- *Corresponding Author:
- M. Tiwari
Department of Pharmacy, Laboratory of Medicinal and Pharmaceutical Chemistry
Shri G. S. Institute of Technology and Science, 23 Park Road, Indore-452 003, India
E-mail: drmeenatiwari@gmail.com
| Date of Submission | 07 Janurary 2017 |
| Date of Revision | 16 April 2017 |
| Date of Acceptance | 26 November 2017 |
| Indian J Pharm Sci 2018;80(1):108-117 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
A series of 6-acetyl-coumarin derivatives (2a-n) were synthesized and evaluated for antiretroviral activity in C8166 T-cell line infected with HxBru-Gluc strain of human immunodeficiency virus-1. Michael Addition followed by re-aromatization and subsequent acid-induced elimination of water leads to the formation of coumarin intermediate, which undergoes Claisen-Schmidt condensation with substituted bezaldehydes using silica sulphuric acid as a catalyst to form 6-acetyl-coumarin derivatives, 2a-n. In silico absorption, distribution, metabolism, excretion and toxicity parameters of compounds 2a-n were found within their reference limits. All synthesized compounds were devoid of cytotoxicity as they have shown cell viability count more than 80 % in cytotoxicity assay. Compounds 2a, 2g and 2h showed potent inhibitory activity against human immunodeficiency virus infection with IC50 value of 4.7, 4.5 and 0.35 μM, respectively. It was found that electron-withdrawing group at phenyl ring, attached to the coumarin nucleus was crucial for activity against human immunodeficiency virus. The present study may be helpful in the development of some potent antiretroviral agents.
Keywords
6-acetyl-coumarin, synthesis, in silico ADMET, in vitro toxicity, antiretroviral activity
Acquired immunodeficiency syndrome (AIDS) caused by human immunodeficiency virus type-1 (HIV-1) is final stage of infection that occurs when CD4 cell (also called T-cells) count of a HIV-1 infected person falls below 200 [1]. Now, it became the fifth leading cause of death among all adults (between 25 to 44 y age-group) in the United States. Globally, about 36.7 million people were living with HIV in year 2015 [2]. Rapid development of viral resistance to currently available antiretroviral drugs and drug combinations invoked an imperative need to develop novel antiretroviral agents. One of the active natural products, coumarin, has attracted attention in recent years due to its diverse pharmacological properties [3-8]. Coumarins are highly variable in structure due to possibility of various types of substitutions in its ring skeleton, which influence a variety of pharmacological actions [9-15]. Various reports showed the antiretroviral potency of coumarins via inhibition of retroviral enzymes i.e. reverse transcriptase, protease and integrase [16-20]. Due to wide range of biological activity, synthesis of coumarins in laboratory has now become the prime interest of medicinal chemists. Previously, several coumarin derivatives were designed as antiHIV agents by quantitative structure-activity relationship (QSAR) and docking studies [21,22]. The present study explores the synthesis and antiretroviral activity of 6-acetylcoumarin derivatives against HIV infection.
The classical synthesis routes of coumarins include condensation reactions such as Knoevenagel, Pechmann, Perkin, Reformatsky and Wittig [23-25]. Phenols, o-hydroxy-benzaldehydes and acetophenones are the commonly used precursors for synthesis of coumarin ring [26-27]. To increase the yield of classical reactions, several variations have been made in terms of catalysts and reaction conditions [28]. It has now became advantageous to use solid catalysts in organic synthesis due to their non-toxicity, operational simplicity, low cost, environmental compatibility, reusability, and ease of isolation [29]. Silica sulphuric acid (SSA) is such a versatile catalyst that makes chemical reactions economic, convenient and environment friendly [30-32]. In the present study, 6-acetyl-coumarin derivatives were synthesized in good yield using SSA as a catalyst.
About 40 % of drug candidates fail in clinical trials because of poor pharmacokinetics, and toxicity. The late-stage detection of such failures significantly increases the cost of new drug development process. In order to determine absorption, distribution, metabolism, excretion and toxicity (ADMET) properties of developed compounds at an early stage, in silico approaches are preferred nowadays. Therefore, a preliminary drug-likeness, in silico ADME profiling, and toxicity risk assessment (i.e. mutagenicity, tumorigenicity, irritant and reproductive effects) studies were performed to determine the pharmacokinetic properties and toxicity of synthesized compounds.
The in vitro toxicity study of synthesized compounds was performed using Trypan Blue exclusion method [33] while the antiretroviral activity of synthesized compounds against HIV-1 infection was determined by measuring the Gaussia luciferase (Gluc) activity [34]. The present study indicated that synthesized compounds have potential of inhibiting the HIV-1 infection in low micromolar concentration.
Materials and Methods
All reactions were carried out under anhydrous conditions and in solvents dried over molecular sieves type 4 Å. Melting points (MP) of all the synthesized compounds were determined by open capillary method and are uncorrected. Reactions were monitored by thin layer chromatography (TLC) using hexane:ethyl acetate (3:7) as mobile phase. Spots on TLC plate were detected in UV cabinet. All synthesized compounds were purified by column chromatography using optimized proportions of hexane and ethyl acetate. The wavelength of maximum absorption (λmax) and LogP (octanol-water partition coefficient) of synthesized compounds was determined by Shimadzu 1700 UV/ Vis double beam spectrophotometer. IR spectra were recorded on Bruker Alpha spectrophotometer. Proton nuclear magnetic resonance (1H-NMR) spectra were recorded on Bruker Avance II NMR spectrometer using tetramethylsilane as an internal reference standard. Chemical shift values are reported in parts per million (δ in ppm), where s, d, dd and m designate singlet, doublet, doublet of doublet and multiplet, respectively. Mass spectra were recorded on Varian 500 Mass spectrometer and elemental analysis was done using Vario EL III CHN elemental analyser.
Synthesis of 5-hydroxy-6-acetyl-4-methylcoumarin (1)
Aluminum chloride (7.5 mmol) and dry nitrobenzene (10 ml) were added to a mixture of resacetophenone (20 mmol) and ethyl acetoacetate (20 mmol) in a three naked round bottom flask. The mixture was protected from moisture and heated around 130-140° for about an hour until the evolution of hydrogen chloride gas ceased. It was then cooled in ice and nitrobenzene was removed by steam distillation. Solid left behind was coumarin intermediate 1 (5-hydroxy-6-acetyl- 4-methylcoumarin), which was re-crystallized from boiling alcohol to give colourless needle shaped crystals (MP=189-195°, % yield=62).
Synthesis of 6-acetyl-coumarin derivatives (2a-n)
Substituted bezaldehydes (10 mmol), intermediate 1 (7.5 mmol) and SSA (0.7 g) were mixed in a glass tube. The mixture was heated at 70-80° for 2 h with intermittent mixing every 15 min with a glass rod. After completion of the reaction, the product was extracted with 50 ml of dichloromethane. The dichloromethane extract was filtered and concentrated under vacuum to give solid mass. Recrystallization from ethanol finally gave 6-acetyl-coumarin derivatives, 2a-n, which was further purified by column chromatography. The physicochemical properties and spectral data of synthesized compounds were as follows:
6-(3-(4-chlorophenyl)-acryloyl)-5-hydroxy-4- methyl-2H-chromen-2-one (2a)
Compound 2a was synthesized by reaction of 1 with 4-chloro-benzaldehyde using SSA as a catalyst; yellow crystals; % yield: 79; MP: 215-218°; Rf: 0.5; UV (λmax): 267; LogP: 3.42; IR (KBr): 3245 (-OH stretch), 2926 (Ar, C-H stretch), 2840 (aliphatic, C-H stretch), 1696 (O-C=O stretch), 1624 (C=O stretch), 1489, 1427 (C=C ring stretch), 1287, 1181, 1142 (Ar, C-H bend, in plane), 1091 (C-Cl stretch), 840, 805, 762 (Ar, C-H bend, out of plane) and 441 cm-1 (C=C ring bend, out of plane); 1H NMR (300 MHz, CDCl3) δ 7.95 (d, J=15.0 Hz, 1H, 3’ –CH), 7.86 (d, J=7.5 Hz, 1H, 7 –CH), 7.48 (dd, J=11.3, 3H, 2’, 2”, 6” –CH), 7.41 (d, J=7.5 Hz, 2H, 3”, 5” –CH), 6.95 (d, J=7.4 Hz, 1H, 8 –CH), 6.05 (d, J=0.5 Hz, 1H, 3 –CH), 4.98 (s, 1H, 5 –OH), 2.54 (s, J=0.6 Hz, 3H, 4 –CH3); calculated mol. wt.: 340; found: EIMS m/z: 340 (M+ peak), 341 (M+1 peak); Molecular formula: C19H13ClO4; Elemental analysis, calculated, C, 66.97; H, 3.85; found, C, 66.85; H, 4.01.
6-(3-(4-fluorophenyl)acryloyl)-5-hydroxy-4- methyl-2H-chromen-2-one (2b)
Compound 2b was synthesized by reaction of 1 with 4-fluoro-benzaldehyde using SSA as a catalyst; light brown crystals; % yield: 78; MP: 212-217°; Rf: 0.7; UV (λmax): 278; LogP: 3.22; IR (KBr): 3385 (O-H), 3056 (Ar, C-H stretch), 2927 (aliphatic, C-H stretch), 1692 (O-C=O stretch), 1624 (C=O stretch), 1599, 1468, 1429 (C=C ring stretch), 1293, 1158 (Ar, C-H bend, in plane), 1229 (C-F stretch), 834 (Ar, C-H bend, out of plane) and 436 cm-1 (C=C ring bend, out of plane); 1H NMR (300 MHz, CDCl3) δ 8.18 (d, J=15.1 Hz, 1H, 3’ –CH), 7.79 (d, J=7.6 Hz, 1H, 7 –CH), 7.62–7.49 (m, 3H, 2’, 2”, 6” –CH), 7.15 (t, J=7.7 Hz, 2H, 3”, 5” –CH), 6.96 (d, J=7.4 Hz, 1H, 8 –CH), 6.05 (d, J=0.9 Hz, 1H, 3 –CH), 5.71 (s, 1H, 5 –OH), 2.54 (s, J=0.5 Hz, 3H, 4 –CH3); calculated mol. wt.: 324; found: EIMS m/z: 324 (M+ peak), 325 (M+1 peak); Molecular formula:C19H13FO4; Elemental analysis: calculated, C, 70.37; H, 4.04; found, C, 69.98; H, 4.43.
5-hydroxy-6-(3-(4-hydroxyphenyl)acryloyl)-4- methyl-2H-chromen-2-one (2c)
Compound 2c was synthesized by reaction of 1 with 4-hydroxy-benzaldehyde using SSA as a catalyst; off white crystals; % yield: 85; MP: 239-243°; Rf: 0.45; UV (λmax): 264; LogP: 2.56; IR (KBr): 3176 (-OH stretch), 3033 (Ar, C-H stretch), 2848 (aliphatic, C-H stretch), 1672 (O-C=O stretch), 1631 (C=O stretch), 1579, 1454 (C=C ring stretch), 1289, 1221, 1159 (Ar, C-H bend, in plane), 855, 789, 706 (Ar, C-H bend, out of plane) and 454 cm-1 (C=C ring bend, out of plane); 1H NMR (300 MHz, CDCl3) δ 7.93 (d, J=15.1 Hz, 1H, 3’ –CH), 7.79 (d, J=7.6 Hz, 1H, 7 –CH), 7.39 (d, J=15.0 Hz, 1H, 2’ –CH), 7.29 (d, J=7.4 Hz, 2H, 2”, 6” –CH), 6.97 (d, J=7.4 Hz, 1H, 8 –CH), 6.87 (d, J=7.4 Hz, 2H, 3”, 5” –CH), 6.48 (s, 1H, 5 –OH), 6.05 (d, J=0.6 Hz, 1H, 3 –CH), 3.94 (s, 1H, 4” –OH), 2.53 (s, J=0.5 Hz, 3H, 4 –CH3); calculated mol. wt.: 322; found: EIMS m/z: 322 (M+ peak), 323 (M+1 peak); Molecular formula: C19H14O5; Elemental analysis; calculated, C, 70.80; H, 4.38; found, C, 70.89; H, 4.54.
5-hydroxy-4-methyl-6-(3-p-tolylacryloyl)-2Hchromen- 2-one (2d)
Compound 2d was synthesized by reaction of 1 with 4-methyl-benzaldehyde using SSA as a catalyst; light red crystals; % yield: 89; MP: 209-214°; Rf: 0.65; UV (λmax): 298; LogP: 3.29; IR (KBr): 3432 (-OH stretch), 3020 (Ar, C-H stretch), 2922 (aliphatic, C-H stretch), 1682 (O-C=O stretch), 1622 (C=O stretch), 1465, 1423 (C=C ring stretch), 1288, 1237, 1183 (Ar, C-H bend, in plane), 847, 809, 756 (Ar, C-H bend, out of plane) and 435 cm-1 (C=C ring bend, out of plane); 1H NMR (300 MHz, CDCl3) δ 7.96 (d, J=15.1 Hz, 1H, 3’ –CH), 7.79 (d, J=7.4 Hz, 1H, 7 –CH), 7.47 – 7.35 (m, 3H, 2’, 2”, 6” –CH), 7.24 (d, J=7.5 Hz, 2H, 3”, 5” –CH), 6.97 (d, J=7.4 Hz, 1H, 8 –CH), 6.36 (s, 1H, 5 –OH), 6.05 (d, J=0.6 Hz, 1H, 3 –CH), 2.53 (s, 3H, 4 –CH3), 2.34 (s, 3H, 4” –CH3); calculated mol. wt.: 320; found: EIMS m/z: 320 (M+ peak), 321 (M+1 peak); Molecular formula: C20H16O4; Elemental analysis: calculated, C, 74.99; H, 5.03; found, C, 75.22, H, 4.89.
5-hydroxy-6-(3-(4-methoxyphenyl)acryloyl)-4- methyl-2H-chromen-2-one (2e)
Compound 2e was synthesized by reaction of 1 with 4-methoxy-benzaldehyde using SSA as a catalyst; reddish brown crystals; % yield: 87; MP: 203-207°; Rf: 0.76; UV (λmax): 287; LogP: 2.89; IR (KBr): 3406 (-OH stretch), 2968 (Ar, C-H stretch), 2932 (aliphatic C-H stretch), 1632 (O-C=O stretch), 1606 (C=O stretch), 1427 (C=C ring stretch), 1248, 1175 (Ar, C-H bend, in plane), 1030 (C-O-C asymmetric stretch), 831 (Ar, C-H bend, out of plane) and 432 cm-1 (C=C ring bend, out of plane); 1H NMR (300 MHz, CDCl3) δ 7.90 (dd, J=28.5, 11.3 Hz, 2H, 7, 3’ –CH), 7.52 (d, J=7.5 Hz, 2H, 2”, 6” –CH), 7.43 (d, J=15.0 Hz, 1H, 2’–CH), 6.99 (dd, J=23.7, 7.4 Hz, 3H, 8, 3”, 5” –CH), 6.05 (d, J=0.9 Hz, 1H, 3 –CH), 4.97 (s, 1H, 5 –OH), 3.81 (s, 3H, 4” – OCH3), 2.54 (s, 3H, 4 –CH3); calculated mol. wt.: 336; found: EIMS m/z: 336 (M+ peak), 337 (M+1 peak); Molecular formula: C20H16O5; Elemental analysis; calculated, C, 71.42; H, 4.79; found, C, 71.59, H, 5.00.
6-(3-(4-(dimethylamino)phenyl)acryloyl)-5- hydroxy-4-methyl-2H-chromen-2-one (2f)
Compound 2f was synthesized by reaction of 1 with p-dimethyl-amino-benzaldehyde using SSA as a catalyst; off white crystals; % yield: 80; MP: 196-200°; Rf: 0.58; UV (λmax): 236; LogP: 3.26; IR (KBr): 3255 (-OH stretch), 2998 (Ar, C-H stretch), 2956 (aliphatic, C-H stretch), 1739 (O-C=O stretch), 1631 (C=O stretch), 1508, 1444 (C=C ring stretch), 1323 (C-N stretch), 1275, 1223, 1175 (Ar, C-H bend, in plane), 1143 (C-N stretch, aliphatic), 879, 839, 730 (Ar, C-H bend, out of plane) and 432 cm-1 (C=C ring bend, out of plane); 1H NMR (300 MHz, CDCl3) δ 7.89 (dd, J=29.1, 11.2 Hz, 2H, 7, 3’ –CH), 7.38 (dd, J=11.3, 6.8 Hz, 3H, 2, 2”, 6” –CH), 6.96 (d, J=7.4 Hz, 1H, 8 –CH), 6.75 (d, J=7.5 Hz, 2H, 3”, 5” –CH), 6.05 (d, J=0.9 Hz, 1H, 3 –CH), 4.77 (s, 1H, 5 –OH), 2.92 (s, 6H, 4” –N(CH3)2), 2.54 (s, 3H, 4 –CH3); calculated mol. wt.: 349; found: EIMS m/z: 349 (M+ peak), 350 (M+1 peak); Molecular formula: C21H19NO4; Elemental analysis: calculated, C, 72.19; H, 5.48; N, 4.01; found, C, 71.98; H, 5.59; N, 3.95.
6-(3-(3,4-dichlorophenyl)acryloyl)-5-hydroxy-4- methyl-2H-chromen-2-one (2g)
Compound 2g was synthesized by reaction of 1 with 3, 4-dichloro-benzaldehyde using SSA as a catalyst; grayish white solid; % yield: 86; MP: 225-230°; Rf: 0.62; UV (λmax): 289; LogP: 3.88; IR (KBr): 3386 (-OH stretch), 3054 (Ar, C-H stretch), 2925 (aliphatic C-H stretch), 1702 (O-C=O stretch), 1623 (C=O stretch), 1469, 1429 (C=C ring stretch), 1287, 1242, 1194 (Ar, C-H bend, in plane), 1054 (C-Cl stretch), 881, 815 (Ar, C-H bend, out of plane) and 436 cm-1 (C=C ring bend, out of plane); 1 H NMR (300 MHz, CDCl3) δ 7.95 (d, J=15.1 Hz, 1H, 3’ –CH), 7.85 (d, J=7.6 Hz, 1H, 7 –CH), 7.60 (d, J=1.1 Hz, 1H, 2” –CH), 7.49 (d, J=15.0 Hz, 1H, 2’ –CH), 7.41 – 7.30 (m, 2H, 5”, 6” –CH), 6.95 (d, J=7.4 Hz, 1H, 8 –CH), 6.05 (d, J=0.9 Hz, 1H, 3 –CH), 5.07 (s, 1H, 5 –OH), 2.54 (s, 3H, 4 –CH3); calculated mol. wt.: 375; found: EIMS m/z: 374 (M+ peak), 375 (M+1 peak); Molecular formula: C19H12Cl2O4; Elemental analysis: calculated, C, 60.82; H, 3.22; found, C, 60.69; H, 3.44.
6-(3-(3,4-dimethoxyphenyl)acryloyl)-5-hydroxy-4- methyl-2H-chromen-2-one (2h)
Compound 2h was synthesized by reaction of 1 with 3, 4-dimethoxy-benzaldehyde using SSA as a catalyst; brown crystals; % yield: 87; MP: 203-207°; Rf: 0.65; UV (λmax): 293; LogP: 2.78; IR (KBr): 3405 (-OH stretch), 3045 (Ar, C-H stretch), 2934, 2837 (aliphatic C-H stretch), 1631 (O-C=O stretch), 1512 (C=O stretch), 1463, 1423 (C=C ring stretch), 1268 (Ar, C-H bend, in plane), 1137 (C-O-C asymmetric stretch), 849, 731 (Ar, C-H bend, out of plane) and 433 cm-1 (C=C ring bend, out of plane); 1H NMR (300 MHz, CDCl3) δ 8.15 (d, J=15.1 Hz, 1H, 3’ –CH), 7.79 (d, J=7.4 Hz, 1H, 7 –CH), 7.57 (d, J=15.1 Hz, 1H, 2’ –CH), 7.23 – 7.14 (m, 2H, 2”, 6” –CH), 6.98 (dd, J=9.8, 7.4 Hz, 2H, 8, 5” –CH), 6.08 – 5.99 (m, 2H, 3 –CH, 5 –OH), 3.82 (d, J=7.8 Hz, 6H, 3”, 4” –OCH3), 2.54 (s, 3H, 4 –CH3); calculated mol. wt.: 366; found: EIMS m/z: 366 (M+ peak), 367 (M+1 peak); Molecular formula: C21H18O6; Elemental analysis: calculated, C, 68.85; H, 4.95; found, C, 68.88; H, 4.99.
5-hydroxy-6-(3-(4-hydroxy-3-methoxyphenyl) acryloyl)-4-methyl-2H-chromen-2-one (2i)
Compound 2i was synthesized by reaction of 1 with 4-hydroxy-3-methoxy-benzaldehyde using SSA as a catalyst; brownish white crystals; % yield: 75; MP: 232-238°; Rf: 0.73; UV (λmax): 276; LogP: 2.56; IR (KBr): 3390 (-OH stretch), 2922 (Ar, C-H stretch), 2910 (aliphatic C-H stretch), 1650 (O-C=O stretch), 1633 (C=O stretch), 1590, 1453, 1433 (C=C ring stretch), 1273, 1175, 1150 (Ar, C-H bend, in plane), 1025 (C-O-C asymmetric stretch), 806, 730 (Ar, C-H bend, out of plane) and 437 cm-1 (C=C ring bend, out of plane); 1H NMR (300 MHz, CDCl3) δ 7.95 (d, J=15.0 Hz, 1H, 3’ –CH), 7.86 (d, J=7.4 Hz, 1H, 7 –CH), 7.43 (d, J=15.0 Hz, 1H, 2’ –CH), 7.08 – 6.99 (m, 2H, 2”, 6” –CH), 6.96 (d, J=7.6 Hz, 1H, 8 –CH), 6.86 (d, J=7.4 Hz, 1H, 5” –CH), 6.05 (d, J=0.9 Hz, 1H, 3 –CH), 4.79 (s, 1H, 5 –OH), 4.33 (s, 1H, 4” –OH), 3.82 (s, 3H, 3” – OCH3 ), 2.54 (s, 3H, 4 –CH3); calculated mol. wt.: 352; found EIMS m/z: 352 (M+ peak), 353 (M+1 peak); Molecular formula: C20H16O6; Elemental analysis: calculated, C, 68.18; H, 4.58; found, C, 68.11; H, 4.70.
5-hydroxy-6-(3-(3-hydroxy-4-methoxyphenyl) acryloyl)-4-methyl-2H-chromen-2-one (2j)
Compound 2j was synthesized by reaction of 1 with 3-hydroxy-4-methoxy-benzaldehyde using SSA as a catalyst; off white solid; % yield: 76; MP: 232- 235°; Rf: 0.62; UV (λmax): 294; LogP: 2.44; IR (KBr): 3299 (-OH stretch), 2935 (Ar, C-H stretch), 2847 (aliphatic C-H stretch), 1673 (O-C=O stretch), 1607 (C=O stretch), 1579, 1443 (C=C ring stretch), 1277, 1246, 1214 (Ar, C-H bend, in plane), 1120 (C-O-C asymmetric stretch), 865, 791, 758 (Ar, C-H, bend, out of plane) and 438 cm-1 (C=C ring bend, out of plane); 1H NMR (300 MHz, CDCl3) δ 7.90 (dd, J=23.1, 11.2 Hz, 2H, 7, 3’ –CH), 7.43 (d, J=15.1 Hz, 1H, 2’ –CH), 7.12 – 7.02 (m, 2H, 2”, 6” –CH), 6.95 (d, J=7.6 Hz, 1H, 8 –CH), 6.88 – 6.81 (m, 1H, 5” –CH), 6.05 (d, J=0.6 Hz, 1H, 3 –CH), 5.16 (s, 1H, 5 –OH), 3.88 (s, 1H, 3” –OH), 3.81 (s, 3H, 4” –OCH3), 2.54 (s, 3H, 4 –CH3); calculated mol. wt.: 352; found EIMS m/z: 352 (M+ peak), 353 (M+1 peak); Molecular formula:C20H16O6; Elemental analysis: calculated, C, 68.18; H, 4.58; found, C, 68.20; H, 4.68.
5-hydroxy-4-methyl-6-(3-(3,4,5-trimethoxyphenyl) acryloyl)-2H-chromen-2-one (2k)
Compound 2k was synthesized by reaction of 1 with 3,4,5-trimethoxy-benzaldehyde using SSA as a catalyst; brownish white crystals; % yield: 84; MP: 202-206°; Rf: 0.54; UV (λmax): 280; LogP: 2.65; IR (KBr): 3372 (-OH stretch), 3008 (Ar, C-H stretch), 2939, 2839 (aliphatic C-H stretch), 1691 (O-C=O stretch), 1623 (C=O stretch), 1590, 1462, 1420 (C=C ring stretch), 1279, 1233, 1184 (Ar, C-H bend, in plane), 1125 (C-O-C asymmetric stretch), 835, 754, 722 (Ar, C-H bend, out of plane) and 438 cm-1 (C=C ring bend, out of plane); 1H NMR (300 MHz, CDCl3) δ 7.96 (d, J=15.1 Hz, 1H, 3’ –CH), 7.79 (d, J=7.6 Hz, 1H, 7 –CH), 7.40 (d, J=15.1 Hz, 1H, 2’ –CH), 7.11 (s, 1H, 5 –OH), 6.96 (d, J=7.6 Hz, 1H, 8 –CH), 6.60 (s, 2H, 2”, 6” –CH), 6.05 (d, J=0.6 Hz, 1H, 3 –CH), 3.80 (s, 9H, 3”, 4”, 5” – OCH3), 2.56 (s, 3H, 4 –CH3); calculated mol. wt.: 396; found EIMS m/z: 396 (M+ peak), 397 (M+1 peak); Molecular formula: C22H20O7; Elemental analysis: calculated, C, 66.66; H, 5.09; found, C, 66.59; H, 5.20.
5-hydroxy-6-(3-(4-hydroxy-3,5-dimethoxyphenyl) acryloyl)-4-methyl-2H-chromen-2-one (2l)
Compound 2l was synthesized by reaction of 1 with 4-hydroxy-3,5-dimethoxy-benzaldehyde using SSA as a catalyst; grayish white solid; % yield: 80; MP: 229- 234°; Rf: 0.51; UV (λmax): 270; LogP: 2.34; IR (KBr): 3267 (-OH stretch), 2968 (Ar, C-H stretch), 2940, 2839 (aliphatic C-H stretch), 1671 (O-C=O stretch), 1631 (C=O stretch), 1586, 1464, 1425 (C=C ring stretch), 1276, 1208, 1142 (Ar, C-H bend, in plane), 1108 (C-O-C asymmetric stretch), 841, 830, 728 (Ar, C-H bend, out of plane) and 433 cm-1 (C=C ring bend, out of plane); 1H NMR (300 MHz, CDCl3) δ 7.92 (d, J=15.1 Hz, 1H, 3’ –CH), 7.80 (d, J=7.4 Hz, 1H, 7 –CH), 7.39 (d, J=15.1 Hz, 1H, 2’ –CH), 6.97 (d, J=7.4 Hz, 1H, 8 –CH), 6.72 (s, 1H, 5 –OH), 6.60 (s, 2H, 2”, 6” –CH), 6.05 (d, J=0.9 Hz, 1H, 3 –CH), 3.87 (s, 1H, 4” –OH), 3.79 (s, 6H, 3”, 5”–OCH3), 2.54 (s, 3H, 4 –CH3); calculated mol. wt.: 382; found EIMS m/z: 382 (M+ peak), 383 (M+1 peak); Molecular formula:C21H18O7; Elemental analysis: calculated, C, 65.96; H, 4.74; O, 29.29; found, C, 67.00; H, 5.00.
6-(3-(1H-indol-3-yl)acryloyl)-5-hydroxy-4-methyl- 2H-chromen-2-one (2m)
Compound 2m was synthesized by reaction of 1 with 1H-indole-3-carbaldehyde using SSA as a catalyst; white solid; % yield: 76; MP: 250-254°; Rf: 0.55; UV (λmax): 298; LogP: 2.56; IR (KBr): 3298 (-OH stretch), 3180 (Ar, C-H stretch), 2931 (aliphatic C-H stretch), 1631 (O-C=O stretch), 1607 (C=O stretch), 1509, 1442 (C=C ring stretch), 1276, 1208, 1143 (Ar, C-H bend, in plane), 879, 839, 729 (Ar, C-H bend, out of plane) and 442 cm-1 (C=C ring bend, out of plane); 1H NMR (300 MHz, CDCl3) δ 7.90 (d, J=15.1 Hz, 1H, 3’ –CH), 7.76 (d, J=7.6 Hz, 1H, 7 –CH), 7.57 (s, 1H, 2” –CH), 7.40 (s, 1H, 3” –NH), 7.32 (d, J=15.1 Hz, 1H, 2’ –CH), 7.21 – 6.99 (m, 4H, 5”, 6”, 7”, 8” –CH), 6.92 (d, J=7.6 Hz, 1H, 8 –CH), 6.00 (d, 1H, 3 –CH), 5.10 (s, 1H, 5 –OH), 2.43 (s, 3H, 4 –CH3); calculated mol. wt.: 345; found EIMS m/z: 345 (M+ peak), 346 (M+1 peak); Molecular formula:C21H15NO4; Elemental analysis: calculated, C, 73.03; H, 4.38; N, 4.06; O, 18.53; found, C, 73.00; H, 4.58; N, 3.99.
6-(3-(1H-pyrrol-2-yl)acryloyl)-5-hydroxy-4- methyl-2H-chromen-2-one (2n)
Compound 2n was synthesized by reaction of 1 with 1H-pyrrole-2-carbaldehyde using SSA as a catalyst; grayish crystals; % yield: 80; MP: 219-224°; Rf: 0.57; UV (λmax): 287; LogP: 1.52; IR (KBr): 3272 (-OH stretch), 3179 (Ar, C-H stretch), 2938 (aliphatic C-H stretch), 1739 (O-C=O stretch), 1630 (C=O stretch), 1508, 1444 (C=C ring stretch), 1275, 1175, 1143 (Ar, C-H bend, in plane), 879, 791, 730 (Ar, C-H bend, out of plane) and 439 cm-1 (C=C ring bend, out of plane); 1H NMR (300 MHz, CDCl3) δ 7.79 (dd, J=15.9, 11.6 Hz, 3H,7, 3’ –CH, 2”-NH), 7.35 (d, J=15.0 Hz, 1H, 2’ –CH), 7.18 (d, J=7.4, 1.3 Hz, 1H, 3” –CH), 6.95 (d, J=7.4 Hz, 1H, 8 –CH), 6.37 (d, J=7.5 Hz,1H, 5” –CH), 6.17 (t, J=7.4 Hz, 1H, 4” –CH), 6.05 (d, J=0.6 Hz, 1H, 3 –CH), 5.85 (s, 1H, 5 –OH), 2.54 (s, 3H, 4 –CH3); calculated mol. wt.: 295; found EIMS m/z: 295 (M+ peak), 296 (M+1 peak); Molecular formula: C17H13NO4; Elemental analysis: calculated, C, 69.15; H, 4.44; N, 4.74; O, 21.67; found, C, 69.33; H, 4.59; N, 4.75.
Drug-likeness and in silico ADME/T study
Drug-likeness and lead-likeness of synthesized compounds was assessed as per Lipinski’s rule of five and Jorgensen’s rule of three, respectively [35,36]. The rule of five states that a compound should have a molecular weight <500 Da, LogP ≤5, hydrogen bond donors (HBD) ≤5 and hydrogen bond acceptors (HBA) ≤10, while the rule of three states that a compound should have LogP ≤3, molecular weight <300 Da, HBD ≤3, HBA ≤3 and rotatable bonds ≤3. Polar surface area (PSA) is also a very good property for characterizing bioavailability, absorption (including intestinal absorption), blood-brain barrier penetration and Caco-2 permeability of a drug [37]. Molecules with PSA less than 140 Å2 (angstroms squared) tends to be good at permeating cell membranes and also possesses better drug-likeness properties [38]. In particular, compounds which meet only two criteria, 10 or fewer rotatable bonds and PSA equal to or less than 140 Å2 are supposed to have good oral bioavailability [39]. In silico prediction of ADME properties and toxicity risks i.e. mutagenicity and tumorigenicity, irritant and reproductive effects, of compounds was done by Qikprop module of Schrödinger suite 2010 [40] and OSIRIS property explorer [41], respectively.
Biological activity
C8166 T-cells were cultured in Dulbecco modified Eagle medium and RPMI 1640 medium, each supplemented with 10 % fetal bovine serum. Peripheral blood mononuclear cells (PBMCs) were isolated from the blood of healthy adult volunteers and cultured as described in previous study [42]. Antiretroviral drug, raltegravir (RAL) was obtained from the NIH AIDS Research and Reference Reagent Program. HIV-1 HxBru-Gluc virus was generated by transfecting 293T cells with corresponding HIV-1 proviral DNAs, as described previously [34,43].
Cytotoxicity assay
The trypan blue exclusion method was used to analyze the cytotoxicity of synthesized compounds, which notably distinguishes non-viable cells from viable cells [33]. C8166 T-cells (0.1×106/well) were seeded in 96-well plate, and treated with corresponding concentrations of compounds (i. e. 0.25-2.0 μg/ml). Negative control was not seeded with C8166 T-cells. After 48 h treatment, viable (unstained) and nonviable (stained) cells were counted by TC 20 automated cell counter (Bio-RAD). The viable cell count was normalized as a percentage of the negative control and organized using following symbols –, +, ++, +++, which represent the mean cell viability of 80-100, 60-79, 40-59 and below 40 %, respectively.
Antiretroviral activity
Synthesized compounds (2 mg/ml) were individually dissolved in dimethyl sulfoxide and stored at –20° until use. To screen the antiHIV-1 activity, each compound was added to a single well of 96-well plates at a final concentration of 0.25, 0.5, 1 and 2 μg/ml, separately. Meanwhile, 1×106 C8166 T cells were infected with HIV-1 HxBru-Gluc virus (HIVp24 10-50 pg/1×106 cells) for two hours, and this cell-virus mixture was immediately transferred to the 96-well plate containing the synthesized compounds such that each well contained a final concentration of 104 cells. Three days post-infection, supernatant were collected and the levels of virus infection were monitored by measuring the Gluc activity in the supernatants from each infected cell culture using a POLARstar OPTIMA microplate luminometer (BMG Labtech, Germany). Gluc activity measurement was used as biomarker to detect the HIV infection [34,44].
Results and Discussion
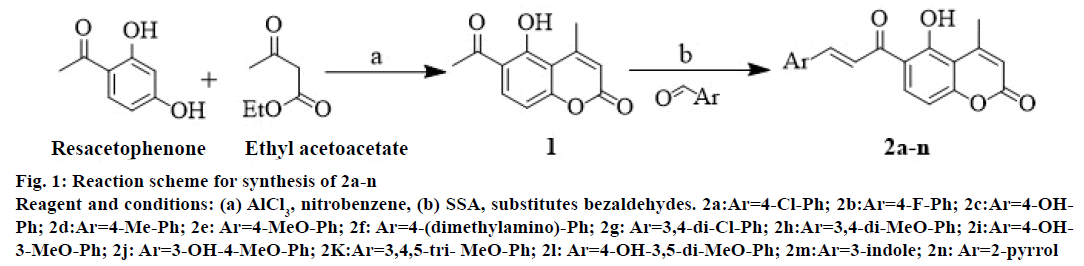
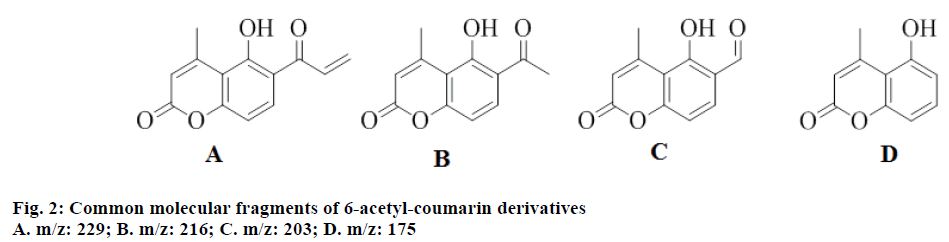
The synthesis of target compounds 2a-n was carried out in two steps (Figure 1). Resacetophenone condensed with ethyl acetoacetate using aluminium chloride as condensing agent, to produce the coumarin intermediate 1 (5-hydroxy-6-acetyl-4-methylcoumarin) in high yield [25]. Claisen-Schmidt condensation of 1 with substituted bezaldehydes using SSA as a catalyst resulted in the formation of 6-acetyl-coumarin derivatives, 2a-n. IR bands at 3344 (-OH stretch), 2942 (Ar, C-H stretch), 2841 (aliphatic, C-H stretch) 1727 (C=O stretch, coumarin), 1662 (C=O stretch), 1585, 1456, 1421 (C=C, ring stretch), 1290, 1234, 1126 (Ar, C-H bend, in plane), 845, 794, 697 (Ar, C-H bend, out of plane), 423 (C=C ring bend, out of plane) showed the characteristic functional groups in 1. NMR spectrum of 1 showed two peaks at δ 2.0-3.0 which were attributed to three protons of -CH3 at 4th position and -COCH3 at 6th position of coumarin ring, respectively. The chemical shifts at δ 5.92, 7.80 and 6.87 were due to single protons at 3, 7 and 8 positions of coumarin ring. Mass spectrum of 1 showed characteristic molecular ion peak at m/z 218. Fragmentation of the molecule showed two peak at m/z 175 (due to removal of –COCH3 fragment from parent ion) and m/z 43 (due to –COCH3 fragment). Compounds 2a-n, which were derived from 1 showed only one peak in NMR spectrum at δ 2.0-3.0 due to three protons of -CH3 group attached at 4 position of coumarin ring. This showed that –COCH3 group of 1 was involved in formation of C=C bond. Mass spectrum of 2a showed M+ peak at 340, M-15 at 325 and M-16 peak at 324, which confirm the molecular weight and CH3 and OH fragments of 2a, respectively. Common fragments of mass 229, 216, 203 and 275 were found in all synthesized compounds (2a-n; Figure 2). The above spectral characterization was further confirmed by elemental analysis, which was in agreement with calculated percent values of C, H and N.
Figure 1: Reaction scheme for synthesis of 2a-n
Reagent and conditions: (a) AlCl3, nitrobenzene, (b) SSA, substitutes bezaldehydes. 2a:Ar=4-Cl-Ph; 2b:Ar=4-F-Ph; 2c:Ar=4-OHPh;
2d:Ar=4-Me-Ph; 2e: Ar=4-MeO-Ph; 2f: Ar=4-(dimethylamino)-Ph; 2g: Ar=3,4-di-Cl-Ph; 2h:Ar=3,4-di-MeO-Ph; 2i:Ar=4-OH-
3-MeO-Ph; 2j: Ar=3-OH-4-MeO-Ph; 2K:Ar=3,4,5-tri- MeO-Ph; 2l: Ar=4-OH-3,5-di-MeO-Ph; 2m:Ar=3-indole; 2n: Ar=2-pyrrol
As per Lipinski’s rule of five, an orally active compound has not more than one violation and as per Jorgensen’s rule of three, if a compound complies with all parameters, then it is more likely to be orally available [45,46]. Compounds 2a-n complied with Lipinski’s rule of five and Jorgensen’s rule of three, as they have shown no rule violations in drug-likeness study (Table 1). All compounds showed values of ADME parameters i.e. LogS (aqueous solubility), #rot (number of rotatable bonds), #met (number of metabolic reactions), CNS activity, Caco2 permeability (permeability through intestinal epithelium), LogBB (ability to cross blood brain barrier), LogKp (permeability through skin), LogKhsa (serum protein binding) and % HOA (percent human oral absorption), within reference limits (Table 2). In silico toxicity study showed that all synthesized compounds have low toxicity risks for mutagenicity, tumorigenicity, irritant and reproductive effects (Table 3).
| Comp. | MW | HBD | HBA | LogP | PSA | ROT | ROF |
|---|---|---|---|---|---|---|---|
| 2a | 340 | 0 | 4 | 3.611 | 81.025 | 0 | 0 |
| 2b | 324 | 0 | 4 | 3.341 | 81.075 | 0 | 0 |
| 2c | 322 | 1 | 5 | 2.597 | 103.380 | 0 | 0 |
| 2d | 320 | 0 | 4 | 3.374 | 81.145 | 0 | 0 |
| 2e | 336 | 0 | 5 | 3.166 | 89.007 | 0 | 0 |
| 2f | 349 | 0 | 5 | 3.499 | 84.496 | 0 | 0 |
| 2g | 375 | 0 | 4 | 4.066 | 81.027 | 0 | 0 |
| 2h | 366 | 0 | 6 | 3.220 | 96.347 | 0 | 0 |
| 2i | 352 | 1 | 6 | 2.746 | 107.210 | 0 | 0 |
| 2j | 352 | 1 | 6 | 2.675 | 110.741 | 0 | 0 |
| 2k | 396 | 0 | 7 | 3.311 | 103.841 | 0 | 0 |
| 2l | 382 | 1 | 7 | 2.885 | 114.227 | 0 | 0 |
| 2m | 345 | 1 | 4 | 3.513 | 94.404 | 0 | 0 |
| 2n | 295 | 1 | 4 | 2.651 | 93.144 | 0 | 0 |
| RAL | 444 | 2 | 11 | 1.673 | 166.711 | 0 | 1 |
| RR | <500 | ≤5 | ≤10 | ≤5 | <140 | 0 | 1 |
ROT: Jorgensen’s rule-of-three violations, ROF: Lipinski’s rule-of-five violations, RR: Reference range for 95 % of known drugs
Table 1: Drug likeness study of synthesized compounds
| Comp. | #rot | #met | LogS | CNS | Caco-2 | LogBB | LogKp | LogKhsa | % HOA |
|---|---|---|---|---|---|---|---|---|---|
| 2a | 5 | 2 | -4.746 | -1 | 573.585 | -0.837 | -2.399 | 0.236 | 100 |
| 2b | 5 | 2 | -4.347 | -1 | 572.893 | -0.881 | -2.365 | 0.154 | 95.871 |
| 2c | 6 | 3 | -4.025 | -2 | 181.524 | -1.563 | -3.268 | 0.119 | 82.585 |
| 2d | 5 | 3 | -4.564 | -2 | 579.804 | -1.014 | -2.427 | 0.282 | 96.160 |
| 2e | 6 | 3 | -4.104 | -2 | 590.902 | -1.061 | -2.306 | 0.079 | 95.089 |
| 2f | 6 | 3 | -4.728 | -2 | 568.606 | -1.123 | -2.428 | 0.233 | 96.737 |
| 2g | 5 | 2 | -5.417 | -1 | 573.912 | -0.716 | -2.529 | 0.358 | 100.000 |
| 2h | 7 | 4 | -4.125 | -2 | 585.011 | -1.138 | -2.367 | 0.046 | 95.327 |
| 2i | 7 | 4 | -4.220 | -2 | 264.441 | -1.491 | -2.972 | 0.108 | 86.378 |
| 2j | 7 | 4 | -4.335 | -2 | 185.853 | -1.669 | -3.329 | 0.131 | 83.221 |
| 2k | 8 | 5 | -4.299 | -2 | 577.417 | -1.239 | -2.449 | 0.032 | 95.756 |
| 2l | 8 | 5 | -4.432 | -2 | 301.893 | -1.528 | -2.938 | 0.111 | 88.225 |
| 2m | 5 | 2 | -5.073 | -2 | 327.267 | -1.280 | -2.601 | 0.466 | 92.528 |
| 2n | 5 | 2 | -3.917 | -2 | 383.962 | -1.141 | -2.725 | 0.115 | 88.720 |
| RAL | 7 | 4 | -4.629 | -2 | 84.453 | -2.04 | -4.262 | -0.278 | 58.268 |
| RR | 0 to 15 | <7 | -6.5to0.5 | -2to2 | <25 poor >500 good |
-3 to1.2 | -8to-1 | ±1.5 | <25 poor |
RR: Reference range for 95 % of known drugs
Table 2: In silico ADME profile of synthesized compounds
| Comp. | Toxicity risks | |||
|---|---|---|---|---|
| MUT | TUM | IRR | RPRD | |
| 2a | Low | Low | Low | Low |
| 2b | Low | Low | Low | Low |
| 2c | Low | Low | Low | Low |
| 2d | Low | Low | Low | Low |
| 2e | Low | Low | High | Medium |
| 2f | High | High | Low | Low |
| 2g | Low | Low | Low | Low |
| 2h | Low | Low | Low | Low |
| 2i | Low | Low | Low | Low |
| 2j | Low | Low | Low | Low |
| 2k | Low | Low | Low | Low |
| 2l | Low | Low | Low | Low |
| 2m | Low | Low | Low | Low |
| 2n | Low | Low | Low | Low |
| RAL | Low | Low | Low | Low |
MUT: mutagenic, TUM: tumorigenic, IRR: irritant, RPRD: reproductive
Table 3: In silico toxicity risk assessment of synthesized compounds
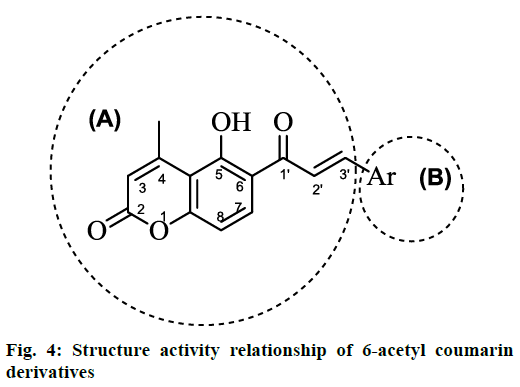
Cytotoxicity assay showed that all synthesized compounds were devoid of cytotoxicity, as their cell viability count was in the range of 80-100 %, except compound no. 2h, 2i and 2j, which showed cell viability count in the range of 60-79 %. The result of in silico toxicity study of synthesized compounds was in agreement with in vitro toxicity study results. The antiretroviral activity showed that most of synthesized compounds were effective in achieving protection against the cytopathic effect of HIV. Compounds with electron withdrawing groups i.e. 2a, 2g and 2h showed higher activity amongst all synthesized compounds as their IC50 value was in the range of 0.35-4.7 μM. Figure 3 showed that activity of compounds 2a, 2g and 2h was comparable with the activity of antiretroviral drug RAL. Structure activity relationship of synthesized compounds was explained by dividing the structure in two parts i.e. A and B. It was found that part B was crucial for activity as change in substitution resulted in variation of antiHIV activity while part A was kept unsubstituted (Figure 4). Compound with para chloro (Cl) substitution on phenyl ring of part B showed high antiHIV activity. As Cl was replaced with flouro (F) group, activity was reduced due to less electron affinity of F as compared to Cl. Introduction of hydroxyl (OH), methyl (CH3), methoxy (OCH3) and dimethylamino (N-(CH3)2) groups further reduced activity as they are less electronegative as compared to Cl and F. The highest activity was found with 3,4-dimethoxy substituent but it showed cell viability count 60-79 % in cell culture, which was less than the cell viability count of compound 2a and 2g (Table 4). Compound with 3,4-dichloro substitution (i.e. 2 g) was equipotent as 2a. This shows that electronegative substituents were favourable for activity.
| Comp. | Cytotoxicity 0.25-2.0 μg/ml |
Mean cell viability (%) |
Anti-HIV activitya IC50 (µM) |
|---|---|---|---|
| 2a | - | 91±1.5 | 4.7±0.12 |
| 2b | - | 87±2.1 | 9.8±1.23 |
| 2c | - | 89±3.6 | 36.33±2.09 |
| 2d | - | 85±2.2 | 10.62±1.07 |
| 2e | - | 84±1.9 | 10.71±1.23 |
| 2f | - | 87±1.7 | 17.47±2.03 |
| 2g | - | 89±1.3 | 4.5±0.23 |
| 2h | + | 75±2.1 | 0.35±0.13 |
| 2i | + | 72±3.9 | 21.02±1.92 |
| 2j | + | 69±2.8 | 12.5±1.11 |
| 2k | - | 85±2.1 | 17.82±2.42 |
| 2l | - | 90±3.2 | 14.65±1.34 |
| 2m | - | 87±2.4 | 20.57±2.09 |
| 2n | - | 81 ± 1.6 | 15.6±1.23 |
| RAL | - | 95 ± 1.1 | 0.09±0.03 |
Symbols - and + represents the mean cell viability of 80-100 % and 60-79 %, respectively. aData represent average results ±standard deviation from at least three separate experiments
Table 4: In vitro cytotoxicity and antiretroviral activity of synthesized compounds
In conclusion, the present study indicated that all synthesized compounds (2a-n) have the potential of inhibiting HIV-1 infection in the low micromolar range. It was also found that early prediction of ADME/T properties reduce the risk of failure of compounds in further studies. Still there is a scope of determination of exact mechanism of action, experimental pharmacokinetic profile and subsequent modification in synthesized compounds to improve activity, which is under progress in our laboratory.
Acknowledgements
Authors thank the Director, SGSITS for providing state-of-art facilities and Dr. Zhujun Ao for technique supports, which helped in successful completion of this research work.
Financial support and sponsorship
This work was partly supported by University Grant Commission, New Delhi as Major Research Project to M. Tiwari and the Canadian Institute of Health Research (CIHR) HIV/AIDS Bridge Funding Biomed/ Clini stream (HBF-131553) to X-J. Yao.
Conflicts of interest
There are no conflicts of interest.
References
- Klimas N, Koneru AOB, Fletcher MA. Overview of HIV. Psychosom Med 2008;70:523-30.
- http://www.unaids.org/sites/default/files/media_asset/global-AIDS-update-2016_en.pdf.
- Borges F, Roleira F, Milhazes N, Santana L, Uriarte E. Simple coumarins and analogues in medicinal chemistry: occurrence, synthesis and biological activity. Curr Med Chem 2005;12:887-916.
- Venugopala KN, Rashmi V, Odhav B. Review on Natural Coumarin Lead Compounds for Their Pharmacological Activity. BioMed Res Int 2013;2013:1-14.
- Soine TO. Naturally occurring coumarins and related physiological activities. J Pharm Sci 1964;53:231-64.
- Al-Soud YA, Al-Sa’doni HH, Amajaour HA, Salih KS, Mubarakb MS, Al-Masoudic NA. Synthesis, characterization and anti-HIV and antitumor activities of new coumarin derivatives. Z Naturforsch B Chem Sci 2008;63:83-9.
- Calcio Gaudino E, Tagliapietra S, Martina K, Palmisano G, Cravotto G. Recent advances and perspectives in the synthesis of bioactive coumarins. RSC Adv 2016;6:46394-405.
- Vijaya Laxmi S, Suresh Kuarm B, Rajitha B. Synthesis and antimicrobial activity of coumarin pyrazole pyrimidine 2,4,6(1H,3H,5H)triones and thioxopyrimidine4,6(1H,5H)diones. Med Chem Res 2013;22:768-74.
- Jameel E, Umar T, Kumar J, Hoda N. Coumarin: A Privileged Scaffold for the Design and Development of Antineurodegenerative Agents. Chem Biol Drug Des 2016;87:21-38.
- Bansal Y, Sethi P, Bansal G. Coumarin: a potential nucleus for anti-inflammatory molecules. Med Chem Res 2013;22:3049-60.
- Spino C, Dodier M, Sotheeswaran S. AntiHIV coumarins from calophyllum seed oil. Bioorg Med Chem Lett 1998;8:3475-8.
- Farinola N, Piller N. Pharmacogenomics: Its Role in Re-establishing Coumarin as Treatment for Lymphedema. Lymphat Res Biol 2005;3:81-6.
- Kaur M, Kohli S, Sandhu S, Bansal Y, Bansal G. Coumarin: a promising scaffold for anticancer agents. Anticancer Agents Med Chem 2015;15:1032-48.
- Kontogiorgis CA, Hadjipavlou-Litina DJ. Synthesis and Antiinflammatory Activity of Coumarin Derivatives. J Med Chem 2005;48:6400-8.
- Kadhum AAH, Al-Amiery AA, Musa AY, Mohamad AB. The antioxidant activity of new coumarin derivatives. Int J Mol Sci 2011;12:5747-61.
- Yu D, Suzuki M, Xie L, Morris‐Natschke SL, Lee KH. Recent progress in the development of coumarin derivatives as potent anti-HIV agents. Med Res Rev 2003;23:322-45.
- Harper JW, Hemmi K, Powers JC. Reaction of serine proteases with substituted isocoumarins: discovery of 3,4-dichloroisocoumarin, a new general mechanism based serine protease inhibitor. Biochemistry 1985;24:1831-41.
- Vlietinck AJ, De Bruyne T, Apers S, Pieters LA. Plant-Derived Leading Compounds for Chemotherapy of Human Immunodeficiency Virus (HIV) Infection. Planta Med 1998;64:97-109.
- Kostova I, Raleva S, Genova P, Argirova R. Structure-Activity Relationships of Synthetic Coumarins as HIV-1 Inhibitors. Bioinorg Chem Appl 2006;2006:68274.
- Garro H, R. Pungitore C. Coumarins as Potential Inhibitors of DNA Polymerases and Reverse Transcriptases. Searching New Antiretroviral and Antitumoral Drugs. Curr Drug Discov Technol 2015;12:66-79.
- Srivastav VK, Tiwari M. QSAR and docking studies of coumarin derivatives as potent HIV-1 integrase inhibitors. Arab J Chem 2017;10:S1081-S94.
- Srivastav VK, Tiwari M. k-nearest neighbor molecular field analysis based 3D-QSAR and in silico ADME/T studies of cinnamoyl derivatives as HIV-1 integrase inhibitors. Med Chem Res 2015;24:684-700.
- Desai RD, Hamid SA. Heterocyclic compounds. Proc Indian Acad Sci Sec A 1937;6:185-90.
- Rao HSP, Sivakumar S. Condensation of α-Aroylketene Dithioacetals and 2-Hydroxyarylaldehydes Results in Facile Synthesis of a Combinatorial Library of 3-Aroylcoumarins. J Org Chem 2006;71:8715-23.
- Sethna SM, Shah NM. The chemistry of coumarins. Chem Rev 1945;36:1-62.
- V. Pechmann H, Duisberg C. Ueber die Verbindungen der Phenole mit Acetessigäther. Ber Dtsch Chem Ges 1883;16:2119-28.
- V. Pechmann H. Neue Bildungsweise der Cumarine. Synthese des Daphnetins. I. Ber Dtsch Chem Ges 1884;17:929-36.
- Dittmer DC, Li Q, Avilov DV. Synthesis of Coumarins, 4-Hydroxycoumarins, and 4-Hydroxyquinolinones by Tellurium-Triggered Cyclizations1. J Org Chem 2005;70:4682-6.
- Parhami A, Khalafi-Nezhad A, Haghighi SM, Bargebid R, Zare A, Moosavi-Zare AR, et al. Silica supported boric tri-sulfuric anhydride as a novel and efficient catalyst for solvent-free synthesis of coumarins via Pechmann condensation. ARKIVOC 2012;9:111-21.
- Nazeruddin GM, Pandharpatte MS, Mulani KB. PEG-SO3H: A mild and efficient recyclable catalyst for the synthesis of coumarin derivatives. C R Chim 2012;15:91-5.
- Pathak S, Debnath K, Pramanik A. Silica sulfuric acid: a reusable solid catalyst for one pot synthesis of densely substituted pyrrole-fused isocoumarins under solvent-free conditions. Beilstein J Org Chem 2013;9:2344-53.
- Dabiri M, Salehi P, Baghbanzadeh M, Zolfigol MA, Agheb M, Heydari S. Silica sulfuric acid: An efficient reusable heterogeneous catalyst for the synthesis of 2,3-dihydroquinazolin-4(1H)-ones in water and under solvent-free conditions. Catal Commun 2008;9:785-8.
- Avelar-Freitas BA, Almeida VG, Pinto MCX, Mourão FAG, Massensini AR, Martins-Filho OA, et al. Trypan blue exclusion assay by flow cytometry. Braz J Med Biol Res 2014;47:307-15.
- Ao Z, Huang J, Tan X, Wang X, Tian T, Zhang X, et al. Characterization of the single cycle replication of HIV-1 expressing Gaussia Luciferase in human PBMCs, macrophages, and in CD4+ T cell-grafted nude mouse. J Virol Methods 2016;228:95-102.
- Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 2001;46:3-26.
- Congreve M, Carr R, Murray C, Jhoti H. A ‘Rule of Three’ for fragment-based lead discovery? Drug Discov Today 2003;8:876-7.
- Ertl P, Rohde B, Selzer P. Fast calculation of molecular polar surface area as a sum of fragment-based contributions and its application to the prediction of drug transport properties. J Med Chem 2000;43:3714-7.
- Ghose AK, Viswanadhan VN, Wendoloski JJ. A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. 1. A qualitative and quantitative characterization of known drug databases. J Comb Chem 1998;1:55-68.
- Veber DF, Johnson SR, Cheng H-Y, Smith BR, Ward KW, Kopple KD. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J Med Chem 2002;45:2615-23.
- http://gohom.win/ManualHom/Schrodinger/Schrodinger_2012_docs/qikprop/qikprop_user_manual.pdf.
- http://www.organic-chemistry.org/prog/.
- Ao Z, Wang X, Bello A, Jayappa KD, Yu Z, Fowke K, et al. Characterization of Anti-HIV Activity Mediated by R88-APOBEC3G Mutant Fusion Proteins in CD4+ T cells, Peripheral Blood Mononuclear Cells, and Macrophages. Hum Gene Ther 2010;22:1225-37.
- Ao Z, Yu Z, Wang L, Zheng Y, Yao X. Vpr14-88-Apobec3G Fusion Protein Is Efficiently Incorporated into Vif-Positive HIV-1 Particles and Inhibits Viral Infection. PLoS ONE 2008;3:e1995.
- Chen L, Ao Z, Jayappa KD, Kobinger G, Liu S, Wu G, et al. Characterization of Antiviral Activity of Benzamide Derivative AH0109 against HIV-1 Infection. Antimicrob Agents Chemother 2013;57:3547-54.
- Ntie-Kang F. An in silico evaluation of the ADMET profile of the StreptomeDB database. SpringerPlus 2013;2:353.
- Jorgensen WL, Duffy EM. Prediction of drug solubility from structure. Adv Drug Deliv Rev 2002;54:355-66.
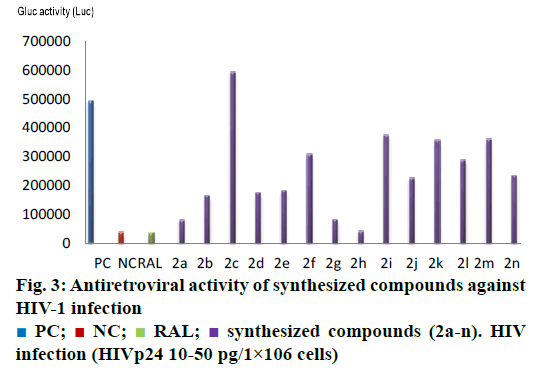
 PC;
PC;  NC;
NC;  RAL;
RAL;  synthesized compounds (2a-n). HIV
infection (HIVp24 10-50 pg/1×106 cells)
synthesized compounds (2a-n). HIV
infection (HIVp24 10-50 pg/1×106 cells)