting solid in each case was collected and recrystallized from methanol (Table 1); 5a: IR (KBr, cm-1): 3225, 3215, 3180 (N-H), 3035, (aromatic C-H str.), 1670 (>C=O), 1625 (C=N), 1605 (aromatic C=C str.), 1450 (>C=S), 718 (C-S-C); MS m/z: 471 (M+); Anal. Calcd. for C25H21N5OS2: C, 63.69; H, 4.45; N, 14.86. Found: C, 63.72; H, 4.37; N, 14.79%; 5b: IR (KBr, cm-1): 3120-3218 (N-H), 3024 (aromatic C-H str.), 1681 (>C=O), 1667 (C=N), 1605 (aromatic C=C str.), 1453 (>C=S), 1255 (C-O-C), 710 (C-S-C); 1H NMR (CDCl3): δ 8.20-10.12 (m, 3H, NH.NH.CS.NH), 7.21-7.92 (m, 15H, aromatic and heterocyclic), 4.21(s, 2H, S-CH2- CO), 3.48 (s, 3H, OCH3); MS m/z: 501 (M+); Anal. Calcd. for C26H23N5O2S2: C, 62.27; H, 4.59; N, 13.97. Found: C, 61.98; H, 4.48; N, 13.89%; 5c: IR (KBr, cm-1): 3340 (OH), 3218, 3205, 3185 (N-H), 3045, (aromatic C-H str.), 1695 (>C=O), 1625 (C=N), 1598 (aromatic C=C str.), 1448 (>C=S), 714 (C-S-C); MS m/z: 487 (M+); Anal. Calcd. for C25H21N5O2S2: C, 61.60; H, 4.31; N, 14.37. Found: C, 61.67; H, 4.39; N, 14.31%.
For preparation of 5-{[(4,6-disubstituted pyrimidine- 2-yl) thio] methyl}-N-phenyl-1,3,4-thiadiazol-2- amine (6a-c), a mixture of the appropriate thiosemicarbazides (5a-c) (0.001 mol) in cold concentrated sulphuric acid (3 ml) was stirred for 10 min the resulting solution was then allowed to reach ambient temperature and poured cautiously into ice cold water. The reaction mixture was made alkaline to pH 8 with aqueous ammonia and the precipitated product in each case was collected washed with cold water and recrystallized from ethanol (Table 1); 6a: 3395 (N-H), 3095 (aromatic C-H str.), 2955 (C-H str.), 1620 (C=N, str.), 1605 (aromatic C=C str.), 740 (C-S-C, thiadiazole), 710 (C-S-CH2); MS m/z: 453 (M+); Anal. Calcd. for C25H19N5S2: C, 66.22; H, 4.19; N, 15.45. Found: C, 66.29; H, 4.14; N, 15.39%; 6b: 3440 (N-H), 3211 (aromatic C-H str.), 2939 (C-H str.), 1665 (C=N, str.), 1599 (aromatic C=C str.), 1248 (C-O-C), 746 (C-S-C, thiadiazole), 708 (C-S-CH2); 1H NMR (CDCl3): δ 9.3 (s, 1H, NH), 7.21-8.32 (m, 15H, aromatic and heterocyclic), 3.38 (s, 3H, OCH3), 3.61 (s, 2H, -CH2-); MS m/z: 483 (M+); Anal. Calcd. for C26H21N5OS2: C, 64.59; H, 4.34; N, 14.49. Found: C, 64.61; H, 4.42; N, 14.51%; 6c: 3380 (N-H), 3345 (OH), 3070 (aromatic C-H str.), 2975 (C-H str.), 1612 (C=N, str.), 1610 (aromatic C=C str.), 726 (C-S-C, thiadiazole), 712 (C-S-C); MS m/z: 469 (M+); Anal. Calcd. for C25H19N5OS2: C, 63.96; H, 4.05; N, 14.92. Found: C, 64.11; H, 4.09; N, 14.89%.
Determination of In vitro antioxidant activity was done by DPPH (1,1-diphenyl-2-picrylhydrazyl) [18] and nitric oxide [19] free radical scavenging methods. The methods were used to screen compounds (2a-i) and (6a-c) for the antioxidant activity. Ascorbic acid and rutin were used as reference standards at a concentration level of 100 μg/ml. Results are presented in Table 2.
| Compd. | Antioxidant activity | Average percent growth values** at 20 M in MCF-7 cell line | |
|---|---|---|---|
| DPPH method IC50 ( g/ml)* | Nitric oxide method IC50 ( g/ml)* | ||
| 2a | >500 | 105 | 67.77 |
| 2b | >500 | 160 | 13.33 |
| 2c | 56 | 95 | 0 |
| 2d | 50.5 | 90 | 79.98 |
| 2e | 84 | >500 | 34.44 |
| 2f | >500 | 125 | 94.44 |
| 2g | 60 | 200 | 77.50 |
| 2h | 84 | >500 | 43.55 |
| 2i | >500 | >500 | 97.4 |
| 6a | 82 | 143 | 68.54 |
| 6b | 92 | 87 | 12.55 |
| 6c | 78 | >500 | 58.50 |
| 18 | 69 | -- | |
| (ascorbic acid) | (rutin) | ||
*Average of three determinations, both test compounds and standard were tested at 100 μg/ml, IC50 concentration of the test compound causing 50% decrease of activity against control. **Mean of two determinations, azero indicates that no cells have died.
Table 2: In Vitro Anticancer And Antioxidant Activities Of Compounds (2a-I) And (6a-C)
Antitumor activity of the compounds was evaluated by tryphan blue dye exclusion technique [20] against human breast cancer MCF-7 cell line at 20 μM concentration. Primary screening of the compounds was done to indicate whether a substance possessed enough activity at this concentration to inhibit cell growth by 50%. Results are presented in Table 2.
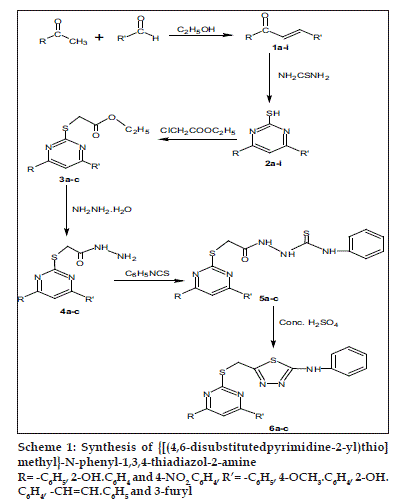
Chalcones (1a-i) required as starting material were prepared [21] by stirring equimolar solution of various substituted acetophenones and araldehydes in the presence of sodium hydroxide in ethanol at room temperature (Scheme 1). Solution in ethanol of chalcones (1a-i) and thiourea in the presence of sodium hydroxide was refluxed on a water bath to yield 4,6-disubstituted pyrimidine-2-thiols (2a-i). When thiols (2a-c) and ethyl chloroacetate was refluxed in the presence of anhydrous sodium carbonate resulted in the formation of ethyl [(4,6-disubstituted pyrimidine-2-yl) thio] acetates (3a-c). Compounds (3a-c), hydrazine hydrate in ethanol as a reaction media afforded 2-[(4,6-disubstitutedpyrimidine- 2-yl) thio] acetohydrazides (4a-c), which on condensation with phenyl isothiocyanate in ethanol gave 2-{[(4,6-disubstituted pyrimidine-2-yl) thio] acetyl}-N-phenylhydrazinecarbothioamides (5a-c). The compounds (5a-c) on treatment with concentrated sulphuric acid yielded 5-{[(4,6-disubstituted pyrimidine-2-yl) thio] methyl}-N-phenyl-1, 3, 4-thiadiazol-2-amines (6a-c). All the compounds synthesized were characterized by their elemental analysis, IR and 1H NMR spectra. The physical and chemical data are presented in Table 1. In the IR spectrum of 2b, the presence of band at 2840 cm-1 (SH) and the absence of band due to >C=O confirmed the formation of pyrimidine-2-thiol moiety. The appearance of singlet at δ 9.72 due to SH also confirmed the formation of 2b. The IR spectrum of 5b exhibited band at 1681 cm-1 (>C=O), 1667 cm-1 (>C=N) and 3120-3218 cm-1 due to N-H. The 1H NMR spectrum of 5b exhibited the aromatic and heterocyclic protons as a multiplet integrating for 15 protons from δ 7.21-7.92 and a multiplet integrating for 3 protons from δ 8.20-10.12 due to NH.NH. CS.NH. In the IR spectrum of 6b, the disappearance of bands at 1681 cm-1 (>C=O) and 1453 cm-1 (>C=S) and the appearance of band at 746 cm-1 (C-S-C) confirmed the formation of thiadiazole ring. The 1H NMR spectrum of 6b exhibited the aromatic and heterocyclic protons as a multiplet integrating for 15 protons from δ 7.21-8.32 and a singlet at δ 9.3 integrating for one proton due to NH. In the mass spectra, the molecular ion peak at 483 (M+) also confirmed the formation of titled compound 6b.
Compound 2c, 2d, 2g and 6b showed moderate DPPH free radical scavenging activity while all other compounds were found to be less active. Compounds 2c, 2d and 6b showed moderate nitric oxide free radical scavenging activity and all other compounds were found to be less active. As shown in Table 2 compounds 2b, 2c and 6b exhibited significant activity against human breast cancer MCF-7 cell line, while compounds 2e and 2h showed moderate cytotoxicity.
Acknowledgements
The authors wish to place their regards to His Holiness Jagadguru Sri Sri Sri Shivarathri Deshikendra Mahaswamigalavaru of Sri Suttur mutt, Mysore for providing facilities.
References
- Singh DV, Misha AR, Misha RM, Pandey AK, Dwivedi AK. Synthesisand fungicidal activity of benzofuran incorporated substitutedpyrimidines. Indian J HetrocyclChem 2005;14:319-22.
- Ahluwalia VK, Madhu B. A facile one pot synthesis of some newderivatives of pyrano [2,3-d] pyrimidines as potential antibacterial andantifungal agents. Indian J Chem 1996;35B:742-44.
- Shamroukh AH, Rashed AE, Sayed HH. Synthesis of some pyrazolo[3,4]pyrimidine derivatives for biological evaluation. Phosphorus, Sulphur,and Silicon and the Related Elements 2005;180:2347-60.
- Pasha TY, Udupi RH, Bhat AR. Synthesis and antimicrobial screeningof some pyrimidine derivatives. Indian J HeterocyclChem 2005;15:149-52.
- Lin TS, Guo JY, Schinazi RF, Chu CK, Xiang JN, Prussof WH.Synthesis and antiviral activity of various 3'-azido analogs of pyrimidinedeoxyribonucleosides against human immunodeficiency virus (HIV-I, HTLV-III/LAV). J Med Chem 1988;31:336-40.
- Holy A, Votruba I, Masojidkova M, Andrei G, Snoeca R, Naesens L, etal. 6-[2-(Phosphonomethoxy)alkoxy]pyrimidines with antiviral activity.J Med Chem 2002;45:1918-29.
- Rakesh K, Nath M, Tyrell DL. Design and synthesis of novel5-substituted acyclic pyrimidine nucleosides as potent and selectiveinhibitors of hepatitis B virus. J Med Chem 2002;45:2032-40.
- Joule JA, Mills K, Smith GF, editors. Heterocyclic chemistry, 3rd ed.London: CRC Press; 1995. p. 189-24.
- Skibo EB, Huang X, Martinez R, Lemus RH, Craigo WA, Derr RT.Pyrimidoquinazoline-based antitumor agents: Design of topoisomeraseII to DNA cross-linkers with activity against protein kinases. J MedChem 2002;45:5543-55.
- Vidal A, Ferrandiz ML, Ubeda A, Guillen I, Riguera R, Quintela JM, etal. Effects of some isoxazolpyrimidine derivatives on nitric oxide andeicosanoid biosynthesis. Life Sci 2000;66:125-31.
- Foroumadi A, Soltani F, Razee MA, MoshafiMH. Synthesis andevaluation of In vitro antimycobacterial activity of some 5-(5-nitro-2-thienyl)-2-(piperazinyl, piperidinyl and morpholinyl)-1,3,4-thiadiazole derivatives. Boll Chim Farm 2003;142:416-9.
- Foroumadi A, Emani S, Hassanzadeh A, Rajaee M, Sokhanvar K,MoshafiMH, et al. Synthesis and antibacterial activity of N-(5-benzylthio-1,3,4-thiadiazol-2-yl)piperazinylquinolone. Bioorg Med ChemLett 2005;15:4488-92.
- Matysiak J, Nasulewicz A, Pelczynska M, Switalska M, JaroszewiczI, Opolski A. Synthesis and antiproliperative activity of some5-substituted 2-(2,4-dihydroxyphenyl)-1,3,4-thiadiazoles. Eur J MedChem 2006;41:475-82.
- Matysiak J. Evaluation of antiproliferative effect In vitro of some2-amino-5-(2,4-dihydroxyphenyl)-1,3,4-thiadiazole derivatives. ChemPharm Bull 2006;54:988-91.
- Martinez A, Alonso D, Castro A, Aran VJ, Cardelus I, Banos JE, etal. Synthesis and potential muscarinic receptor binding and antioxidantproperties of 3-(thiadiazolyl)pyridine 1-oxide compounds. Arch Pharm (Weinheim) 1999;332:191-4.
- Furniss BS, Hannaford AJ, Smith PW, Tatchell AR, editors. Vogel’sText Book of Practical Organic Chemistry. 5th ed. Singapore: PearsonEducation Pvt. Ltd.; 2005. p. 395-69.
- Gilman H, Adams R, Marvel CS, Clarke HT, Noller CR, Conant JB,et al. editors. Organic Syntheses, Collective Vol. I. 2nd ed. New York:John Wiley and Sons, Inc; 1988. p. 447.
- Hemant R, Jadav, Bhutani KK. Antioxidant properties of Indianmedicinal plants.Phytother Res 2002;16:771-73.
- Bishayee S, Balasubramanium AS. Lipid peroxide formation in ratbrain. J NeuroChem 1971;18:902-20.
- Bracht K, Boubakari R, Grunert P, Bednarski PJ. Correlations betweenthe activities of 19 antitumor agents and the intracellular glutathioneconcentrations in a panel of 14 human cancer cell lines: Comparisonswith the national cancer institute data. Anticancer Drugs 2006;17:41-21.
- Furniss BS, Hannaford AJ, Smith PW, Tatchell AR, editors. Vogel’sText Book of Practical Organic Chemistry. 5th ed. Singapore: PearsonEducation Pvt. Ltd.; 2005. p. 1028-34.