- Corresponding Author:
- B. B. Subudhi
University Department of Pharmaceutical Sciences, Utkal University, Vanivihar, Bhubaneshwar-751 004, India. E-mail: bharatsubudhi@yahoo.co.in
| Date of Submission | 1 November 2006 |
| Date of Revision | 17 December 2007 |
| Date of Acceptance | 17 June 2008 |
| Indian J Pharm Sci, 2008, 70 (3): 381-383 |
Abstract
Certain 2-(1'-iminothioimido substituted)-1'-substituted phenybenzoic acids (P 1-9 ) were synthesized by reaction of phthalic anhydride with benzotriazole, 2-mercapto benzothiazole and 2-p-amino phenyl benzimidazole, respectively (A 1-3 ) followed by imine formation with Schiff bases of thiourea with salicylaldehyde, furfuraldehyde and 1-phenyl-3-methyl-5-pyrazolone. Antiulcer activity was evaluated using reduction in total acidity, free acidity and ulcer index as parameters. Compounds P 3 , P 6 , P 7 and P 9 (100 mg/kg) showed significant (P<0.001) antiulcer action compared to control and omeprazole (40 mg/kg).
Keywords
Benzothiazole, benzotriazole, benzimidazole, total acidity, free acidity, ulcer index
The long-term acid suppression by irreversible H+/ K+-ATPase inhibitors because of covalent binding to the specific enzyme is reported to increase C. difficalies infection [1], improper absorption of Vit-B12[2], calcium [3] and other opportunistic infections [4]. SCH 28080 [5] is the prototype of a reversible proton pump inhibitor, which is effective in ulcer healing yet avoid prolonged achlorhydria. Derivatives of imidazole [6] quinoline [7], isoquinoline [8], quinazoline [9], pyrazine [10] and thiazole11 that lack the sulfinyl group but contain a basic group have been reported as reversible proton pump inhibitors. Keeping in view this structural flexibility and capitalizing on the basic structural feature, we thought to explore several benzimidazole, benzotriazole and benzothiazole derivatives to find some alternatives to the present irreversible proton pump inhibitors.
With the above aim, different 2-(1′-iminothioimido substituted)-1′-substituted phenyl benzoic acids (P1-9) were prepared. Melting points were determined in open capillary tubes and are uncorrected. The purity of the compounds was checked on silica gel-G coated plates using iodine as visualizing agent. Infrared spectra were recorded on Perkin Elmer model 600 spectrophotometer using KBr pellet. 1H NMR (DMSO d-6) spectra of title compounds were recorded on a Bruker DRX-300 NMR spectrophotometer (300 MHz) using TMS as internal standard. The C, H and N elemental analysis was carried out using a Perkin Elmer-2400 elemental analyser. The animal experiments were carried out following the protocols approved by the Institutional Animal Ethics Committee of the University Department of Pharmaceutical Sciences, Utkal University (Registration no-990/c/06/ CPCSEA). The title compounds (P 1-9) at a dose of 100 mg/kg were tested for antiulcer activity [12] using reduction in total acidity, free acidity and ulcer index as parameters. Omeprazole (40 mg/kg) was used as the standard drug.
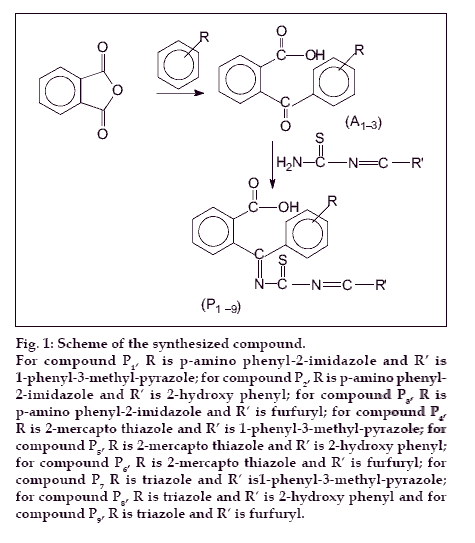
The compounds (A1-3) were prepared according to standard procedure (fig. 1) [13]. Schiff bases of thiourea with salicylaldehyde, furfuraldehyde and 1-phenyl- 3-methyl-5-pyrazolone were synthesized as per the standard laboratory method [14]. A mixture of compound A (0.015 mol) and corresponding Schiff base (0.015 mol) were taken in 25 ml of ethanol. Few drops of glacial acetic acid were added to it. The mixture was refluxed for 2 h. It was allowed to cool and then mixed with ice-cold water. The precipitate was filtered, dried, and recrystallised with 1, 4-dioxan. Nine such compounds P(1-9) were synthesized and characterized (Table 1). Following the above procedure compound 2-(1′-imino thioimido-o-hydroxy benzyl)- 1′-(2″-mercaptobenzthiazolyl) benzoic acid (P5) was obtained as a crystalline product. IR (KBr, cm-1) spectrum of compound P5 exhibited bands at 3366 cm-1, (O-H), 1469 cm-1 (C=C, ring of benzene), 1632 cm-1 (C=O) and 1511 cm-1 (C=N). 1H NMR (DMSO d-6, δ ppm) spectra of P5 exhibited peaks at δ 7.0-7.5(m, 4H, Ar-H), δ 11.5(s, 1H, -OH), δ 8.1(s, 1H, CH=N). Other compounds of the series P1-9 displayed broad IR absorption bands corresponding to Ar-OH superimposed on that of NH in the range of 3285-3368 cm-1. Another sharp band appeared at 1645-1687 cm-1 due to carbonyl function. Bands assigned to stretching vibrations of carbon-carbon double bonds of aromatic ring were observed at 1442-1489 cm-1. 1H NMR (DMSO d-6, δ ppm) spectral studies exhibited a broad singlet at δ 11.7-13.2 due to proton of aromatic carboxylic acid group. Another broad singlet appeared at δ 4.8-5.45 due to N-H proton. The aromatic protons appeared as multiplets in the range δ 7.05-7.65.
Figure 1: Scheme of the synthesized compound. For compound P1, R is p-amino phenyl-2-imidazole and R′ is 1-phenyl-3-methyl-pyrazole; for compound P2, R is p-amino phenyl-2-imidazole and R′ is 2-hydroxy phenyl; for compound P3, R isp-amino phenyl-2-imidazole and R′ is furfuryl; for compound P4,R is 2-mercapto thiazole and R′ is 1-phenyl-3-methyl-pyrazole; for compound P5, R is 2-mercapto thiazole and R′ is 2-hydroxy phenyl; for compound 6, R is 2-mercapto thiazole and R′ is furfuryl; for compound P7 R is triazole and R′ is1-phenyl-3-methyl-pyrazole;for compound P8, R is triazole and R′ is 2-hydroxy phenyl and for compound P9, R is triazole and R′ is furfuryl.
| Compd | Mol. Formula | Mol. Wt. | Yield % | Mp | % of C, H, N calculated (found) | ||
|---|---|---|---|---|---|---|---|
| C | H | N | |||||
| P1 | C32H25N7O2S | 571.1 | 51.59 | 167 | 67.25(68.12) | 4.37(4.28) | 17.15(17.89) |
| P2 | C29H21N5O3S | 519.1 | 54.23 | 162 | 67.03(67.45) | 4.04(4.26) | 13.48(13.22) |
| P3 | C27H19N5O3S | 493.1 | 48.82 | 179 | 65.70(66.1) | 3.85(4.21) | 14.19(14.32) |
| P4 | C26H19N5O2S3 | 529.3 | 47.72 | 155 | 58.94(59.12) | 3.58 (4.23) | 13.22(13.20) |
| P5 | C23H15N3O3S3 | 477.3 | 53.16 | 176 | 57.82(57.80) | 3.14(3.28) | 8.79(9.21) |
| P6 | C21H13N3O3S3 | 451.3 | 46.98 | 173 | 55.83(55.13) | 2.88(2.67) | 9.30 (9.54) |
| P7 | C25H19N7O2S | 481.1 | 51.49 | 159 | 62.35(63.08) | 3.94(4.01) | 20.36(20.81) |
| P8 | C22H15N5O5S3 | 429.1 | 55.48 | 170 | 61.52(61.29) | 3.49(3.45) | 16.31(16.95) |
| P9 | C20H13N5O3S | 403.1 | 58.74 | 184 | 59.53(59.24) | 3.22(3.14) | 17.36(17.69) |
Table 1: Physical and analytical data of compounds p(1-9)
Male rats weighing between 140 and 175 g were selected for pyloric ligation ulcer model [12]. Rats were divided into eleven groups consisting of six animals each and were fasted overnight. One group received normal saline 2 ml/kg (negative control). The second group received omeprazole 40 mg/kg (positive control) and the other groups received test compounds (100 mg/kg) by oral route 30 min prior to pyloric ligation. Animals were sacrificed 4 h later and the stomach was opened to collect the gastric contents. The gastric contents were centrifuged at 1000 rpm for 10 min. One ml of the supernatant liquid was pipetted out and diluted to 10 ml with distilled water. The solution was titrated against 0.01 N sodium hydroxide solution using Topfer′s reagent as indicator to the end point when the solution turned to orange color. The volume of sodium hydroxide consumed was taken as corresponding to the free acidity. Titration was further continued till the solution regained pink color. The volume of sodium hydroxide solution required was noted and total acidity calculated. After opening the stomach the ulcer index was calculated. The results are expressed as mean±SEM. The difference between groups was determined using the one way analysis of variance (ANOVA) followed by Dunnett′s test and p<0.05 was considered significant.
From the data in Table 2 it can be inferred that all the compounds at the tested dose level exhibited varying degree of antiulcer activity. The compound P3, P6, P7 and P9 exhibited good gastro protective actions as indicated by their very low ulcer index, free acidity and total acidity values. The inhibition of gastric acid secretion was better for benzotriazole and benzothiazole derivatives than substituted benzimidazoles. However benzimidazole derivatives exhibited significant capacity for neutralization of gastric acid compared to control. The present study indicates the necessity of a basic group but shows structural flexibility among the compounds to exert the antiulcer effect. Presence of iminothioimido derivatives of substituted pyrazole, fufuryl and o-hydroxy phenyl does not seem to affect activity significantly.
| Compound | Total acid (mEq/l) | Free acid (mEq/l) | Ulcer index |
|---|---|---|---|
| Control | 11.4± 1.6 | 3.18±0.29 | 4.4±0.35 |
| Omeprazole | 3.2± 1.25* | 0.42±0.25* | 0.33±0.06* |
| P1 | 8.69±0.21* | 2.52±0.42* | 3.66±0.81* |
| P2 | 8.31±0.29* | 2.18±0. 38* | 3.36±0.65* |
| P3 | 6.4±0.75* | 2.11±0.30* | 2.33±0.22* |
| P4 | 9.04±0.34* | 2. 92±0.12* | 3.4±0.29* |
| P5 | 7.3±0.57* | 2.41±0.17* | 2.83±0.34* |
Each value represents the mean± SEM. No of animals in each group were 6. *p<0.05 as compared to control (Dunnett′s test)
Table 2: Antiulcer activity data of compounds p(1-9)
Acknowledgements
The authors wish to extend thanks to Prof. B. B. Barik, HOD, University Department Pharmaceutical Sciences, Utkal University, Bhubaneshwar for the encouragement and facilities provided for the research work and Mr. L. Rout, IIT, Guwahati for helping analysis and characterization of the compounds.
References
- Cunningham R, Dale B, Undy B, Gaunt N. Proton pump inhibitors as a risk factor for C. difficalies diarrhoea. J Hosp Infect 2003;54:243-5.
- Termanini P, Gibril F, Sutliff V. Effect of long term gastric acid suppressive therapy on serum Vit B12 levels in patients with Zollinger- Ellison syndrome. Am J Med 1998;104:422-30.
- Connell MB, Modern DM, Murray AM. Effects of proton pump inhibitors on calcium carbonate absorption: A randomized crossover trial. Am J Med 2005;118:778-81.
- Laheji RJ, Strukenboom MC, Hassing RJ, Dieleman J, Stricker BH. Risk of community-acquired pneumonia and use of gastric acidsuppressive drugs. J Am Med Assoc 2004;292:1955-60.
- Andreas HW, Klaus W. Gastric proton pump inhibitors. In: Manfred WE, editor. Burger.s medicinal chemistry and drug discovery. Vol. II.5th ed. New York: John Willey and Sons; 1996. p. 142-5.
- Kaminiski JJ, Wallmark S, Briving C, Anderson BM. Inhibition of gastric (H+/K+)-ATPase by substituted imidazo [1,2-a] pyridines and related analogues and its implication on modeling the high affinity K+ binding site of gastric proton pump enzyme. J Med Chem 1991;34:533-41.
- Leach CA, Brown TH, Robert JI, Keeling DJ, Laing SM, Parsons ME, et al. Reversible inhibitors of the gastric (H+/K+)-ATPase.1-Aryl pyrolo [3,2-c] quinolines: Effect of the 4-substituent. J Med Chem 1992;35:1845-52.
- Yu KS, Bae KS, Shon JH, Cho JY, Yi SY, Chung JY, et al. Pharmacokinetic and pharmacodynamic evaluation of a novel proton pump inhibitor YH1885, in healing volunteers. J Clin Pharmacol 2004;44:73-82.
- Robert JI, Brown TH, Blurton P, Keeling DJ, Meeson ML, Wiggal KJ. Reversible inhibition of gastric (H+/K+)-ATPase. Substituted 2,4-diaminoquinazolines and thienopyramidines. J Med Chem 1995;38:2763-73.
- Perlin DS. Ion pumps as targets for therapeutic intervention: Old and new paradigms. Electronic J Biotechnol 1998;1:55-64.
- Lamattina JL, McCarthy PA, Reiter LA, Holt WF, Myeni L. Antiulcer agents, 4-substituted-2-guanidinothiazoles: Reversible, competitive and selective inhibitors of gastric (H+/K+)-ATPase. J Med Chem 1990;33:543-52.
- Kulkarni SK. Hand book of experimental pharmacology. 3rd ed. Delhi: Vallabh Prakashan; 1999.
- Kar A. Advanced practical medicinal chemistry, theory, methodology, purification and usage.1st ed. New Delhi: New Age International; 2004.
- Furniss BS, Hannaford AJ, Smith PW, Tatchell AR, editors. Vogel.s text book of practical organic chemistry. Singapore: Pearson Education; 1988.