| Date of Submission | 9 December 2009 |
| Date of Revision | 28 August 2010 |
| Date of Acceptance | 15 November 2010 |
| Indian J Pharm Sci, 2010, 72 (6): 801-806 |
Abstract
Chalcones and their synthetic analogues appear to have the same binding site of tubuline as phenstatin, combretastatin steganacin and podophylotoxin and are therefore capable to inhibit cancer cell proliferation. The phenyl rings with appropriate substitutions maintain a fixed distance between two centers of aryl rings. The two aromatic rings in these molecules are arranged like the two wings of a butterfly having certain dihedral angle between them, therefore a "butterfly model" is proposed an important structural feature responsible for their antitubulin activity. In this sequence a series of chalcones were synthesized and evaluated for in vitro cytotoxic activity against a panel of human cancer cell lines. In addition the synthetics reduced MIC of ciprofloxacin upto four fold this indicates their bioavailability enhancing potential.
Keywords
Antimicrobial, chalcones, in vitro cytotoxicity
Chalcone (trans-1,3-diphenyl-2-propen-1-one) is an α,β-unsaturated ketone that has the skeletal makeup of so-called “chalcones”. Chalcones are open-chain flavonoids in which two aromatic rings, joined by a three carbon linker, are synthesized by chalcone synthetase from 3-malonyl-CoA and a starter CoA ester such as 4-coumaronyl-CoA in plants [1]. Chalcone synthetase functions as a key enzyme of flavonoid biosynthesis, utilizing the same substrates as stilbene synthetase [2]. Chalcones are also called anthochlor pigments. This term was coined to identify a group of yellow pigments which turn red in the presence of alkali. In some plants, chalcones contribute significantly to the corolla pigmentation. They are also found in naturally occurring compounds, such as plant allelochemicals, and insect hormones and pheromones [3].
Chalcones are prepared by condensing aryl ketones with aromatic aldehydes in the presence of suitable condensing agents. They undergo a variety of chemical reactions and are found useful in synthesis of variety of heterocyclic compounds [4]. α,β-unsaturated ketones, which are structures in which the double bond is adjacent to the carbonyl group, have been used as starting materials for the synthesis of various chemicals, including plastics, resins, pesticides, dyes, and pharmaceuticals [5].
The compounds with the backbone of chalcones have been reported to possess various biological activities such as antimicrobial [6], antiinflammatory [7], analgesic [8], antiplatelet [9], antiulcerative [10], antimalarial [11], anticancer [12], antiviral [13], antileishmanial [14], antioxidant [15], antitubercular [16], antihyperglycemic [17], immunomodulatory [18], inhibition of chemical mediators release [19], inhibition of leukotriene B4 [20], inhibition of tyrosinase [21] and inhibition of aldose reductase [22], estrogenic activities [23]. Since anticancer compounds like phenstatin, combretastatin, colchicines, steganacin and certain other synthetic analogues of these compounds have a common structural feature of possessing two rings with appropriate substitutions. We therefore propose a butterfly model with two wings represented by two aromatic rings connected by n number of carbon atoms (n= 1-4).
Due to the rapid development of bacterial resistance to antibacterial agents, it is vital to discover novel scaffold for the design and synthesis of the new antibacterial agents to help in the battle against pathogenic microorganisms [24].
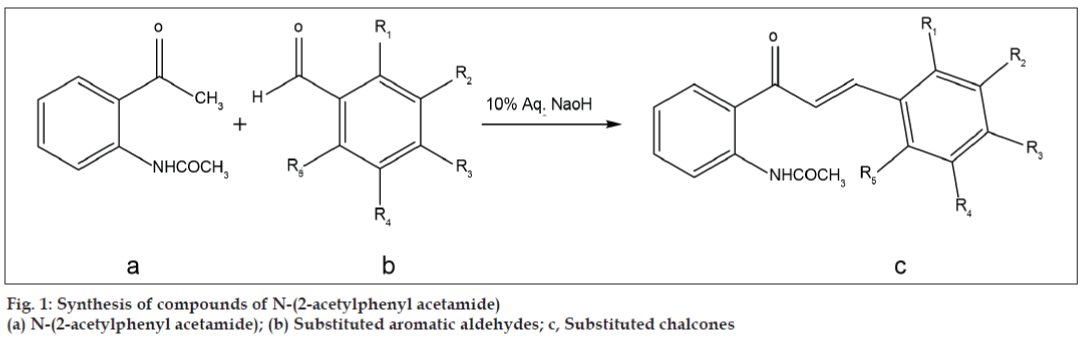
N-(2-acetylphenyl acetamide) (0.5 g, 0.003 M) was dissolved in methanol (10 ml) in a 100 ml conical flask. To the solution substituted aromatic aldehyde (0.003 M) and 10% aq. NaOH solution (2 ml) were added respectively. Reaction mixture was kept in stirred condition. Temperature was maintained below 15° (in any case not more than 20°). The progress of reaction was monitored on TLC (0.2 % methanol in chloroform). After completion of reaction, the reaction mixture was poured in ice cold water and the solid was separated by filtration, washed with cold ice water and crystallized from alcohol (fig. 1 and Table 1). Spots were visualized by spraying the chromatogram with vanillin boric acid spray reagent (methanol:vanillin:boric acid:H2SO4::1000 ml, 1 g, 1 g, 20 ml) followed by heating the plate at 120°. From 1H NMR spectra it was found that the synthetic compounds were pure and with trans-configuration (J= 15.45-15.51Hz).
| Code | R1 | R2 | R3 | R4 | R5 |
|---|---|---|---|---|---|
| SN-1 | H | H | H | H | H |
| SN-2 | H | OCH3 | OCH3 | OCH3 | H |
| SN-3 | OCH3 | OCH3 | OCH3 | H | H |
| SN-4 | H | OCH3 | OCH3 | H | H |
| SN-5 | H | NO2 | H | H | H |
| SN-6 | H | Br | H | H | H |
| SN-7 | H | H | Br | H | H |
| SN-8 | OCH3 | OCH3 | OH | H | H |
| SN-9 | H | H | H | OCH3 | H |
| SN-10 | Cl | Cl | H | H | H |
Table 1: Various R groups
N- [2-(3-Phenylacryloyl)-phenyl]-acetamide (SN-1): Yield 63%; m.p. 54-56; 1H NMR (CDCl3) δ: 2.26 (3H, s, COCH3), 7.17 (1H, dd, J=7.81 and 7.42 Hz, H-5′), 7.42 (1H, d, J=15.51Hz, H-2), 7.65-7.38 (6H, m, H= 4′,2′′,3′′,4′′,5′′,6′′), 7.81 (1H, d, J=15.51Hz, H-3), 7.98 (1H, d, J= 7.55Hz, H-3′), 8.71 (1H, d, J=8.31Hz, H-6′); IR (KBr,cm-1)-1680.35 (-C=O), 1650.45 (-NHCOCH3), 1585.72 (aromatic); MS (m/e): 265 M+ calculated for C17H15O2N; UV- λmax: 321 nm (CH3OH)
N-{2- [3-(3,4,5-Trimethoxy-phenyl)-acryloyl]-phenyl}- acetamide (SN-2): Yield 72%; m.p. 81-82; 1H NMR (CDCl3)δ: 2.25 (3H, s, -COCH3), 3.93 (9H, s, -OCH3), 6.86(2H, s, H-2′′,6′′), 7.18 (2H, t, J=7.54 and 7.91Hz, H-5′), 7.4 (1H, d, J=15.51Hz, H-2), 7.58 (1H, dd, J=7.34 and 7.58Hz, H-4′), 7.72 (1H, d, J=15.51Hz, H-3), 7.97 (1H, d, J=7.98 Hz, H-3′), 8.70 (1H, d, J=8.45Hz, H-6′); IR (KBr, cm-1)- 1693.37 (-C=O), 1649.01 (-NHCOCH3), 1585.01 (aromatic); MS (m/e): 355 M+ calculated for C20H21O5N; UV- λmax: 362.50 nm (CH3OH)
N-{2- [3-(2,3,4-Trimethoxy-phenyl)-acryloyl]-phenyl}- acetamide (SN-3). Yield 65%; m.p. 82-83; 1H NMR (CDCl3) δ: 2.25 (3H, s, -COCH3), 3.93 (9H, s, -OCH3), 6.17 (1H, d, J=8.32, H-5′′), 6.64 (1H, d, J=8.25, H-6′′), 7.18 (1H, dd, J=7.54 and 7.93Hz, H-5′), 7.4 (1H, d, J=15.51Hz, H-2), 7.58 (1H, t, J=7.34 and 7.58Hz, H-4′), 7.72 (1H, d, J=15.51Hz, H-3), 7.97 (1H, d, J=7.98Hz, H-3′), 8.70 (1H, d, J=8.45Hz, H-6′); IR (KBr, cm-1)- 1682.57(-C=O), 1648.51 (-NHCOCH3), 1585.66(aromatic); MS (m/e): 355 M+ calculated for C20H21O5N; UV- λmax: 362.50 nm (CH3OH)
N-{2- [3-(3,4-Dimethoxy-phenyl)-acryloyl]-phenyl}- acetamide (SN-4).Yield 62%; m.p. 79-81; 1H NMR (CDCl3) δ: 2.7 (3H, s, -COCH3), 3.9 (6H, s, 2-OCH3), 6.61 (1H, d, J=8.62 Hz, H-5′′), 6.70 (1H, s, H-2′′), 6.75 (1H, d, J=8.41Hz, H-6′′), 6.9 (2H, m, H-4′,5′), 7.2 (1H, d, J=15.45Hz, H-2), 7.6 (1H, d, J=15.45Hz, H-3), 8.7 (1H, d, J =7.42Hz, H-6′); IR (KBr,cm-1)- 1682.53(- C=O), 1686.4(-NHCO CH3), 1585.72(aromatic); MS (m/e): 325 M+ calculated for C19H19O4N; UV- λmax: 342.40 nm (CHCl3)
N-{2- [3-(3-Nitro-phenyl)-acryloyl]-phenyl}-acetamide (SN-5). Yield 65%; m.p. 84-86; 1H NMR (CDCl3) δ: 2.7 (3H, s, -COCH3), 6.9 (2H, m, H-4′,5′), 7.2 (1H, d, J=15.45Hz, H-2), 7.47 (1H, dd, J=8.66, 8.71Hz, H-5′′), 7.6 (1H, d, J=15.45Hz, H-3), 7.69 (1H, d, J=8.4Hz, H-6′′), 8.07 (1H, m, H-4′′), 8.23 (1H, s, H-2′′), 8.7 (1H, d, J=7.44Hz, H-6′); IR (KBr,cm-1)- 1683.09(-C=O), 1654.48(-NHCOCH3), 1588.65(aromatic); MS (m/e): 310 M+ calculated for C17H14O4N2; UV- λmax: 300.60 nm (CH3OH)
N-{2- [3-(3-Bromo-phenyl)-acryloyl]-phenyl}-acetamide (SN-6). Yield 78%; m.p. 89-91; 1H NMR (CDCl3)δ: 2.7 (3H, s, -COCH3), 6.9 (1H, m, H-4′,5′), 7.10 (1H, dd, J=8.68, 8.71Hz, H-5′′), 7.2 (1H, d, J=15.45Hz, H-2), 7.24 (1H, d, J=8.42Hz, H-6′′), 7.31 (1H, d, J=8.53Hz, H-4′′), 7.47 (1H, s, H-2′′), 7.6 (1H, d, J=15.45Hz, H-3), 8.7 (1H, d, J=7.44 Hz, H-6′); IR (KBr, cm-1)- 1680.77(- C=O), 1646.31(-NHCOCH3), 1585.15(aromatic); MS (m/e): 344 M+ calculated for C17H14O2NBr; UV- λmax: 313.80 nm (CHCl3)
N-{2- [3-(4-Bromo-phenyl)-acryloyl]-phenyl}-acetamide (SN-7). Yield 76%; m.p. 91-94; 1H NMR (CDCl3) δ: 2.7 (3H, s, -COCH3), 6.9 (1H, m, H-4′,5′), 7.19 (2H, d, J=7.54Hz, H-2′′,6′′), 7.2 (1H, d, J=15.45Hz, H-2), 7.47 (2H, dd, J=8.43Hz, H-3′′,5′′), 7.6 (1H, d, J=15.45Hz, H-3), 8.7 (1H, d, J=7.41Hz, H-6′); IR (KBr,cm-1)- 1686.07(-C=O), 1646.31(-NHCOCH3), 1589.44(aromatic); MS (m/e): 344 M+ calculated for C17H14O2NBr; UV- λmax: 326.40 nm (CHCl3)
N-{2- [3-(4-Hydroxy-3-methoxy-phenyl)-acryloyl]- phenyl}-acetamide (SN-8). Yield 65%; m.p. 128- 130; 1H NMR (CDCl3) δ: 2.7 (3H, s, -COCH3), 3.9 (3H, s, -OCH3), 6.57 (1H, d, J=8.44, H-5′′), 6.64 (1H, d, J=15.45Hz, H-2), 6.9 (2H, m, H-4′,5′), 7.19 (2H, s, H-2′′), 7.6 (1H, d, J=15.45Hz, H-3), 8.7(1H, d, J=7.45 Hz, H-6′); IR (KBr,cm-1)- 1687.17(-C=O), 1654.48(-NHCOCH3), 1588.56 (aromatic); MS (m/e): 311 M+ calculated for C18H17O4N; UV- λmax: 330.20 nm (CHCl3)
N-{2- [3-(2,5-Dimethoxy-phenyl)-acryloyl]-phenyl}- acetamide (SN-9). Yield 65%; m.p. 77-79; 1H NMR (CDCl3) δ: 2.7 (3H, s, -COCH3), 3.9 (6H, s, 2, -OCH3), 6.54(1H, d, J=8.52Hz, H-4′′), 6.70 (1H, s, H-6′′), 6.61 (1H, d, J=8.72Hz,H-3′′), 6.9 (2H, m, Hz, H-4′,5′), 7.2 (1H, d, J=15.45Hz, H-2), 7.6 (1H, d, J=15.45Hz, H-3), 8.7 (1H, d, J=7.43Hz, H-6′); IR (KBr,cm-1)- 1682.35(- C=O), 1648.45 (-NHCOCH3), 1588.72 (aromatic); MS (m/e): 325 M+ calculated for C19H19O4N; UV- λmax: 340.20 nm (CHCl3)
N-{2- [3-(2,3-Chloro-phenyl)-acryloyl]-phenyl}- acetamide (SN-10). Yield 76%; m.p. 82-84; 1H NMR (CDCl3) δ: 2.7 (3H, s, -COCH3), 6.7 (1H, m, H-5′′), 6.9 (2H, m, H-4′,5′), 7.09 (1H, d, J=8.23Hz, H-4′′), 7.12 (1H, d, J=8.45Hz, H-6′′), 7.2 (1H, d, J=15.45Hz, H-2), 7.6 (1H, d, J=15.45 Hz, H-3), 8.7 (1H, d, J=7.42 Hz, H-6′); IR(KBr,cm-1)-1681.35 (-C=O), 1648.45 (-NHCOCH3), 1585.72 (aromatic); MS (m/e): 265 M+ calculated for C17H13O2NCl2; UV- λmax: 345.00 nm (CHCl3)
The synthetic compounds (SN-1 to SN-10) were assayed for in vitro cytotoxicity against A-549 (lung), IGR-OV-1 (ovary), PC-3 (prostate), SF-295 (CNS) cell lines using sulforhodamine B. All compounds showed significant response against A-549, IGR-OV-1, PC-3, SF-295 cell lines. The results of cytotoxic activity against human cancer cell lines are given in (Table 2).
| Code | Conc.(M) | %Growth Inhibition | |||
|---|---|---|---|---|---|
| Lung | Ovary | Prostate | CNS | ||
| A-549 | IGR-OV-1 | PC-3 | SF-295 | ||
| SN-1 | 1×10-5 | 92 | 59 | 96 | 95 |
| 1×10-4 | 95 | 87 | 98 | 98 | |
| SN-2 | 1×10-5 | 94 | 63 | 97 | 86 |
| 1×10-4 | 95 | 68 | 100 | 95 | |
| SN-3 | 1×10-5 | 87 | 60 | 72 | 75 |
| 1×10-4 | 89 | 73 | 74 | 83 | |
| SN-4 | 1×10-5 | 57 | 12 | 35 | 12 |
| 1×10-4 | 60 | 34 | 37 | 20 | |
| SN-5 | 1×10-5 | 91 | 45 | 68 | 76 |
| 1×10-4 | 93 | 48 | 70 | 79 | |
| SN-6 | 1×10-5 | 94 | 93 | 99 | 97 |
| 1×10-4 | 96 | 97 | 100 | 98 | |
| SN-7 | 1×10-5 | 91 | 49 | 97 | 74 |
| 1×10-4 | 93 | 51 | 100 | 85 | |
| SN-8 | 1×10-5 | 50 | 30 | 32 | 37 |
| 1×10-4 | 71 | 64 | 67 | 62 | |
| Paclitaxel | 1×10-5 | 59 | 62 | 50 | |
| Mitomycin | 1×10-5 | 65 | |||
| Adriamycin | 1×10-6 | 72 | |||
Table 2: Inhibition of various human cancer cell lines by compounds
These synthetic compounds were also evaluated for antimicrobial activity and SN-6 having bromo substituent at meta position of ring B showed four fold reduction in MIC of ciprofloxacin when tested against S. aureus. The study revealed that the synthetics being reported potentiate antimicrobial activity of ciproflaxacin (Table 3).
| Code | MIC alone (µg/ml) ciprofloxacin | MIC of ciprofloxacin with the synthetics concentration (mg/ml) | ||||||
|---|---|---|---|---|---|---|---|---|
| 50 | 25 | 12.5 | 6.25 | 3.12 | 1.5 | 0.75 | ||
| SN-1 | 2 | 2 | 4 | 8 | 8 | 8 | ||
| SN-2 | 8 | 2 | 2 | 4 | 8 | 8 | 8 | 8 |
| SN-3 | 8 | 2 | 2 | 4 | 8 | 8 | 8 | 8 |
| SN-4 | 8 | 2 | 2 | 4 | 4 | 8 | 8 | 8 |
| SN-5 | 8 | 4 | 4 | 8 | 8 | 8 | 8 | 8 |
| SN-6 | 8 | 2 | 2 | 2 | 4 | 4 | 8 | 8 |
| SN-7 | 8 | 2 | 2 | 4 | 4 | 8 | 8 | 8 |
| SN-8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 |
Table 3: Results of antimicrobial activity of synthesized compounds against S. Aureus
A comparative assessment of the activity revealed that unsubstituted compound (SN-1) showed appreciable in vitro cytotoxic activity against all cell lines. However SN-6 and SN-7 having bromo substituent at meta and para positions respectively in ring B are more potent than SN-1
Protocol for anticancer activity: Human cancer cell lines were grown in complete growth medium (RPMI-1640) in a carbon dioxide incubator (37°, 5% CO2, 90% RH). Stock solutions (2×10-2 M) of compounds were prepared in DMSO. In vitro cytotoxicity against six human cancer cell lines was determined using 96-well tissue culture plate. The cell growth was determined by substracting mean OD value of respective blank from mean OD value of experimental set. Percent growth in presence of test material was calculated considering the growth in absence of any test material.
Protocol for antimicrobial activity: The combination studies were performed by a broth checkboard method [25]. The experiment was performed in a 96-well microtitre plate. Ciprofloxacin was used as control. The bacterial inocula of the test bacteria S. aureus 1199B were prepared from the overnight grown culture by adjusting the bacterial turbidity to 0.1 OD at 625 nm using a spectrophotometer in sterile normal saline. The inoculum was added to the wells of columns 1-12 (leaving 4 wells of column 12 as negative control). The plates were incubated at 37° for 24 h, read visually and the lowest concentration well in each row showing no turbidity was recorded.
In summary, we have synthesized a number of novel chalcone derivatives by condensation of N-(2-acetyl phenyl acetamide) with different substituted aromatic aldehydes. The compounds were purified by repeated crystallization from appropriate solvent. The yields of synthetic ranged between 62-78 %w/w. The structure of synthetics was established by spectral data (IR, NMR, MASS). NMR analysis of all the chalcones obtained showed that the trans isomers were formed. Screening of synthetics for cytotoxic activity revealed that all compounds showed significant response against A-549, IGR-OV-1, PC-3, SF-295 cell lines. Screening of synthetics for antimicrobial activity revealed that SN-6 showed four fold reduction in MIC of ciprofloxacin when tested against S. aureus.
Acknowledgements
We wish to express our thanks to Parveen Garg, Chairman of I. S. F. College of Pharmacy, Moga for his support and inspiration. We also express our thanks to IIIM, Jammu for sophisticated instrumentation facility.
References
- Schroder G, Schroder J. Stilbeneand chalcone synthases: Related enzymes with key functions in plant-specific pathways. Z NaturforschC 1990;45:1-8.
- Schroder G, Brown JW, Schroder J. Molecular analysis of resveratrol synthase. cDNA, genomic clones and relationship with chalconesynthase. Eur J Biochem 1988;172:161-9.
- Wadleigh RW, Yu SJ. Glutathione transferase activity of fall armyworm larvae toward α,β-unsaturated carbonyl allelochemicals and its induction by allelochemicals. Insect Biochem 1987;17:759-64.
- Bhat BA, Dhar KL, Puri SC, Saxena AK, Shanmugavel M, Qazi GN. Synthesis and biological evaluation of Chalcones and their derived Pyrazoles as potential cytotoxic agents.Bioorg Med ChemLett2005;15:3177-80.
- Opdyke DL. Monographs on fragrance raw materials. Food CosmetToxicol 1973;11:1011-81.
- Mokle, SS, Sayyee MA, Chopde K. Studies on synthesis and antimicrobial activity of some new iodochalcones, flavones and flavonols. Int J ChemSci 2004;2:96-100.
- Hsieh HK, Tsao LT, Wang JP, Lin CN.Synthesis and anti-inflammatory effect of chalcones. J Pharm Pharmacol 2000;52:163-70.
- Viana GS, Bandeira MA, Matos FJ. Analgesic and anti-inflammatory effects of chalcones isolated from Myracrodruonurundeuvaallemao. Phytomedicine 2003;10:189-95.
- Zhao LM, Jin HS, Sun LP, Piao HR, Quan ZS. Synthesis and evaluation of antiplatelet activity of trihydroxychalcone derivatives.Bioorg Med ChemLett 2005;15:5027-9.
- Mukarami S, Muramatsu M, Aihara H,Otomo S. Inhibition of gastric H+,K+-ATPase by the antiulcer agent, sofalcone. BiochemPharmacol1991;42:1447-51.
- Liu M, Wilairat P, Go ML. Antimalarial alkoxylated and hydroxylatedchalones: Structure-activity relationship analysis. J Med Chem2001;44:4443-52.
- Pati HN, Holt Jr. HL, Blanc RL, Dickson J, Stewart M, Brown T, et al. Synthesis and cytotoxic properties of nitro and aminochalcones. Med Chem Res 2005;14:19-25.
- Trivedi JC, Bariwal JB, Upadhyay KD, Naliapara YT, Joshi SK, Pannecouque CC, et al. Improved and rapid synthesis of new coumarinylchalcone derivatives and their antiviral activity. Tetrahedron Lett 2007;48:8472-4.
- Nielsen SF, Chen M, TheanderTG, Kharazmi A, Christensen SB. Synthesis of antiparasiticlicoricechalcones. Bioorg Med ChemLett1995;5:449-52.
- Miranda CL, Stevens JF, Ivanov V, McCall M, Frei B, DeinzerML, et al. Antioxidant and prooxidant actions of prenylated and nonprenylatedchalcones and flavanones in vitro. J Agric FoodChem2000;48:3876-84.
- Ali MA, Shaharyar M, Siddiqui AA.Synthesis, structural activity relationship and antitubercular activity of novel pyrazoline derivatives.Eur J Med Chem 2007;42:268-75.
- Satyanarayana M, Tiwari P, Tripathi BK, Srivastava AK, PartapR. Synthesis and antihyperglycemic activity of chalcone based aryloxypropanolamines. Bioorg Med ChemLett 2004;12:883-9.
- Barford L, Kemp K, Hansen M, Kharazmi A. Chalcones from Chinese liquorice inhibit proliferation of T cells and production of cytokines. IntImmunopharmacol 2002;2:545-55.
- Ko HH, Tsao LT, Yu KL, Liu CT, Wang JP, Lin CN. Structure-activity relationship studies on chalcone derivatives the potent inhibition of chemical mediators release. Bioorg Med Chem 2003;11:105-8.
- Deshpande AM, Argade NP, Natu AA, Eckman J. Synthesis and screening of a combinatorial library of naphthalene substitutedchalcones: Inhibitors of leukotriene B-4. Bioorg Med Chem1999;7:1237-40.
- Khatib S, Nerya O, Musa R, Shmnel M, Tamir S, Vaya J. Chalconesas potent tyrosinase inhibitors: The importance of a 2,4-disubstituted resorcinol moiety. Bioorg Med Chem 2005;13:433-6.
- Severi F, Benvenuti S, Costantino L, Vampa G, Melegari M, AntoliniL. Synthesis and activity of a new series of chalcones as aldose reductase inhibitors. Eur J Med Chem 1998;33:859-62.
- Kohno Y, Kitamura S, Sanoh S, Sugihara K, Fujimoto N, Ohta S. Metabolism of the α, β-unsaturated ketones, chalcone and trans-4-Phenyl-3-buten-2-one, by rat liver microsomes and estrogenic activity of metabolites. J PharmacolExpTher 2005;33:1115-23.
- Baviskar B, Patel S, Baviskar B, Khadabadi SS, Shiradkar M. Design and synthesis of some novel chalcones as potent antimicrobial Agent. Asian J Res Chem 2008;1:67-9.
- Alam MI, Gomes A. Snake venom neutralization by Indian medicinal plants Vitexnegundo and Emblicaofficinalis root extracts. J Ethnopharmacol 2003;1:75-80.