- *Corresponding Author:
- S. Patil
Organic Research Laboratory, Department of Chemistry, P. D. V. P. College, Tasgaon, Sangli 416312, India.
E-mail: sanyujapatil@yahoo.com
| Date of Submission | 28 October 2009 |
| Date of Decision | 2 March 2010 |
| Date of Acceptance | 1 August 2010 |
| Indian J Pharm Sci, 2010, 72 (4): 500-504 |
Abstract
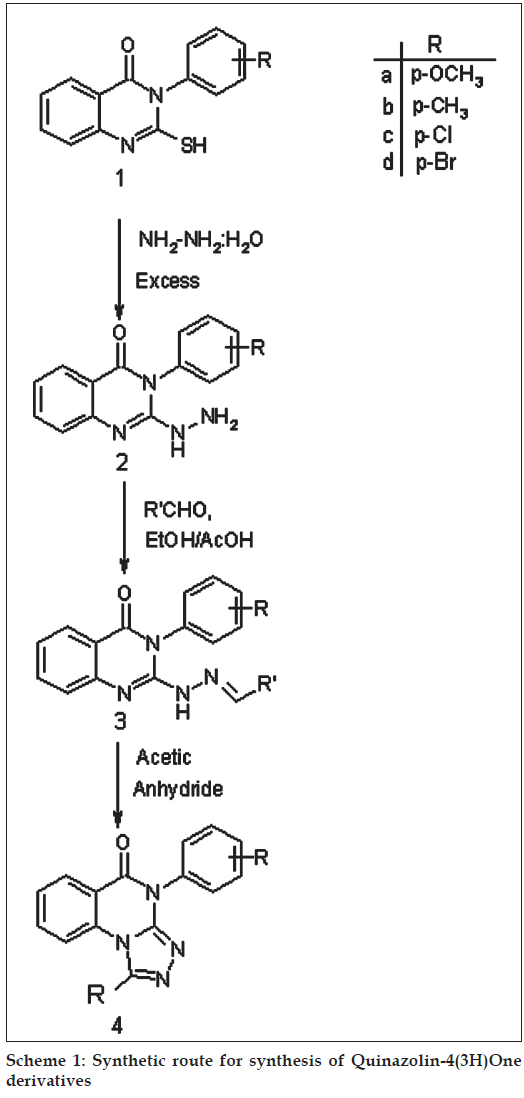
2-thio-3-aryl quinazolin-4(3H)one (1) was synthesized by reacting anthranilic acid with thiocarbamate salts of substituted aniline and carbon disulphide, which on refl ux with excess of hydrazine hydrate to form 2-hydrazino quinazolin-4(3H)one derivatives (2). The reaction of (2) with variously substituted aryl aldehydes gave the corresponding hydrazones (3). Further, the cyclization of compound (3) in acetic anhydride gave tricyclic pyrazoloquinazolinones (4). All newly synthesized compounds have been tested for their antibacterial activity against gram +ve bacteria B. substilis, S. aureus and gram –ve bacteria E. coli, P. vulgaris. The species used for antifungal activity are Aspergillus niger and Phytophora. Introduction of -OCH3, -OH and -Cl groups to the heterocyclic frame work enhanced antibacterial and antifungal activities.
Keywords
Acetic anhydride, hydrazino, hydrazones, pyrazolo-quinazolinones, quinazolinones
Derivatives quinazolines are of special importance because of their versatile biological activities [1,2], especially antihistaminic [3], antiinflammatory [4], antihypertensive [5], antiHIV [6], antifungal [6,7], antimicrobial [8,9], anticonvulsant [10], antithrombotic [11], antitubercular [12,13], antitumor [14], analgesic [15], antibacterial [6,15] and insecticidal [16]. In this paper, a new route for the synthesis of pyrazolo quinazolinones is reported.
reported. The strategy employed for the synthesis of desired compounds involved the sequential treatment of anthranilic acid with thiocarbamate salts of substituted aniline and carbon disulphide to give substituted 2-thio-3-aryl quinazol-4(3H)ones (1). The appearance of broad band at 3330-3110 cm-1 in IR spectrum and a singlet displayed at δ, 10-13 ppm in the PMR spectrum due to –SH supports their formation. Compound (1) were refluxed with excess hydrazine hydrate to form 2-hydrazino derivative (2), the formation witch has been explained by the appearance of IR band at 3390-3100 cm-1 due to -NHNH2 and disappearance of signal observed at δ, 10-13 ppm due to -SH and the appearance of two additional singlet between δ, 9-11 and δ, 2-7 ppm due to -NH and -NH2 protons, respectively in their PMR spectra. The condensation of (2) with variously substituted aryl aldehydes gave the corresponding hydrazones (3). The appearance -NH and =CH protons at δ, 5.1 and δ, 8.2 in PMR spectrum and also disappearance of -NH2 band 3390-3200 cm-1 in IR spectrum indicated their formation. Further, the cyclization of compound (3) in acetic anhydride gave tricyclic triazolo quinazolones (4). The formation of these compounds have been established by the disappearance of the PMR singlet due to –NH and =CH displayed at δ, 5.1 and δ, 8.2, respectively in the PMR spectrum of (3). (Scheme 1)
All chemicals used were of AR grade and are used without further purification. Melting points were determined by open capillary method and are uncorrected. 1H NMR spectra in DMSO-d6 were scanned on a Bruker A-300 F-NMR spectrometer. IR spectra were recorded on a Perkin-Elmer 783 (FTIR) spectrophotometer. Purity of the products in addition to the elemental analysis was checked by TLC.
The starting compound (1) was prepared by reported method [17]. 2-Hydrazino 3-p-methoxy phenyl quinazolin-4(3H)one (2a) was synthesized as follows; The compound (la) (5.0 g, 0.018 mole) was refluxed with excess of hydrazine hydrate (15 ml) with constant stirring at 100oC for about 1 ½ h, cooled and the solid obtained was filtered and recrystallized from ethanol to furnish (2a), IR(KBr); 3386-3328 (-HNNH2), 1664 (cyclic amido C=O) cm-1; 1H NMR (DMSO-d6): δ, 3.81(3H,s,Ar-OCH3), 6.25(2H,s,-NH2), 6.9-7.8(8H,m, Ar-H), 10.9(1H,s, br, -NH).
2-(p-Methoxybenzylidene)hydrazine-3-(p methoxyphenyl) quinazolin-4-(3H)one (3a-1) was synthesized using following procedure; the mixture of compound (2a) (0.2 g, 0.0007 mole) and p-methoxy benzaldehyde (0.1 g, 0.0007 mole) in ethanol (10 ml) to which two drops of acetic acid were added and the reaction mixture heated on oil bath for 5 h. The separated solid was filtered under vacuum and further recrystallized from DMF, IR(KBr); 3100-3350(-NH), 1665 cm-1 (cyclic amido >C=O), 1600 cm-1 (-C=N); 1H NMR (DMSO-d2): 3.82 (3H, s, Ar-OCH3), 3.84 (3H, s, another Ar-OCH3), 6.00 (1H, s, br, -NH), 8.21 (1H, s, =CH), 6.8-8.1 (12H, m, Ar-H), 8.20 (=CH) ppm.
3,5’-(p-Dimethoxyphenyl) pyrazolo- [3’,4’-a] quinazolin-4(3H)one (4a-1) was synthesized as follows; To a solution of compound (3a-1) (0.1 g, 0.00025 mole) in acetic anhydride (10 ml) was refluxed for about 2 h then poured in ice-cold water and separated solid was filtered, recrystallized from DMF to get desired tricyclic pyrazolo quinazolinones, IR (KBr): 1665 cm-1 (cyclic amido > C=O) and 1620 cm-1 (C=N); 1H NMR: (DMSO-d6): 3.79 (3H, s, Ar-OCH3) 3.95(3H, s, another Ar-OCH3), 6.8-8.2(12H, m, Ar-H) ppm. (Table 1)
| S. No. | R Groups | M.P.(o) | Yield(%) | Mol. Formula | % C | % H | % N | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Cal. | Found | Cal. | Found | Cal. | Found | |||||
| 2a | p-OCH3 | 205 | 76 | C15H14O2N4 | 63.82 | 63.76 | 5.00 | 5.10 | 19.85 | 19.90 |
| 2b | p-CH3 | 215 | 86 | C15H14ON4 | 67.65 | 67.59 | 5.30 | 5.38 | 21.04 | 21.10 |
| 2c | p-Cl | 218 | 82 | C14H11ON4Cl | 58.65 | 58.58 | 3.87 | 4.95 | 19.54 | 19.47 |
| 2d | p-Br | 196 | 83 | C14H11ON4Br | 50.78 | 50.62 | 3.35 | 3.40 | 16.92 | 16.86 |
| R’ groups | ||||||||||
| 4a-1 | p-OCH3C6H4 | 230 | 83 | C23H18O3N4 | 69.34 | 69.45 | 4.55 | 4.41 | 14.06 | 13.86 |
| 4a-2 | o-NO2C6H4 | 193 | 78 | C22H15O4N5 | 63.92 | 63.85 | 3.66 | 3.70 | 16.94 | 16.82 |
| 4a-3 | 3,4,5(OCH3)3-C6H2 | 211 | 68 | C25H22O5N4 | 65.49 | 67.00 | 4.84 | 4.89 | 12.22 | 12.18 |
| 4a-4 | o-OHC6H4 | 216 | 77 | C22H16O3N4 | 68.74 | 68.80 | 4.20 | 4.16 | 14.58 | 14.49 |
| 4a-5 | p-OHC6H4 | 239 | 71 | C22H16O3N4 | 68.74 | 68.65 | 4.20 | 4.18 | 14.58 | 14.50 |
| 4a-6 | o-ClC6H4 | 241 | 70 | C22H15O2N4Cl | 65.60 | 65.60 | 3.75 | 3.68 | 13.91 | 14.00 |
| 4a-7 | p- ClC6H4 | 235 | 72 | C22H15ON4Cl | 65.60 | 65.58 | 3.75 | 3.81 | 13.91 | 13.86 |
| 4a-8 | p-OH,m-OCH3 -C6H3 | 243 | 78 | C23H18O4N4 | 66.66 | 66.70 | 4.38 | 4.40 | 13.52 | 13.60 |
| 4b-1 | p-OCH3C6H4 | 198 | 86 | C23H18O2N4 | 72.24 | 72.30 | 4.74 | 4.81 | 14.65 | 14.72 |
| 4b-2 | o-NO2C6H4 | 175 | 81 | C22H15O3N5 | 66.49 | 66.40 | 3.80 | 3.75 | 17.62 | 17.70 |
| 4b-3 | 3,4,5(OCH3)3-C6H2 | 203 | 78 | C25H22O4N4 | 67.86 | 67.70 | 5.01 | 5.11 | 12.66 | 12.73 |
| 4b-4 | o-OHC6H4 | 218 | 68 | C22H16O2N4 | 71.73 | 71.62 | 4.38 | 4.29 | 15.21 | 15.30 |
| 4b-5 | p-OHC6H4 | 221 | 62 | C22H16O2N4 | 71.73 | 71.66 | 4.38 | 4.30 | 15.21 | 15.16 |
| 4b-6 | o-ClC6H4 | 216 | 72 | C22H15ON4Cl | 68.31 | 68.40 | 3.91 | 3.83 | 14.48 | 14.53 |
| 4b-7 | p-ClC6H4 | 235 | 72 | C22H15ON4Cl | 68.31 | 68.39 | 3.91 | 4.00 | 14.48 | 14.51 |
| 4b-8 | p-OH,m-OCH3 -C6H3 | 235 | 81 | C23H18O3N4 | 69.34 | 69.28 | 4.55 | 4.49 | 14.06 | 14.10 |
| 4c-1 | p-OCH3C6H4 | 246 | 86 | C22H15O2N4Cl | 65.60 | 64.30 | 3.75 | 3.81 | 13.91 | 13.84 |
| 4c-2 | o-NO2C6H4 | 198 | 82 | C21H12O3N5Cl | 60.37 | 60.45 | 2.89 | 2.81 | 16.76 | 16.68 |
| 4c-3 | 3,4,5(OCH3)3-C6H2 | 261 | 79 | C24H24O4N4Cl | 62.27 | 62.32 | 4.14 | 4.21 | 12.10 | 12.05 |
| 4c-4 | o-OHC6H4 | 232 | 71 | C21H13O2N4Cl | 64.87 | 64.91 | 3.37 | 3.42 | 14.41 | 14.35 |
| 4c-5 | p-OHC6H4 | 222 | 69 | C21H13O2N4Cl | 64.87 | 65.00 | 3.37 | 3.40 | 14.41 | 14.50 |
| 4c-6 | o-ClC6H4 | 248 | 65 | C21H12ON4Cl2 | 61.93 | 61.85 | 2.97 | 3.05 | 13.76 | 13.81 |
| 4c-7 | p-ClC6H4 | 242 | 80 | C21H12ON4Cl2 | 61.93 | 61.86 | 2.97 | 3.10 | 13.76 | 13.82 |
| 4c-8 | p-OH,m-OCH3 -C6H3 | 243 | 65 | C22H15O3N4Cl | 63.09 | 63.13 | 3.61 | 3.53 | 13.38 | 13.31 |
| 4d-1 | p-OCH3C6H4 | 246 | 86 | C21H15O2N4Br | 59.08 | 59.10 | 3.38 | 3.42 | 12.53 | 12.60 |
| 4d-2 | o-NO2C6H4 | 198 | 82 | C21H12O3N5Br | 54.56 | 54.50 | 2.62 | 2.56 | 15.15 | 15.10 |
| 4d-3 | 3,4,5(OCH3)3 -C6H2 | 261 | 79 | C24H19O4N4Br | 56.82 | 56.89 | 3.77 | 3.68 | 11.04 | 11.12 |
| 4d-4 | o-OHC6H4 | 232 | 71 | C21H13O2N4Br | 58.22 | 58.16 | 3.02 | 3.11 | 12.93 | 12.84 |
| 4d-5 | p-OHC6H4 | 222 | 69 | C21H13O2N4Br | 58.22 | 58.31 | 3.02 | 3.12 | 12.93 | 12.86 |
| 4d-6 | o-ClC6H4 | 248 | 65 | C21H12ON4ClBr | 55.84 | 55.91 | 2.68 | 2.61 | 12.40 | 12.32 |
| 4d-7 | p-ClC6H4 | 218 | 73 | C21H12ON4ClBr | 55.84 | 55.80 | 2.68 | 2.73 | 12.40 | 12.46 |
| 4d-8 | p-OH,m-OCH3 C6H3 | 226 | 78 | C23H18O4N4Br | 57.04 | 57.12 | 3.26 | 3.20 | 12.09 | 12.11 |
Table 1: Characterization Data of Compounds 2, 3 and 4
The antimicrobial screening of synthesized compounds was carried out by paper disc diffusion method [18] at 100 ppm against Gram +ve bacteria B. substilis, S. aureus and Gram –ve bacteria like E. coli, P. vulgaris. The antifungal activity of the compounds was assayed using fungal species Aspergillus niger and Phytophora. Standard antibacterial streptomycin and antifungal griseofulvin were also screened under similar condition for comparison. (Table 2)
| Comp | Bacteria | Fungi | ||||
|---|---|---|---|---|---|---|
| E. coli | P. valgaris | B. subtilis | S. aureus | Aspergillusniger | Phytophora spp. | |
| 4a-1 | 17 | 16 | 12 | 17 | 17 | 15 |
| 4a-2 | 5 | 7 | 15 | 14 | 5 | 11 |
| 4a-3 | 20 | 14 | 19 | 14 | 16 | 12 |
| 4a-4 | 7 | 10 | 7 | 4 | 8 | 1 |
| 4a-5 | 14 | 5 | 9 | 11 | 13 | 8 |
| 4a-6 | 16 | 20 | 17 | 15 | 17 | 20 |
| 4a-7 | 18 | 18 | 17 | 12 | 19 | 18 |
| 4a-8 | 12 | 15 | 11 | 12 | 12 | 14 |
| 4b-1 | 20 | 19 | 11 | 16 | 19 | 12 |
| 4b-2 | 4 | 8 | 9 | 7 | 4 | 8 |
| 4b-3 | 25 | 20 | 16 | 17 | 20 | 16 |
| 4b-4 | 9 | 4 | 14 | 4 | 9 | 7 |
| 4b-5 | 5 | 9 | 12 | 8 | 7 | 9 |
| 4b-6 | 17 | 11 | 16 | 11 | 19 | 19 |
| 4b-7 | 16 | 20 | 16 | 13 | 18 | 11 |
| 4b-8 | 11 | 12 | 14 | 11 | 12 | 12 |
| 4c-1 | 16 | 20 | 13 | 16 | 20 | 15 |
| 4c-2 | 9 | 4 | 8 | 4 | 8 | 10 |
| 4c-3 | 20 | 18 | 17 | 20 | 18 | 20 |
| 4c-4 | 8 | 6 | 12 | 6 | 8 | 5 |
| 4c-5 | 5 | 5 | 11 | 7 | 6 | 8 |
| 4c-6 | 22 | 19 | 18 | 17 | 23 | 16 |
| 4c-7 | 19 | 22 | 17 | 16 | 24 | 17 |
| 4c-8 | 11 | 11 | 7 | 12 | 9 | 12 |
| 4d-1 | 20 | 20 | 11 | 19 | 18 | 11 |
| 4d-2 | 14 | 14 | 6 | 8 | 12 | 7 |
| 4d-3 | 22 | 24 | 18 | 25 | 20 | 18 |
| 4d-4 | 7 | 8 | 11 | 5 | 7 | 7 |
| 4d-5 | 12 | 12 | 14 | 9 | 13 | 10 |
| 4d-6 | 25 | 23 | 20 | 16 | 24 | 20 |
| 4d-7 | 20 | 21 | 25 | 20 | 22 | 24 |
| 4d-8 | 15 | 12 | 14 | 11 | 15 | 11 |
Diameter of zone of inhibition in milimeters
Table 2: Antimicrobial Screening Data of the Derivatives of 4
The result indicated that some compounds exhibit good antimicrobial activity against the above mentioned bacterial and fungal species, while some compounds have moderate antimicrobial activity against both Gram +ve and Gram –ve bacterial and fungal species. It was absovered that introduction of –OCH3 and –Cl groups to the heterocyclic frame work enhanced antibacterial and antifungal activities.
References
- El-Sharief AM, Ammar YA, Zahran MA, Ali AH, El-Gaby MS. Aminoacids in the synthesis of heterocyclic systems: The synthesis of triazinoquinazolinones, triazepinoquinazolinones and triazocinoquinazolinones of potential biological interest. Molecules 2001;6:267-78.
- El-Sharief AM, Ammar YA, Zahran MA, Ali AH. Oxidation of 3-aminoquinazolinones with lead tetraacetate: A novel synthesis of naphtho-fused azirino-pyrazolo- and 1,4,5-oxadiazepino-quinazolinones. J Chem Res 2002;5:205-8.
- Raju MB, Singh SD, Raghu Ram Rao A, Rajan KS. New antihistaminic agents: Synthesis and evaluation of h1-antihistaminic actions of 3- [(n,n-dialkylamino)alkyl]-1,2,3,4-tetrahydro-(1h)-thioquinazolin-4(3H)-ones and their oxo analogues. Indian J Pharm Sci 2007:69;853-6.
- Alexander EJ. 4,5-Dihydro-5-oxopyrazolo [1,5-a]quinazoline-3-carboxylic acid derivatives. U S Patent 1978, 4105766.
- Wright WB, Tomcufcik AS, Chan PS, Marsico JW, Press JB. Thromboxane synthetase inhibitors and antihypertensive agents: 4. N- [(1H-imidazol-1-yl)alkyl] derivatives of quinazoline-2,4(1H,3H)-diones, quinazolin-4(3H)-ones, and 1,2,3-benzotriazin-4(3H)-ones. J Med Chem 1987;30:2277-83.
- Alagarsamy V, Giridhar R, Yadav MR, Revati R, Ruckmani K, Chercq ED. AntiHIV, antibacterial and antifungal activities of some novel 1,4-disubstituted-1,2,4-triazolo [4,3-a]quinazolin-5(4H)-ones. Indian J Pharm Sci 2006;68:532-5.
- Ghorab MM, Abdel-Gawad SM, El-Gaby MS. Synthesis and evaluation of some new fluorinated hydroquinazoline derivatives as antifungal agents. Farmco 2000;55:249.
- Raghvendra NM, Thampi PP, Gurubasavarajswamy PM. Synthesis and antimicrobial activity of some novel substituted piperazinyl-quinazolin-3(4H)-ones. E-J Chem 2008;5:23-33.
- Singh S, Dave U, Parikh AR. Studies on imidazolinones: Synthesisand antimicrobial activity of 6-(4’-substituted-benzylidene-2’-methyl/ phenyl-5’- imidazolinon-1’-yl)-2-methyl-4(3H)-quinazolinone J Indian ChemSoc 1994;71:159-60.
- El-Helby AG, Wahab MH. Design and synthesis of some new derivatives of 3H-quinazolin-4-one with promising anticonvulsant activity. Acta Pharm 2003;53:127-38.
- Lacefield WB. 2,4-diaminoquinazolines as antithrombotic agents. US Pat 1977;4048312.
- Kunes J, Bazant J, Pour M, Waisser K, Slosarek M, Janota J. Quinazoline derivatives with antitubercular activity. Farmaco 2000;55:725-9.
- Pattan SR, Krishna Reddy VV, Manvi FV, Desai BG, Bhat AR. Synthesis of N-3(4-(4-chlorophenyl thiazole-2-yl)-(2-(amino)methyl)-quinazoline-4(3H)-one and their derivatives for antitubercular activity. Indian J Chem 2006;45B:1778.
- Martin JD, Jeremiah PM, Alicia MB, Bredly DS. One-step synthesis of 4(3H)-quinazolinones. Tetrahedron Lett 2001;42:1851-4.
- Alagarsamy V, Pathak US, Goyal RK. Synthesis and evaluation of some novel 2-Mercapto-3-(substituted methylamino)quinazolin-4(3H)-ones as analgesic, antiinflammatory and antibacterial agents. Indian J Pharm Sci 2000;62:63-6.
- Singh T, Sharma S, Kishore V, Shrivastava Kumar A. Synthesis, insecticidal and antimicrobial activities of some heterocyclic derivatives of quinazolinone. Indian J Chem 2006;45B:2558-65.
- Deshmukh MB, Deshmukh DS, Shirke SD. Synthesis of some new 2-thioxoquinazolin-4-ones. J Indian ChemSoc 1997;74:422-3.
- Bauer RW, Kirby MD, Sherris JC, Turck M. Antibiotic susceptibility testing by standard single disc diffusion method. Am J ClinPathol 1966;45:493-6.