- Corresponding Author:
- N. C. Desai
Medicinal Chemistry Division, University Department of Chemistry, Bhavnagar University, Bhavnagar-364 002, India
E-mail: dnisheeth@rediffmail.com
| Date of Submission | 7 April 2008 |
| Date of Revision | 27 August 2008 |
| Date of Acceptance | 14 February 2009 |
| Indian J Pharm Sci, 2009, 71 (1): 90-94 |
Abstract
Several 4-arylidene-2-phenyl-1-(2,4,5-trichlorophenyl)-1H-imidazol-5(4H)-ones (4a-q), N-(4-benzylidene-5-oxo-2-phenyl-4,5-dihydroimidazol-1-yl)-4-chlorobenzamides (5a-o) and N-(4-benzylidene-5-oxo-2-phenyl-4,5-dihydroimidazol-1-yl)-2,4-dichlorobenzamides (6a-m) were prepared. All newly synthesized compounds have been tested for their antibacterial activity against gram (+)ve and gram (-)ve bacteria and also on different strains of fungi. Introduction of OH, OCH 3 , NO 2 , Cl and Br groups to the heterocyclic frame work enhanced antibacterial and antifungal activities.
Keywords
5-Imidazolinone, antibacterial activity, antifungal activity
Imidazolinone ring system is of biological and chemical interest since long. The imidazolinones [1] are associated with a wide range of therapeutic activities [2-7] such as anticonvulsant, sedative and hypnotic, potent CNS depressant, antihistamine, antifilarial, bactericidal, fungicidal, antiinflammatory, MAO inhibitory, antiparkinsonian, antihypertensive and anthelmintic. Recently some new imidazolinone derivatives have been reported as antiinflammatory, herbicidal and hypertensive activities. Some workers have recognized 5-imidazolone as having anticancer activity [8]. The therapeutic importance of the compounds inspired us to synthesize some potential imidazolinones [9-13].
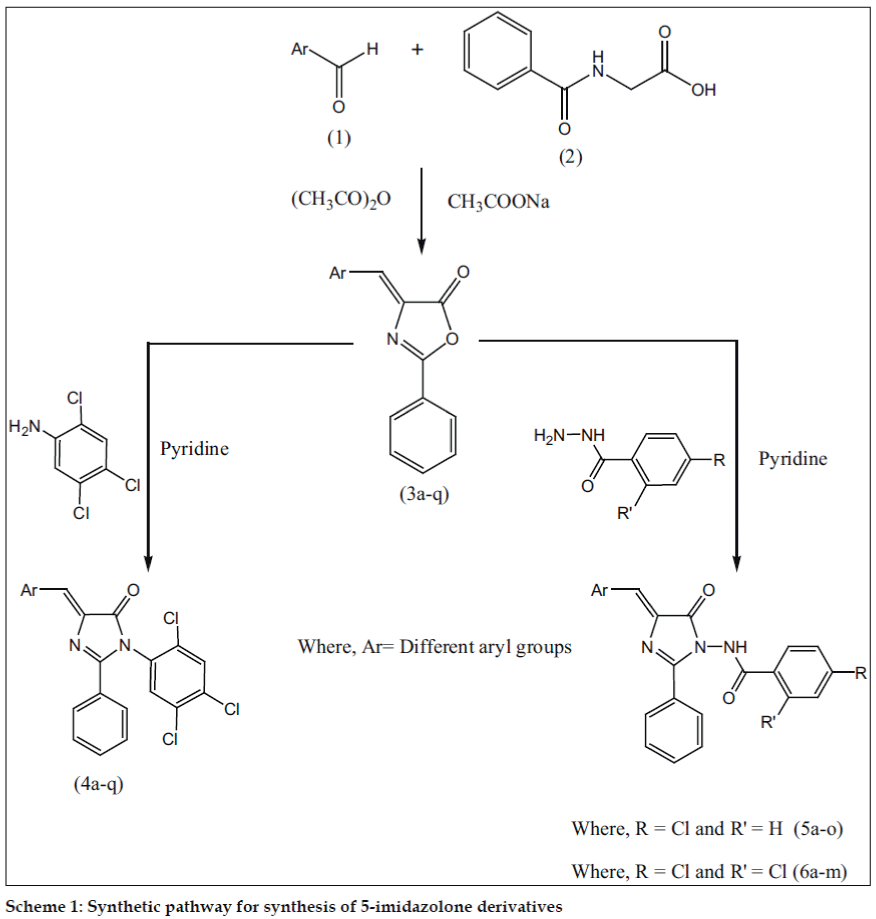
Desai et al. [14] have synthesized 4-benzylidene-2-phenyloxazole-5-one based on the methods descried in the literature which is a special type of Perkin condensation in which reaction between aldehyde and benzoylglycine proceeds first followed by ring closer. It is observed that aldehyde condenses under the influence of a base with the reactive methylene group in the azalactone which is formed by the dehydration of benzoylglycine, when the latter reacts with Ac2O in presence of sodium acetate. In view of these observations, we have synthesized imidazol-5-ones (Scheme I, Table 1).
| Sr No | Ar- | Molecular Formula | M.P. | Yield (%) | Elemental Analysis | |||
|---|---|---|---|---|---|---|---|---|
| % Carbon | % Nitrogen | |||||||
| Cal. | Found | Cal. | Found | |||||
| 4a | C6H5 | C22H13Cl3N2O | 173 | 65 | 61.78 | 61.69 | 6.55 | 6.41 |
| 4b | 2-OH-C6H4 | C22H13Cl3N2O2 | 170 | 60 | 59.55 | 59.47 | 6.31 | 6.25 |
| 4c | 4-OCH3-C6H4 | C23H15Cl3N2O2 | 160 | 55 | 60.35 | 60.28 | 6.12 | 6.03 |
| 4d | 3-Cl-C6H4 | C22H12Cl4N2O | 185 | 54 | 57.17 | 57.06 | 6.06 | 6.01 |
| 4e | 3-OCH3-C6H4 | C23H15Cl3N2O2 | 190 | 50 | 60.35 | 60.21 | 6.12 | 6.03 |
| 4f | 2-Cl-C6H4 | C22H12Cl4N2O | 195 | 53 | 57.17 | 57.06 | 6.06 | 5.98 |
| 4g | 4-Cl-C6H4 | C22H12Cl4N2O | 210 | 54 | 57.17 | 57.05 | 6.06 | 6.01 |
| 4h | 2-NO2-C6H4 | C22H12Cl3N3O3 | 180 | 57 | 55.9 | 55.79 | 8.89 | 8.8 |
| 4i | 3-NO2-C6H4 | C22H12Cl3N3O3 | 230 | 55 | 55.9 | 55.74 | 8.89 | 8.78 |
| 4j | 3-OCH3-4-OH-C6H3 | C23H15Cl3N2O3 | 170 | 65 | 58.31 | 58.21 | 5.91 | 5.7 |
| 4k | 5-Br-3OCH3-4-OH-C6H2 | C23H14BrCl3N2O3 | 235 | 68 | 49.99 | 49.85 | 5.07 | 4.98 |
| 4l | 4-OH-C6H4 | C22H13Cl3N2O2 | 145 | 57 | 59.55 | 59.36 | 6.31 | 6.21 |
| 4m | 5-Br-2OH-C6H 3 | C22H12BrCl3N2O2 | 175 | 50 | 50.56 | 50.45 | 5.36 | 5.22 |
| 4n | 3-OC6H5-C6H4 | C28H17Cl3N2O2 | 160 | 48 | 64.7 | 64.64 | 5.39 | 5.34 |
| 4o | 2,4,5 (OCH3)3-C6H2 | C25H19Cl3N2O4 | 198 | 45 | 57.99 | 57.9 | 5.41 | 5.33 |
| 4p | 3,4,5 (OCH3)3-C6H2 | C25H19Cl3N2O4 | 185 | 45 | 57.99 | 57.89 | 5.41 | 5.35 |
| 4q | 3-OH-C6H4 | C22H13Cl3N2O2 | 165 | 58 | 59.55 | 57.47 | 6.31 | 6.26 |
| 5a | C6H5 | C23H16ClN3O2 | 233 | 60 | 68.74 | 68.62 | 10.46 | 10.35 |
| 5b | 2-OH-C6H4 | C23H16ClN3O3 | 235 | 65 | 66.11 | 66.05 | 10.06 | 9.97 |
| 5c | 3-Cl-C6H4 | C23H15Cl2N3O2 | 237 | 66 | 63.32 | 63.2 | 9.63 | 9.51 |
| 5d | 3-OCH3-C6H4 | C24H18ClN3O3 | 246 | 55 | 66.75 | 66.66 | 9.37 | 9.29 |
| 5e | 2-Cl-C6H4 | C23H15Cl2N3O2 | 212 | 62 | 63.32 | 63.19 | 9.63 | 9.51 |
| 5f | 4-Cl-C6H4 | C23H15Cl2N3O2 | 214 | 64 | 63.32 | 63.23 | 9.63 | 9.54 |
| 5g | 2-NO2-C6H4 | C23H15ClN4O4 | 248 | 50 | 61.82 | 61.73 | 12.54 | 12.45 |
| 5h | 3-NO2-C6H4 | C23H15ClN4O4 | 223 | 56 | 61.82 | 61.69 | 12.54 | 12.47 |
| 5i | 3-OCH3-4-OH-C6H3 | C24H18ClN3O4 | 226 | 45 | 64.36 | 64.25 | 9.38 | 9.25 |
| 5j | 4-OH-C6H4 | C23H16ClN3O3 | 231 | 47 | 66.11 | 66.01 | 10.06 | 9.98 |
| 5k | 3-OC6H5-C6H4 | C29H20ClN3O3 | 186 | 48 | 70.52 | 70.4 | 8.51 | 8.39 |
| 5l | 2,4,5 (OCH3)3-C6H2 | C26H22ClN3O5 | 245 | 46 | 63.48 | 63.4 | 8.54 | 8.47 |
| 5m | 3,4,5 (OCH3)3-C6H2 | C26H22ClN3O5 | 210 | 50 | 63.48 | 63.41 | 8.54 | 8.45 |
| 5n | 3-OH-C6H4 | C23H16ClN3O3 | 182 | 57 | 66.11 | 66.02 | 10.06 | 9.98 |
| 5o | 4-N(C2H5)2-2-OH-C6H3 | C27H25ClN4O3 | 176 | 43 | 66.21 | 66.21 | 11.46 | 11.4 |
| 6a | 2-OH-C6H4 | C23H15Cl2N3O3 | 175 | 60 | 61.08 | 61.01 | 9.29 | 9.93 |
| 6b | 3-Cl-C6H4 | C23H14Cl3N3O2 | 208 | 58 | 58.68 | 58.61 | 8.39 | 8.88 |
| 6c | 3-OCH3-C6H4 | C24H17Cl2N3O3 | 202 | 55 | 61.82 | 61.7 | 9.01 | 5.9 |
| 6d | 2-Cl-C6H4 | C23H14Cl3N3O2 | 205 | 56 | 58.68 | 58.58 | 8.93 | 8.85 |
| 6e | 4-Cl-C6H4 | C23H14Cl3N3O2 | 244 | 55 | 58.68 | 58.59 | 8.93 | 8.87 |
| 6f | 2-NO2-C6H4 | C23H14Cl2N4O4 | 216 | 58 | 57.4 | 57.31 | 11.64 | 11.55 |
| 6g | 3-NO2-C6H4 | C23H14Cl2N4O4 | 233 | 60 | 57.4 | 57.32 | 11.64 | 11.56 |
| 6h | 3-OCH3-4-OH-C6H3 | C24H17Cl2N3O4 | 237 | 48 | 59.77 | 59.65 | 8.74 | 8.65 |
| 6i | 4-OH-C6H4 | C23H15Cl2N3O3 | 236 | 45 | 61.08 | 61.01 | 9.29 | 9.22 |
| 6 j | 3-OC6H5-C6H4 | C29H19Cl2N3O3 | 224 | 48 | 65.92 | 65.8 | 7.95 | 7.81 |
| 6k | 2,4,5 (OCH3)3-C6H2 | C26H21Cl2N3O5 | 238 | 43 | 59.33 | 59.27 | 7.98 | 7.9 |
| 6l | 3,4,5 (OCH3)3-C6H2 | C26H21Cl2N3O5 | 212 | 45 | 59.33 | 59.26 | 7.98 | 7.88 |
| 6m | 3-OH-C6H4 | C23H14Cl2N3O3 | 190 | 48 | 61.08 | 61.01 | 9.29 | 9.2 |
Table 1: Physical constants and elemental analysis of 5-imidazolnes4a-q, 5a-o and 6a-m.
Various 4-arylidene-2-phenyl-1-(2,4,5-trichlorophenyl)- 1H-imidazol-5(4H)-ones (4a-q) were prepared by the reaction of 2,4,5-trichlorobenzenamine with 4-arylidene-2-phenyloxazol-5(4H)-ones (3a-q). N-(4- benzylidene-5-oxo-2-phenyl-4,5-dihydroimidazol- 1-yl)-4-chlorobenzamide (5a-o) were synthesized by the reaction of 4-chlorobenzohydrazide and 4-arylidene-2-phenyloxazol-5(4H)-ones (3a-q). N-(4- benzylidene-5-oxo-2-phenyl-4,5-dihydroimidazol-1- yl)-2,4-dichlorobenzamides (6a-m) were obtained by the reaction of 2,4-dichlorobenzohydrazide with 4-arylidene-2-phenyloxazol-5(4H)-ones (3a-q).
Melting points were taken in open capillaries using paraffin bath and are uncorrected. IR spectra were recorded on FTIR-Perkin-Elmer spectrometer (Vmax cm-1); 1H NMR spectra were recorded on Bruker Avance 300 FT-NMR spectrometer using CDCl3 as a solvent and mass spectra carried out on JEOL SX 102/DA-600 mass spectrometer, respectively. All the compounds were analyzed for carbon, hydrogen and nitrogen and the results were within ±0.4% of theoretical values. Purity was checked by TLC using TLC aluminum sheets silica gel 60, supplied by E. Merck, Mumbai, India. The spots were located by keeping the plates in iodine vapor and 2,4,5-trichlorobenzenamine was supplied by S. D. Fine Chem Limited, Mumbai, India. 4-Chlorobenzohydrazide, 2,4-dichlorobenzo hydrazide and 4-arylidene-2-phenyloxazol-5(4H)-one (3a-q), were prepared as given in literature method [15-20].
4-Arylidene-2-phenyl-1-(2,4,5-trichlorophenyl)-1H-imidazol- 5(4H)-one (4) were synthesized as follows; A mixture of 2,4,5-trichloroaniline (0.01 mol) and 4-(arylidene)-2-phenyloxazol-5(4H)-ones (0.01 mol) was placed in a round bottom flask and 10 ml of pyridine were added to it. The reaction mixture was refluxed on a sand bath for 6 h. (scheme I) and the mixture was poured into ice-cold water and then a required amount of conc. hydrochloric acid was added to neutralize the reaction mixture. The progress of the reaction and the purity of compounds were routinely checked on TLC. The solid obtained was left overnight, filtered and washed with water. The product was dried and recrystallized from ethanol (99%). m.p.195° Yield 53% anal. found: C, 57.06; N, 5.98; calc for C22H12Cl4N2O: C, 57.17; N, 6.06%.
Compound 4f: IR (KBr): 3062 cm-1 (-C-H str., aromatic), 1643 cm-1 (>C=O str., cyclic ring), 1359 cm-1 (>C=N str., imidazol ring), 1284 cm-1 (-C-N tertiary amine), 1074 cm-1 (-C-Cl str., aromatic), 744 cm-1 (>C=CH medium), 704, 688, 613 cm-1 (trisubstituted aromatic). 1H NMR (CDCl3): δ7.2 (s, 1H, -CH), 7.26-8.54 (m, 11H, Ar-H, C=C-Ar) ppm. MS: m/z 461 with 62% relative intensity (base peak) & 462 with 47% relative intensity (M+). Other compounds of the series were prepared by using a similar method and their physical data are recorded in Table 1.
N-(4-benzylidene-5-oxo-2-phenyl-4,5-dihydroimidazol-1-yl)-4-chlorobenzamides (5)/N-(4-benzylidene-5-oxo-2-phenyl-4,5-dihydroimidazol- 1-yl)-2,4-dichlorobenzamides (6) were prepared using the following procedure; A mixture of 4-chlorobenzohydrazide/ 2,4-dichlorobenzohydrazide (0.01 mol) and 4-(arylidene)-2-phenyloxazol-5(4H)- ones (0.01 mol) was placed in a round bottom flask and 10 ml of pyridine was added to this mixture. The reaction mixture was refluxed on a sand bath for 6 h (Scheme I). The mixture was poured into ice-cold water and then required amount of con. hydrochloric acid was added to neutralize the reaction mixture. The solid obtained was left overnight, filtered and washed with water. The product was dried and recrystallized from ethanol (99%).
Compound 5f: IR (KBr): 3249 cm-1 (medium –CONH-), 3033 cm-1 (-C-H str., aromatic), 1656 cm-1 (>C=O str., cyclic ring), 1625 cm-1 (>C=N str., imidazol ring), 1490 cm-1 ( >NH weak), 1299 cm-1 (-C-N tertiary amine), 1095 cm-1 (-C-Cl str., aromatic), 754 cm-1 (>C=CH medium ), 707 cm-1 (monosubstituted aromatic). 1H NMR (CDCl3): δ7.28 (s, 1H, -CH), 7.26-8.54 (m, 13H, Ar-H, -C=C-Ar), 10.02 (s, 1H, -NH-CO-) ppm. MS: m/z 436 with 45% relative intensity (base peak) & 437 with 32% relative intensity (M+).
Compound 6e: IR (KBr): 3213 cm-1 (medium, –CONH-), 2993 cm-1 (-C-H str., aromatic), 1662 cm-1 (>C=O str., cyclic ring), 1635 cm-1 (>C=N str., imidazol ring), 1473 cm-1 (>NH weak), 1305 cm-1 (-C-N tertiary amine), 1109 cm-1 (-CCl str., aromatic), 925 cm-1 (>C=CH medium), 825, 713 cm-1 (disubstituted aromatic), 707 cm-1 (monosubstituted aromatic). 1H NMR (CDCl3): δ7.2 (s, 1H ,-CH), 7.32-8.05 (m, 12H, Ar-H,C=C-Ar), 10.02 (s,1H, -NH-CO-) ppm. MS: m/z 471 with 79% relative intensity (base peak) and 472 with 51% relative intensity (M+). Other compounds of the series were prepared by using a similar method and their physical data are recorded in Table 1.
Antibacterial activity was carried out by broth dilution method [21]. The strains used for the activity were procured from Institute of Microbial Technology, Chandigarh. The compounds 4a-q, 5a-o and 6a-m were screened for their antibacterial activity against Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa and Staphylococcous pyogenes at concentrations of 1000, 500, 200, 100, 50, 25, 12.5 μg/ml respectively (Table 2). Same compounds were tested for antifungal activity against C. albicans, A. niger and A. clavatus at concentrations of 1000, 500, 200, and 100 μg/ml respectively (Table 2). The results are recorded in the form of primary and secondary screening.
| Sr. No. | Minimal bactericidal concentration (MBC) in μg/ml | Minimal fungicidal concentration (MFC) in μg/ml | |||||
|---|---|---|---|---|---|---|---|
| E. coli | P. aeruginosa | S. aureus | S. pyogenus | C. albicans | A. niger | A. clavatus | |
| MTCC-443 | MTCC-1688 | MTCC-96 | MTCC-442 | MTCC-227 | MTCC-282 | MTCC-1323 | |
| 4a | 25 | – | – | – | – | – | – |
| 4b | 25 | 50 | – | – | – | – | – |
| 4f | 100 | – | – | – | 100 | 100 | 100 |
| 4i | 25 | – | – | – | – | – | – |
| 4j | 25 | – | – | – | – | – | – |
| 4k | 50 | 100 | 50 | – | – | – | – |
| 4q | 50 | 100 | – | – | – | – | – |
| 5b | – | – | – | – | 100 | 100 | 100 |
| 5e | 50 | 50 | – | – | – | – | – |
| 6f | 100 | – | – | – | 100 | 100 | 100 |
| 6j | – | – | – | – | – | – | 100 |
| 6l | – | – | – | – | 100 | – | 100 |
| 6m | – | – | – | – | 100 | – | – |
Table 2: Antibacterial and antifungal activities of the synthesized compounds*.
The synthesized compounds found to be active in the primary screening were further tested in a second set of dilution against all microorganisms. The compounds found active in primary screening were similarly diluted to obtain 100, 50, 25 μg/ml concentrations. Ten microlitres suspensions from each well were further inoculated on appropriate media and growth was noted after 24 and 48 h. The lowest concentration, which showed no growth after spot subculture was considered as MBC/MFC for each drug. The highest dilution showing at least 99% inhibition was taken as MBC/MFC. The result of this test is affected by the size of the inoculums. The test mixture should contain 108 organisms/ml. For antibacterial activity, in present protocol 50 μg/ml is considered as active as compared to the standard drug gentamycin. For antifungal activity, 100 μg/ml is considered as active as compared to standard nystatin. Compounds 4a, 4b, 4i, 4j, 4k, 4q and 5e are active on E. coli where as 4b and 5e are active on P. aeruginosa. Compound 4k is active on S. aureus and 6m is also active on S. pyogens. Compounds 4f, 5b, 6f, 6l, and 6m are active on fungi strains. On the basis of biological activity results, it may be concluded that the introduction of OH, OCH3, NO2, Cl and Br groups to the heterocyclic frame work enhanced antibacterial and antifungal activities.
Acknowledgements
The Authors are thankful to the Bhavnagar University for providing research facilities. Authors are also thankful to director, FSL Gandhinagar for the spectral data.
References
- Duschinsky R (1995) Imidazolone derivatives. US patent US2707186.
- Luigi A, Alfonso M, Pierluigi R, Afro G, Enzo Z, Nicola DT, et al. (1969) Derivatives of imidazoles. III. Synthesis and pharmacological activities of nitrile, amides, and carboxylic acid derivatives of imidazo [1,2-a] pyridine. J Med Chem12:122-126.
- Godefroi EF, Platje JTJ (1972) DL- l-(alpha-methylbenzyl)-2-methylimidazole-5-carboxylate esters. Synthesis and pharmacological properties. J Med Chem15:336.
- Upadhyay PS, Joshi HD, Baxi AJ, Parikh AR (1991) Synthesis and pharmacological evaluation of some new imidazolinones as anticonvusants. Indian J Heterocyc Chem1:71.
- Harfenist M, Soroko EF, Mckenzie GM (1978) 2-(Alkoxyaryl)-2-imidazoline monoamine oxidase inhibitors with antidepressant activity. J Med Chem21:405.
- Mukerji D, Nautiyal SR, Prasad CR (1981) Synthesis of some new 1-(2’-methoxy-4’-nitrophenyl)-2-methyl-4-substituted benzylidine-5-imidazolones as CNS depression compounds. Indian Drugs18:125.
- Meenakshi S, Reena K, Renu S, Dixit KS, Nath C, Barthwal JP (1990) Synthesis, anticonvusant and enzyme inhibitory activities of some indolyl-1,3,4-thiadiazoles. Indian J Chem29(B):85.
- Solankee A, Kapadiya K, Thakor I, Patel J, Lad S (2004) Synthesis and antimicrobial activity of 1-(4’-Trifluoromethylphenyl)-2-phenyl-4-(benzylidene/substituted benzylidene/2’-furylidene/2’-thienylidene)-imidazolin-5-ones. Asian J Chem16:917.
- Naithani DK, Srivastava VK, Barthwal JP, Saxena AK, Gupta TK, Shanker K (1989) Synthesis and antiparkinsonian activity of newer imidazolones. Indian J Chem28(B):990.
- Desai NC, Dave D, Shah MD, Vyas GD (2000) Synthesis and antibacterial activity of some novel 4-oxo-1,3-thiazolidines, 2-oxoazetidines and 5-oxoimidazolines. Indian J Chem39(B):277-282.
- Tripathy PK (2004) Microwave assisted one pot synthesis of (z)-1,2-disubstituted-4-arylmethylene-2 imidazolin-5-ones using solid support under solvent free condition. Indian J Heterocycl Chem14: 77-78.
- Hirpara KV, Patel SP, Parikh KA, Bhimani AS, Parekh HH (2004) Preparation, characterisation and antimicrobial activities of some novel nitriles and imidazolines. J Sci Islam Rep Iran15:135.
- Rama Sharma, GVS, Reddy VM (1993) Synthesis of 1,2 disubstituted-4- [(chromon-3-yl) methylene]imidazolin-5(4H)-ones as potential pharmacological agents. Indian J Heterocycl Chem3:111-116.
- Shah MD, Desai NC, Awasthi KK, Saxena AK (2001) Synthesis and QSAR studies of 5-imidazolinone derivatives as potential antibacterial agents. Indian J Chem40(B):201
- Shah HP, Shah BR, Bhatt JJ, Deasi NC, Trivedi PB, Undavia NK (1998) Synthesis of 2,5-disubstituted 1,3,4-oxadiazoles as potential antimicrobial, anticancer and anti-HIV agents. Indian J Chem37(B):180.
- Furniss BS, Hannaford AJ, Smith PWG, Tatchell AR (2004) Vogel’s text book of practical organic chemistry. 5th Ed. London: Pearson Education 1156.
- Monk KA, Dusan S, Mohan RS (2000) Bismuth (III) acetate: A new catalyst for preparation of azalactones via the Erlenmeyer synthesis. Synth Comm 30:3167-3170.
- Imtiaz HM, Vinaykumar (1992) Synthesis of 2-(2-benzothiazolyl/ benzoxazolylthio)-N- [4,5-dihydro-4-((substitutedphenyl)methylene)-5-oxo-2-phenyl/methyl-1H-imidazole-1-l]acetamides as possible anthelmintics. Indian J Chem31(B):285-288.
- Shah BR, Bhatt JJ, Patel HH, Undavia NK, Trivedi PB, Desai NC (1995) Synthesis of 2,3-disubstituted-3,1-quinazolin-4(4H)-ones as potential anticancer and anti-HIV agents. Indian J Chem34(B):201.
- Bhatt KN, Dave AM, Desai NC, Undavia NK, Trivedi PB (1992) Studies on synthesis and antimicrobial evaluation of some new thiophenol derivatives. J Indian Chem Soc69:785.
- (1997) National Committee for Clinical Laboratory Standard. Reference method for broth dilution antifungal susceptibility testing of yeasts Approved standard M27A NCCLS, Wayne, PA.