- *Corresponding Author:
- O. Prakash
Departments of Chemistry Kurukshetra University, Kurukshetra-136 119, India
E-mail: dromprakash50@rediffmail.com
| Date of Submission | 7 April 2010 |
| Date of Revision | 28 September 2011 |
| Date of Acceptance | 2 October 2011 |
| Indian J Pharm Sci, 2011, 73 (5): 586-590 |
Abstract
Seven new 2-(3-(4-aryl)-1-phenyl-1H-pyrazol-4-yl) chroman-4-ones (4a-4g) have been synthesized by cyclization of 2-hydroxychalcone analogues of pyrazole 3a-3g using conc. HCl in acetic acid. The structures of the compounds 4a-4g were established by the combined use of 1HNMR, IR and mass spectra. All the seven compounds were tested in vitro for their antibacterial activity against two Gram positive bacteria namely Staphylococcus aureus and Bacillus subtilis and two Gram negative bacteria Escherichia coli and Pseudomonas aeruginosa. The compounds 4b, 4c, 4e, 4f, 4g have displayed good antibacterial activity when compared with commercially available antibiotic, ciprofloxacin. These compounds also were screened for their antifungal activity against two ear pathogenic fungi, namely Aspergillus Niger and A. flavus. The compounds 4a, 4c, 4d, 4g exhibited good antifungal activity when compared with commercially available antifungal, fluconazole.
Keywords
2-(3-(4-aryl)-1-phenyl-1H-pyrazol-4-yl) chroman-4-ones, antibacterial activity, antifungal activity, chalcone, pyrazole
There is resurgence in research activity in the flavanoid class of molecules due to excellent biological profiles reported in the recent times. These are used in numerous pharmacological applications such as antimalarial [1], anticancer [2], antiinflammatory [3], antibacterial [4], antifungal [5-7], and antiproliferative [8] activities. On the other hand, pyrazole and its derivatives, a class of well known nitrogen containing heterocyclic compounds, occupy an important position in the medicinal and pesticide chemistry with having a wide range of bioactivities such as antimicrobial, [9] anticancer [10], antiinflammatory [11], antibacterial [12], antifungal [13], and herbicidal [14,15]. A literature survey revealed that the title compounds 2-(3-(4-aryl)-1- phenyl-1H-pyrazol-4-yl) chroman-4-ones (4a-4g) remain unknown. Led by these observations, the synthesis of some new 2-(3-(4-aryl)-1-phenyl-1H-pyrazol-4-yl) chroman-4-ones (4a-4g) was undertaken with a view to evaluate their antibacterial and antifungal activities.
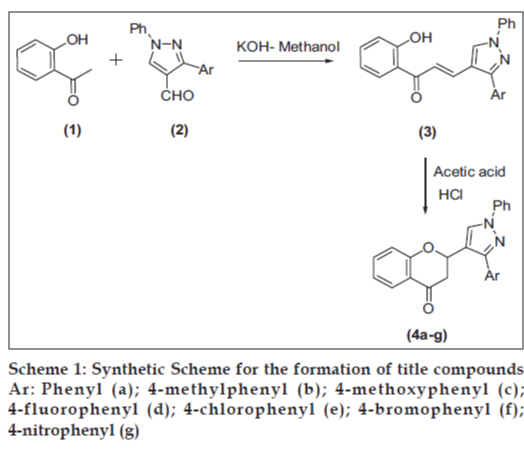
2-(3-(4-Aryl)-1-phenyl-1H-pyrazol-4-yl) chroman- 4-ones (4a-4g) were obtained byf cyclization of 2’-hydroxychalcones (3) using conc HCl in acetic acid. The chalcone derivatives of pyrazole analogues 3a-3g were obtained by reacting pyrazole aldehydes (2) with 2’-hydroxyacetophenone (1) in methanolic KOH (Scheme 1).
All melting points were determined in open capillaries in electrical melting point apparatus and are uncorrected. The IR spectra were recorded with a buck scientific IR M-500 spectrophotometer. The 1H NMR spectra were scanned on a Bruker (300 MHz) spectrometer in CDCl3 using tetramethylsilane as an internal standard. TLC was run on silica gel G plates using chloroform-methanol (9:1) as irrigant. All the new compounds gave satisfactory analytical results.
Pyrazole aldehydes (2) and 2’-hydroxychalcones (3) were synthesized according to the literature method [16]. The general procedure for the synthesis of 2-(3-(4-aryl)-1-phenyl-1H-pyrazol-4-yl) chroman-4-ones (4a-4g) is as follows (scheme 1). To a solution of 2’-hydroxychalcone (0.01mol) in acetic acid (20 ml) was added 1 ml of conc HCl. The reaction mixture was refluxed for 6-8 h and was then poured on to crushed ice with vigorous stirring. The solid material, which separated, was washed with water and crystallized from alcohol to give the pure chromanones 4a-4g.
The antibacterial activity of seven new compounds 4a-4g was evaluated by agar well diffusion method. All the cultures were adjusted to 0.5 McFarland standard, which is visually comparable to a microbial suspension of approximately 1.5×108 cfu/ml. Twenty millilitres of Mueller Hinton agar medium was poured into each Petri plate and plates were swabbed with 100 μl inocula of the test microorganisms and kept for 15 min for adsorption. Using sterile cork borer of 8 mm diameter, wells were bored into the seeded agar plates and these were loaded with a 100 μl volume with concentration of 4.0 mg/ml of each compound reconstituted in the dimethylsulphoxide (DMSO). All the plates were incubated at 37° for 24 h. Antibacterial activity of compounds 4a-4g was evaluated by measuring the zone of growth inhibition against the test organisms with zone reader (Hi Antibiotic zone scale). DMSO was used as a negative control whereas ciprofloxacin was used as positive control. This procedure was performed in three replicate plates for each organis [17,18]. Minimum inhibitory concentration (MIC) of the various compounds 4a-4g against bacterial strains was tested through a macrodilution tube method as recommended by NCCLS. In this method, various test concentrations of synthesized compounds 4a-4g were made from 128 to 0.25 μg/ml in sterile tubes No. 1 to 10. One hundred microlitre of sterile Mueller Hinton Broth (MHB) was poured in each sterile tube followed by addition of 200 μl test compound in tube 1. Two fold serial dilutions were carried out from the tube 1 to the tube 10 and excess broth (100 μl) was discarded from the last tube No. 10. To each tube, 100 μl of standard inoculum (1.5×108 cfu/ml) was added. Ciprofloxacin was used as control. Turbidity was observed after incubating the inoculated tubes at 37° for 24 h [19].
The antifungal activity of the synthesized compounds 4a-4g was evaluated by poison food technique .The moulds were grown on Sabouraud dextrose agar (SDA) at 25° for 7 days and used as inocula. The 15 ml of molten SDA (45°) was poisoned by the addition of 100 μl volume of each compound having concentration of 4.0 mg/ml reconstituted in the DMSO and poured into a sterile Petri plate and allowed it to solidify at room temperature. The solidified poisoned agar plates were inoculated at the center with fungal plugs (8 mm diameter) obtained from the actively fungus growing on margins of the SDA plates and incubated at 25° for 7 days. DMSO was used as the negative control whereas fluconazole was used as the positive control. The experiments were performed in triplicates. Diameter of fungal colonies was measured and expressed as percent mycelial inhibition by applying the formula [20]. Percent inhibition of myelial growth = (dc-dt)/dc×100, where dc = average diameter of fungal colony in negative control sets, dt = average diameter fungal colony in experimental sets.
The structures of all the newly synthesized 2-(3-(4-aryl)- 1-phenyl-1H-pyrazol-4-yl)chroman-4-ones (4a-4g) were confirmed by their spectral (IR, 1H NMR and mass) and elemental analytical data. The lack of absorption band at 1640 cm-1 in the IR spectra of compounds 4a-4g and appearance of characteristic absorption band at 1680- 1690 cm-1 due to carbonyl group showed the absence of α,β-unsaturated carbonyl group, thereby suggesting the cyclic structure. The 1H NMR spectra of compounds 4a-4g showed three characteristic doublets of doublet due to C(2) and C(3) protons in the regions δ 2.90-3.16 and 3.13-3.74, 5.56-5.68, respectively. The C(5)–H of pyrazole ring appeared as a singlet at δ 8.12.
All the tested compounds 4a-4g possessed variable antibacterial activity against both gram-positive (S. aureus, B. subtilis) and gram negative (E. coli, P. aeruginosa) bacteria. On the basis of maximum inhibitory activity shown against gram positive bacteria compounds 4d, 4e and 4g were found to be most effective against S. aureus with zone of inhibition of 25.1, 24.1 and 24.2 mm and in case of B. subtilis, compound 4d, 4e and 4g were most effective with zone of inhibition 22.6, 22.6 and 23.6 mm respectively. However in case of gram negative bacteria, compounds 4a-4g displayed moderate to low activity against E. coli and P. aeruginosa (Table 1).
| Compounds | Diameter of growth of inhibition zone (mm)a | |||
|---|---|---|---|---|
| Staphylococcus Bacillus Escherichia Pseudomonas | ||||
| aureus | subtilis | coli | aeruginosa | |
| 4a | 22.6 (16) | 22.3 | 15.3 (>128) | 17.6 (64) |
| (16) | ||||
| 4b | 19.3 (64) | 21.6 | 16 (128) | 15.3 (>128) |
| (32) | ||||
| 4c | 20.6 (32) | 20.3 | 18.6 (64) | 16.6 (128) |
| (16) | ||||
| 4d | 25.1 (8) | 22.6 | 18.3 (64) | 16.3 (128) |
| (16) | ||||
| 4e | 24.1 (8) | 22.6 (8) | 16.3 (128) | 17.6 (64) |
| 4f | 20.6 (32) | 19.3 | 18.6 (64) | 18 (64) |
| (64) | ||||
| 4g | 24.2 (8) | 23.6 (8) | 18.3 (64) | 16.6 (128) |
| Ciprofloxacin | 26.0 (5) | 24.0 (5) | 25.0 (5) | 22.0 (5) |
a Values, including diameter of the well (8mm), are means of three replicates MIC values are given in parenthesis
Table 1: In Vitro antibacterial activity of synthesized compounds 4a-4g
The MIC values of compounds ranged between 8 and 64 μg/ml against gram positive bacteria, compounds 4d, 4e and 4g showed highest MIC of 8 μg/ml against S. aureus whereas compounds 4e, 4g also showed highest MIC of 8 μg/ml against B. subtilis.
However, in case of gram negative bacteria, MIC of compounds ranged between 64 and 128 μg/ml and compounds 4c, 4d, 4f and 4g showed highest MIC of 64 μg/ml against E. coli and compound 4c, 4d and 4e showed highest MIC of 64 μg/ml against P. aeruginosa (Table 1). All the newly synthesized compounds showed low activity against gram negative bacteria as compared to gram positive bacteria.
All the seven compounds 4a-4g were also screened for antifungal activity. Compounds 4c, 4d, 4g displayed good antifungal activity against Aspergillus flavus, whereas compounds 4a, 4c, 4d, 4g showed good activity against Aspergillus niger when compared with commercially available antifungal fluconazole (Table 2). From above discussion, it can be concluded that presence of substituents such as fluoro, chloro and nitro in the aryl ring of pyrazole moiety of compounds 4a-4g enhance antibacterial activity.
| Compounds | Mycelial growth inhibition (%) | |
|---|---|---|
| Aspergillus niger | Aspergillus flavus | |
| 4a | 55.5 | 61.1 |
| 4b | 55.5 | 55.5 |
| 4c | 66.6 | 66.6 |
| 4d | 61.1 | 66.6 |
| 4e | 33.3 | 50 |
| 4f | 38.8 | 55.5 |
| 4g | 66.6 | 61.1 |
| Fluconazole | 81.1 | 77.7 |
Table 2: In Vitro antifungal activity of synthesized compounds 4a-4g
The characterization data of all new compounds 4a-4g are summarized in Table 3 and spectral data are as follows; 4a: IR (KBr, cm-1): 1682; 1HNMR (CDCl3,δ): 3.16 (dd, 1H, J=3.3, 13.9 Hz), 3.74 (dd, 1H, J=12.7, 13.9 Hz), 5.68 (dd, 1H, J=3.3, 12.1 Hz), 8.11 (s, 1H), 8.25 (dd, 1H, J=1.5, 7.8 Hz), 7.87 (d, 2H, J=7.8 Hz), 7.30 (d, 2H, J=7.8 Hz), 6.98 (d, 1H, J=8.4 Hz), 7.61- 7.72 (m, 5H), 7.36-7.40 (m, 3H); MS m/z: 367.1; 4b: IR (KBr;cm-1) 1682; 1HNMR (CDCl3,δ): 2.31 (s, 3H), 2.91 (dd, 1H, J=3.3, 16.8 Hz), 3.15 (dd, 1H, J=16.8, 12.1 Hz), 5.57 (dd, 1H, J=3.3, 12.1 Hz), 8.04 (s,1H), 7.96 (dd, 1H, J=1.5, 7.9 Hz), 7.76 (d, 2H, J=8.0 Hz), 7.73 (d, 2H, J=8.1 Hz), 7.43-7.55 (m, 6H), 7.04-7.11 (m, 2H); MS m/z: 381.15; 4c: IR (KBr;cm-1) 1687.4; 1HNMR (CDCl3,δ): 3.48 (s, 3H), 3.03 (dd, 1H, J=3.3, 16.8 Hz), 3.15 (dd, 1H, J=12, 16.8 Hz), 5.65 (dd, 1H, J=3.3, 12 Hz), 8.12 (s, 1H), 7.97 (dd, 1H, J=1.5, 7.9 Hz), 7.76 (d, 2H, J=8 Hz), 7.73 (d, 2H, J=8.1 Hz), 7.43-7.55 (m, 6H), 7.05-7.10 (m, 2H); MS m/z: 397.15; 4d: IR (KBr; cm-1) 1685.1; 1HNMR (CDCl3,δ): 3.04 (dd, 1H, J=3.3, 16.2 Hz), 7.31 (dd, 1H, J=12, 16.2 Hz), 5.62 (dd, 1H, J=3.3, 12 Hz), 8.12 (s,1H), 7.97 (dd, 1H, J=1.5, 7.8 Hz), 7.75-7.82 (m, 4H), 7.47-7.55 (m, 3H), 7.35 (m, 1H), 7.05-7.19 (m, 4H); MS m/z: 384.13; 4e: IR (KBr; cm-1) 1685.3; 1HNMR (CDCl3,δ): 3.03 (dd, 1H, J=3.3, 16.6 Hz), 3.25 (dd, 1H, J=11.7, 16.6 Hz), 5.65 (dd, 1H, J=3.3, 11.7 Hz), 8.12 (s, 1H), 7.97 (dd, 1H, J=1.5, 8.1 Hz), 7.76 (d, 2H, J=8 Hz), 7.73 (d, 2H, J=8.1 Hz), 7.43- 7.55 (m, 6H), 7.05-7.10 (m, 2Hz); MS m/z: 403.11, 401.13; 4f: IR (KBr; cm-1) 1682.3; 1HNMR (CDCl3,δ): 3.03 (dd, 1H, J=3.3, 18.0 Hz), 3.23 (dd, 1H, J=12.0, 18.0 Hz), 5.65 (dd, 1H, J=3.3, 12.0 Hz), 8.12 (s, 1H), 7.97 (dd, 1H, J=1.5, 7.8 Hz), 7.60 (d, 2H, J=8.1 Hz), 7.71 (d, 2H, J=8.1 Hz), 7.47-7.61 (m, 5H), 7.35 (m, 1H), 7.04-7.13 (m, 2H); MS m/z: 445.01, 447.05; 4g: IR (KBr; cm-1) 1689.3; 1HNMR (CDCl3,δ): 3.02 (dd, 1H, J=3.3, 18.0 Hz), 3.19 (dd, 1H, J=18.0, 12.1 Hz), 5.56 (dd, 1H, J=3.3, 12.1 Hz), 8.26 (s, 1H), 8.38 (d, 1H, J=8.4 Hz), 8.00 (m, 3H), 7.80 (m, 2H), 7.48-7.59 (m, 4H), 7.51 (m, 3H); MS m/z: 411.12.
| Compound | M.P.(o) | Yield(%) | Mol. Formula | Elemental analysis Calcd/(found)% | ||||
|---|---|---|---|---|---|---|---|---|
| C | H | N | ||||||
| 4a | 170 | 68 | C24H18N2O2 | (366.41) | 78.67 (78.32) | 4.95 (4.70) | 7.65 | (7.55) |
| 4b | 206 | 79 | C25H20N2O2 | (380.44) | 78.93 (78.82) | 5.30 (5.20) | 7.36 | (7.23) |
| 4c | 175 | 72 | C25H20N2O3 | (396.44) | 75.74 (76.02) | 5.08 (4.98) | 7.07 | (7.23) |
| 4d | 190 | 74 | C24H17N2O2F (384.4) | 74.99 (75.15) | 4.46 (4.40) | 7.29 | (7.11) | |
| 4e | 226 | 78 | C24H17N2O2Cl (400.86) | 71.91 (71.85) | 4.27 (4.20) | 6.99 | (7.22) | |
| 4f | 220 | 81 | C24H17N2O2Br (445.31) | 64.73 (64.66) | 3.85 (3.63) | 6.29 | (6.02) | |
| 4g | 214 | 65 | C24H17N3O2 (411.41) | 70.07 (69.93) | 4.16 (4.00) | 10.21 | (10.00) | |
Table 3: Characterization Data Of Compounds 4a-4g
Acknowledgements
We are thankful to CSIR, New Delhi for the award of Junior Research Fellowship to Khalid Hussain. Thanks are also due to RSIC, CDRI Lucknow, India, for providing mass and elemental analyses.
References
- Li R, Kenyon GL, Cohen FE, Chen X, Gong B, Dominguez JN, et al.In vitro antimalarial activity of chalcones and their derivatives. J MedChem 1995;38:5031-7.
- Du J, He ZD, Jiang RW, Ye WC, Xu HX, But PP. Antiviral flavonoids from the root bark of Morus alba L. Phytochemistry 2003;62:1235-8.
- Ballesteros JF, Sanz MJ, Ubeda A, Miranda MA, Iborra S, Paya M, et al. Synthesis and pharmacological evaluation of 2’-hydroxychalconesand flavones as inhibitors of inflammatory mediators generation. J Med Chem 1995;38:2794-7.
- Martini ND, Katerere DR, Eloff JN. Biological activity of five antibacterial flavonoids from Combretumerythrophyllum (combretaceae). J Ethnopharmacol 2004;93:207-12.
- Machado KR, Norbert A, Ludger W. Antifungal flavonoids from Tibouchinagrandifolia. BiochemSystEcol 2009;37:63-5.
- Kujumgiev A, Tsvetkova I, Serkedjieva Y, Bankova V, Christov R, Popov S. Antiviral activities on some nigerian medicinal plants extracts. J Ethnopharmacol 1999;64:235-40.
- Aziz NH, Farag SE, Mousa LA, Abo-Zaid MA. Comparative antibacterial and antifungal effects of some phenolic compounds. Microbios 1998;93:43-54.
- Ferry DR, Smith A, Malkhandi J, Fyfe DW, deTakats PG, Anderson D, et al. Phase I clinical trial of the flavonoid quercetin pharmacokineticsand evidence for in vivo tyrosine kinase inhibition. Clin Cancer Res 1996;2:659-68.
- Sullivan TJ, Truglio JJ, Boyne ME, Novichenok P, Zhang X, Stratton CF, et al. High affinity InhA inhibitors with activity against drug-resistant strains of Mycobacterium tuberculosis. ACS ChemBiol 2006;1:43-53.
- Magedov IV, Manpadi M, Slambrouck SV, Steelant WF, Rozhkova E, Przheval’skii NM, et al. Discovery and investigation of antiproliferative and apoptosis-inducing properties of new heterocyclic podophyllotoxin analogues accessible by a one-step multicomponent synthesis. J Med Chem 2007;50:5183-92.
- Rovnyak GC, Millonig RC, Schwartz J, Shu V. Synthesis and antiinflammatory activity of hexahydrothiopyrano[4,3-c] pyrazoles and related analogues. J Med Chem 1982;25:1482-8.
- Liu XH, Cui P, Song BA, Bhadury PS, Zhu HL, Wang SF. Synthesis, structure and antibacterial activity of novel 1-(5-substituted-3-substituted-4,5-dihydropyrazol-1-yl)ethanoneoxime ester derivatives. Bioorg Med Chem 2008;16:4075-82.
- Akbas E, Berber I. Antibacterial and antifungal activities of new pyrazolo[3,4-d]pyridazin derivatives. Eur J Med Chem 2005; 40:401-5.
- Meazz G, Bettarini F, La Porta P, Piccardi P, Signorini E, Portoso Det al. Synthesis and herbicidal activity of novel heterocyclicprotoporphyrinogen oxidase inhibitors. Pest ManagSci 2004; 60:1178-88.
- Jung JC, Walkins EB, Avery MA. Synthesis of 3-substituted and 3,4-disubstituted pyrazolin-5-ones. Tetrahedron 2002;58:3639-46.
- Prakash O, Kumar R, Parkash V. Synthesis and antifungal activity of some new 3-hydroxy-2-(1-phenyl-3-aryl-4-pyrazolyl) chromones. Eur J Med Chem 2008;43:435-40.
- Ahmad I, Beg AJ. Antimicrobial and phytochemical studies on 45 Indian medicinal plants against multi-drug resistant human pathogens. J Ethnopharmacol 2001;74:113-23.
- Andrews JM. Determination of minimum inhibitory concentrations. J AntimicrobChemother 2001;48Suppl 1:5-16.
- Al-Burtamani SK, Fatope MO, Marwah RG, Onifade AK, Al-Saidi SH. Chemical composition, antibacterial and antifungal activities of the essential oil of Haplophyllumtuberculatum from Oman. J Ethnopharmocol 2005;96:107-12.
- NCCLS. Method for dilution antimicrobial susceptibility test for bacteria that grow aerobically, Approved Standards, 5th ed. Villanova, PA: National Committee for Clinical Standards; 2000.