- *Corresponding Author:
- K. K. Srinivasan
Department of Pharmaceutical Chemistry, Manipal College of Pharmaceutical Sciences, MAHE, Manipal - 576 104, India
E-mail: pansrini@yahoo.co.in
| Date of Submission | 11 September 2006 |
| Date of Revision | 22 January 2007 |
| Date of Acceptance | 18 June 2007 |
| Indian J Pharm Sci 2007, 69 (3): 470-473 |
Abstract
Chalcones (2a and 2b) were prepared from 2-acetyl benzofuran (1) and condensed with different aromatic acid hydrazides (3a-o) to get the corresponding pyrazolines (4a-o and 5a-o). The structures of all these compounds have been established on the basis of analytical and spectral data. Compounds have been screened for antiinflammatory, antioxidant and antibacterial studies. Among the 7 compounds that were screened for antiinflammatory activity, compounds 4g and 5m showed 83.4% and 80.5% inhibition of oedema volume, while the standard drug (ibuprofen) showed inhibition of 91.9%. Compounds 4k and 5h showed moderate activity of 72.8% and 59.6% respectively. All the 30 compounds were tested for antioxidant activity at 1000, 500, 250, 100, 50, 25 and 10 mg/ml concentrations against standard drug ascorbic acid. Compounds 4g, 4h, 4k, 4m, 5g, 5h, 5k and 5m showed excellent antioxidant activity as compared with ascorbic acid. Among the 30 compounds that were screened against two Gram +ve ( Staphylococcus aureus and Bacillus subtilis ) and two gram -ve ( Escherichia coli and Pseudomonas aeruginosa ) organisms, compounds possessing p-chloro, p-fluoro, 2-amino-5-bromo, 2-hydroxy-5-nitro and 3,5-dichloro substitutions on the phenyl ring showed good activity against Escherichia coli and Bacillus subtilis . The activity is comparable with that of the standard drug ciprofloxacin.
Keywords
Benzofuran, pyrazoline, antioxidant, antiinfl ammatory, antibacterial
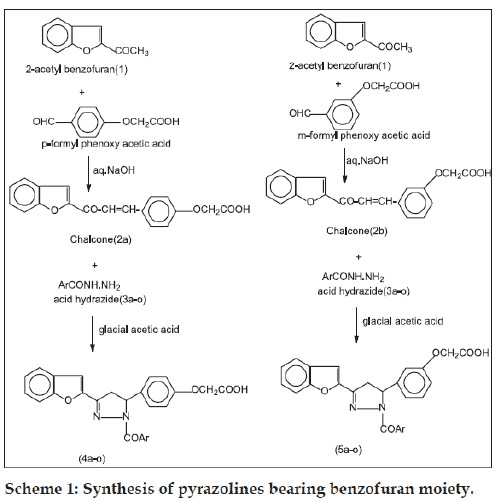
In view of the various activities reported for compounds possessing benzofuran [1-2] and pyrazoline moiety [3-5], in the present study we attempted to synthesize benzofuran pyrazolines, as they appeared to be highly promising. Since the compounds are mainly targeted for antiinflammatory activity, an acidic group is introduced at the 5th position on the pyrazoline ring in the form of m (or) p-phenoxyacetic acid. Benzofuran chalcones (2a and 2b) were synthesized by reacting 2-acetyl benzofuran (1) with m (or) p-formyl phenoxyacetic acid in the presence of aqueous sodium hydroxide (10%). The chalcones on refluxing with substituted acid hydrazides (3a-o) in the presence of glacial acetic acid at above 130o for a period of 10 h afforded different pyrazolines (4a-o and 5a-o) and were recrystallized from boiling aqueous ethanol. The structure of chalcones and pyrazolines were confirmed by mp, tlc and spectral data.
The melting points of the compounds were determined on a Toshniwal electric melting point apparatus and the values were uncorrected. IR spectra of the compounds were recorded on a Shimadzu-FTIR 8300 using the KBr disc method. 1H NMR spectra were recorded on a Joel-GSX 400, (IIT Chennai) using DMSO-d6 as solvent. Mass spectra were recorded on a Shimadzu-GCMS 50508. All the solvents used were of analytical grade.
2-acetylbenzofuran (1) was prepared following the literature method [6]. Chalcone (2a/2b) was prepared by adding a solution of sodium hydroxide (8 ml, 10% w/v solution of NaOH in water) to a well-stirred solution of 2-acetyl benzofuran (0.01 mol) and m/pformyl phenoxy acetic acid (0.01 mol) in 20 ml of ethanol at room temperature. The solution was stirred at room temperature for 24h using a magnetic stirrer. The reaction mixture was diluted with ice-cold water and acidified with concentrated HCl. The product obtained was filtered and washed with ice-cold water and recrystallized from aqueous ethanol. Solvent system used for tlc was chloroform:acetone (1:1). Pyrazolines (4a-o and 5a-o) were prepared as shown in scheme 1 by taking chalcone (0.01 mol) and aromatic acid hydrazide (0.02 mol) in 20 ml glacial acetic acid and refluxing at 130o for a period of 10 h. The reaction mixture was concentrated, poured into 300 ml of ice-cold water and the product was recrystallized from aqueous ethanol. The solvent system used for tlc was a 1:1 mixture of acetone: methanol. The yield and melting points are given in Table 1. 1 - b e n z o y l - 3 - ( b e n z o f u r a n - 2 - y l ) - 5 - ( 4 ’ - carboxymethyleneoxyphenyl)-2-pyrazoline (4a): IR (KBr): 1735.8 (C=Ostr of COOH), 1662.5 (N-C=Ostr), 1608.5 (C=Nstr), 1548.7 (C=Cstr), 1139.9 (C-O-C Str), 713.6, 750.3 (mono substituted phenyl); 1H NMR (DMSO) δppm: 3.1-3.5 (d, 2H, CH2 of pyrazoline); 4.5-5.5 (t, 1H, N-CH-Ar); 10.5 (s, 1H of COOH); 6-9 (m, 14H, Ar-H); 4.6-4.9 (s, 2H of OCH2COOH); MS: (m/z): 440(M+), 262.2 (100%), 248.1,186.
| Comp. | Molecular formula | Ar | M.P. (O) | Yield (%) | Rf* |
|---|---|---|---|---|---|
| 4a | C26 H20 N2 O5 | Phenyl | 96-98 | 62 | 0.60 |
| 4b | C26 H20N2 O6 | 4-hydroxy phenyl | 85-90 | 45 | 0.9 |
| 4c | C26 H19 N2 O5 Cl | 4-chloro phenyl | 80-85 | 75 | 0.66 |
| 4d | C26 H19 N2 O5 Cl | 2-chloro phenyl | 106-108 | 78 | 0.7 |
| 4e | C26 H19 N3O7 | 4-nitro phenyl | 95-97 | 63 | 0.82 |
| 4f | C26 H19 N3 O7 | 2-nitro phenyl | 93-96 | 32 | 0.88 |
| 4g | C26 H19 N2 O5 F | 4-fluoro phenyl | 89-93 | 57 | 0.87 |
| 4h | C27 H22N2 O5 | o-tolyl | 120-122 | 67 | 0.8 |
| 4i | C26 H20 N2 O6 | 2-hydroxy phenyl | 80-83 | 68 | 0.89 |
| 4j | C26 H21 N3 O5 | 4-amino phenyl | 120-122 | 54 | 0.83 |
| 4k | C25 H20 N3 O5 | isonicotinyl | 98-102 | 58 | 0.78 |
| 4l | C27 H21N2 O6 | 4- methoxy phenyl | 89-92 | 72 | 0.85 |
| 4m | C26 H20 N3O5 Br | 2-amino-5-bromo phenyl | 116-120 | 76 | 0.9 |
| 4n | C26 H19 N3O8 | 2-hydroxy- 5-nitro phenyl | 123-125 | 66 | 0.89 |
| 4o | C26 H18N2 O5 Cl2 | 3,5 dichloro phenyl | 100-112 | 62 | 0.75 |
| 5a | C26 H20 N2 O5 | Phenyl | 100-103 | 60 | 0.62 |
| 5b | C26 H20 N2 O6 | 4-hydroxy phenyl | 90-93 | 42 | 0.89 |
| 5c | C26H19N2O5Cl | 4-chloro phenyl | 91-93 | 73 | 0.65 |
| 5d | C26 H19 N2 O5 Cl | 2-chloro phenyl | 100-105 | 73 | 0.7 |
| 5e | C26 H19 N3O7 | 4-nitro phenyl | 90-94 | 35 | 0.82 |
| 5f | C26 H19 N3O7 | 2-nitro phenyl | 89-91 | 52 | 0.87 |
| 5g | C26 H19 N2 O5 F | 4-fluoro phenyl | 115-120 | 67 | 0.86 |
| 5h | C27 H22N2 O5 | o-tolyl | 118-120 | 68 | 0.8 |
| 5i | C26 H20 N2 O6 | 2-hydroxy phenyl | 89-90 | 69 | 0.82 |
| 5j | C26 H21N3O5 | 4-amino phenyl | 116-118 | 72 | 0.83 |
| 5k | C25H20N3O5 | isonicotinyl | 113-115 | 70 | 0.78 |
| 5l | C27 H21N2 O6 | 4- methoxy phenyl | 96-99 | 32 | 0.85 |
| 5m | C26 H20 N3O5 Br | 2-amino-5-bromo phenyl | 112-116 | 35 | 0.9 |
| 5n | C26 H19 N3O8 | 2-hydroxy- 5-nitro phenyl | 120-122 | 64 | 0.89 |
| 5o | C26 H18N2 O5 Cl2 | 3,5 dichloro phenyl | 113-116 | 60 | 0.74 |
Table 1: Physical Data of Synthesized Compounds
1-(2’’-methylbenzoyl)-3-(benzofuran-2-yl)-5-(4’- carboxymethyleneoxyphenyl)-2-pyrazoline (5h): IR (KBr): 2923.9 (C-H str), 1666.4 (N-C=O str), 1610.5 (C=N str), 1548.7 (C=Cstr), 1139.9 (C-O-Cstr.); 1H NMR(DMSO) δppm: 3.1-3.5 (d, 2H, CH2 of pyrazoline); 4.5-5.5 (t, 1H, N-CH-Ar); 10.5 (s, 1H of OH), 10.1 (s, 1H of COOH); 6-9 (m, 13H, Ar-H); 4.6-4.9 (2s, 2H of OCH2COOH); MS: (m/z): 454 (M+), 336, 262, 248, 186, 158, 144.
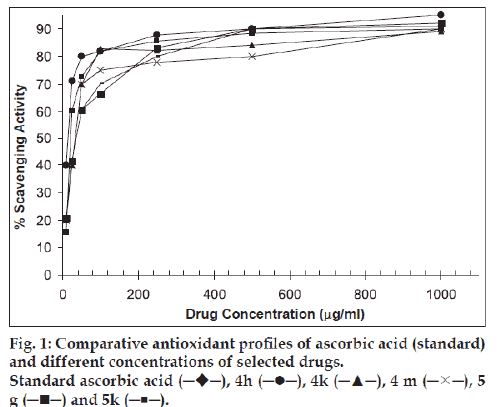
All the compounds were screened for antioxidant activity using DPPH method [7]. Stock solutions of synthetic compounds have been diluted in 95% ethanol to obtain 1000, 500, 250, 100, 50, 25 and 10 mg concentrations. DPPH solution (2 mmol) was prepared in 95% ethanol. To 0.5 ml of drug solution, 0.5 ml of DPPH solution (freshly prepared) was added, mixed and the reaction was allowed for 20 min. UV absorbance was measured at 517 nm. Ascorbic acid was used as a standard drug. Percentage scavenging for each drug was calculated using the formula, (control absorbance-test absorbance/ control absorbance)×100. The results were presented graphically in fig. 1.
Compounds with high antioxidant activity (7 compounds) were selected and screened for antiinflammatory activity using carrageenan-induced paw oedema method [8]. Ibuprofen was used as a standard drug and the results are shown in Table 2.
| Synthetic compound code | Dose (mg/kg) | Mean oedema volume ± S.E.(0-3 hrs) | % reduction in oedema volume |
|---|---|---|---|
| Control | - | 0.42 ± 0.192 | |
| Ibuprofen | 200 | 0.0416 ± 0.017 | 91.13 |
| 4c | 200 | 0.26 ± 0.19a | 32.89 |
| 4e | 200 | 0.28 ± 0.171a | 40.14 |
| 4g | 200 | 0.075 ± 0.03a | 83.89 |
| 4k | 200 | 0.19 ± 0.077a | 59.57 |
| 5b | 200 | 0.27 ± 0.17a | 40.39 |
| 5h | 200 | 0.12 ± 0.049a | 72.79 |
| 5m | 200 | 0.09 ± 0.037a | 80.49 |
Table 2: Antiinflammatory studies of Synthesized compounds
All the 30 synthesized compounds were screened against two gram+ve (S. aureus and B. subtilis) and two gram-ve (E. coli and P. aeuruginosa) organisms using cup-plate method [9] at a concentration of 10 mg and 25 mg of drug per cup. Ciprofloxacin was used as a standard drug at a concentration of 10 mg. The results are shown in Tables 3 and 4.
| Comp. | Amount of drug per cup | Zone of inhibition (mm) | |||
|---|---|---|---|---|---|
| Staph. aureus | Bacillus subtilis | E. coli | P. aeruginosa | ||
| 4a | 10 μg | 12 | - | - | - |
| 25 μg | 14 | - | - | - | |
| 4b | 10 μg | 13 | - | 14 | 11 |
| 25 μg | 14 | - | 15 | 12 | |
| 4c | 10 μg | 14 | 16 | 12 | - |
| 25 μg | 15 | 18 | 13 | - | |
| 4d | 10 μg | - | - | - | - |
| 25 μg | - | - | - | - | |
| 4e | 10 μg | 14 | 14 | 14 | - |
| 25 μg | 15 | 16 | 15 | - | |
| 4f | 10 μg | - | - | - | - |
| 25 μg | - | - | - | - | |
| 4g | 10 μg | 14 | 16 | 12 | 12 |
| 25 μg | 15 | 18 | 16 | 13 | |
| 4h | 10 μg | - | - | - | - |
| 25 μg | - | - | - | - | |
| 4i | 10 μg | - | - | - | - |
| 25 μg | - | - | - | - | |
| 4j | 10 μg | - | - | - | - |
| 25 μg | - | - | - | - | |
| 4k | 10 μg | 11 | 10 | 10 | - |
| 25 μg | 12 | 12 | 11 | - | |
| 4l | 10μg | - | - | - | - |
| 25 μg | - | - | - | - | |
| 4m | 10 μg | 13 | 15 | 13 | - |
| 25 μg | - | - | - | - | |
| 4n | 10 μg | 12 | 16 | 14 | - |
| 25 μg | 13 | 17 | 16 | - | |
| 40 | 10 μg | - | 14 | 14 | - |
| 25 μg | - | 17 | 17 | - | |
Table 3: Antibacterial Studies of Synthesized Compounds
| Comp. | Amount of drug per cup | Zone of inhibition (mm) | |||
|---|---|---|---|---|---|
| Staph. Aureus | Bacillus subtilis | E. coli | P. aeruginosa | ||
| 5a | 10 μg | 10 | - | - | - |
| 25 μg | 11 | - | - | - | |
| 5b | 10 μg | 12 | - | 13 | 10 |
| 25 μg | 13 | - | 14 | 11 | |
| 5c | 10 μg | 12 | 14 | 10 | - |
| 25 μg | 14 | 15 | 12 | - | |
| 5d | 10 μg | - | - | - | - |
| 25 μg | - | - | - | - | |
| 5e | 10 μg | 14 | 14 | 14 | - |
| 25 μg | 15 | 16 | 15 | - | |
| 5f | 10 μg | - | - | - | - |
| 25 μg | - | - | - | - | |
| 5g | 10 μg | 14 | 16 | 12 | 12 |
| 25 μg | 15 | 18 | 16 | 13 | |
| 5h | 10 μg | - | - | - | - |
| 25 μg | - | - | - | - | |
| 5i | 10 μg | - | - | - | - |
| 25 μg | - | - | - | - | |
| 5j | 10 μg | - | - | - | - |
| 25 μg | - | - | - | - | |
| 5k | 10 μg | 11 | 10 | 10 | - |
| 25 μg | 12 | 12 | 11 | - | |
| 5l | 10 μg | - | - | - | - |
| 25 μg | - | - | - | - | |
| 5m | 10 μg | 11 | 12 | 14 | - |
| 25 μg | 12 | 13 | 16 | - | |
| 5n | 10 μg | 10 | 14 | 13 | - |
| 25 μg | 11 | 16 | 17 | - | |
| 5o | 10 μg | - | 14 | 13 | - |
| 25 μg | - | 16 | 16 | - | |
| Std. | 10 μg | 21 | 20 | 20 | 19 |
Table 4: Antibacterial Studies Of Synthesized Compounds
Among the 30 compounds synthesized, compounds 4g, 4h, 4k, 4m, 5g, 5h, 5k and 5m have shown good antioxidant activity, which was comparable with that of standard drug, ascorbic acid. Among the seven compounds screened for antiinflammatory activity, compounds 4g and 5m showed 83.9% and 80.5% inhibition of oedema volume, while the standard drug (ibuprofen) showed 91.9% inhibition. Compounds 4k and 5h showed moderate activity (72.8% and 59.6%). A good correlation was observed between antioxidant activity and antiinflamatory activity among the above compounds. The results clearly indicated that benzofuran pyrazolines with m/p-phenoxyacetic acid moiety at 5th position and with suitably substituted phenyl ring at 1st position on pyrazoline ring can exhibit good antioxidant and antiinflammatory activities. Among the 30 compounds that were screened against two gram +ve (S. aureus and B. subtilis) and two gram –ve (E. coli and P. aeruginosa) organisms, compounds possessing electron releasing groups (p-chloro, p-fluoro, 2-amino-5-bromo, 2-hydroxy-5-nitro and 3,5- dichloro) on the phenyl ring showed good activity against E. coli and B. subtilis and the activity is comparable with that of the standard drug ciprofloxacin.
Acknowledgements
The authors are thankful to Dr. Udupa, Principal, Manipal College of Pharmaceutical Sciences, Manipal for providing necessary facilities to carry out the research. The authors are thankful to Dr. Vasanthkumar, HOD, Pharmacology, MAHE, Manipal and to Mr. J. Venkata Rao, for providing laboratory facilities to conduct pharmacological and microbiological activities. The authors are also thankful to IIT, Madras for providing NMR and Mass spectral data.
References
- Nasef AM, El Naem SI, El Shabrawy OA. Synthesis and analgesic activity of benzofuran carboxamide derivatives. Egypt J Pharm Sci 1992;33:463.
- Chakrabarthi KJ, Richard EJ, Peter GT, Janette H, Terrence HA. 5-acyl-3- substituted benzofuran-2(3H)-ones as potential antiinflammatory agents. J Med Chem 1987;30:1663.
- Shah M, Patel P, Korgaokar S, Parekh H. Synthesis of pyrazolines, isoxazoles and cynopyridines as potential antimicrobial agents. Indian J Chem 1996;35:1282.
- Shenoy GG, Bhat AR, Bhat VG, Kotian M. Synthesis and antimicrobial activities of 1,3,5-trisubstituted -2- pyrazolines. Indian J Heterocyclic Chem 2001;10:197.
- Singh GB, Andatra Singh SJ, Khajuria CS. synthesis and antiinflammatory activity of 1-acetyl-3-(2,4- dimethoxyphenyl)-5-phenyl-2-pyrazolines. J. Indian ChemSoc 1993;70:266.
- Vidya VP, Agasimuddin YS. Synthesis of some benzofuro(3,2-d)-pyramidines and benzofuro(3',2':4,5)pyramido(1,2-b) benzo(d)thiazoles. Indian J Chem 1981;20:114-7.
- Sreejayan N Rao. MNA.Int J Pharma 1993;100:93.
- Winter CA, Risely EA, Nuss GW. Proc Soc Exp Biol 1962;111:54719.
- Gillespie SH. Medical Microbiology-Illustrated. 1st ed. Butterworth Heinemann; 1994.