- *Corresponding Author:
- A. Jamal Abdul Nasser
P. G. and Research Department of Chemistry, Jamal Mohamed College, Tiruchirappalli - 620 020
E-mail: jamal_abdulchem@ymail.com
| Date of Submission | 22 February 2010 |
| Date of Revision | 22 October 2010 |
| Date of Acceptance | 13 November 2010 |
| Indian J Pharm Sci 2010, 72 (6): 719-725 |
Abstract
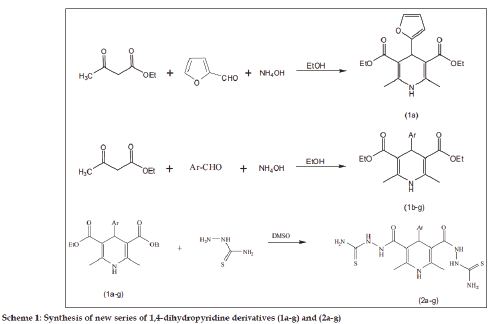
A series of 1,4-dihydropyridine derivatives (1a-g) were prepared from three compounds condensation of Hantzsch synthesis. A new series of 2,2'-{[4-(aryl)-2,6-dimethyl-1,4-dihydropyridine-3,5-diyl]dicarbonyl}dihydrazinecarbothioamide (2a-g) were prepared from compounds diethyl 4-(aryl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate (1a-g) reacted with thiosemicarbazide to give the corresponding compounds (2a-g) by hydrazinolysis method. The synthesized compounds were confirmed by IR, 1HNMR, 13CNMR, mass spectral and elemental analyses. The newly synthesized compounds (2a-g) were screened for anticonvulsant activity against in swiss albino rat. The test was evaluated by maximal electrode induced convulsion method. Synthesized compounds were used two (50 and 100 mg/kg) concentrations. Compounds (1a-g) were inactive while compounds (2a-g) have moderate anti-convulsant activity compared with standard phenytoin drug. The compound 2,2'-{[4-(furan-2-yl)-2,6-dimethyl-1,4-dihydropyridine-3,5-diyl]dicarbonyl} dihydrazinecarbothioamide (2a) has highly active compared with other compound (2b-2g).
Keywords
1,4-dihydropyridine, anticonvulsant activity, condensation, thiosemicarbazide
1,4-dihydropyridine derivatives are of interest because of their potential biological activity such as antihypertensive [1-4], antiinflammatory [5] and antiischemic activities [6] and also as calcium channel modulators of the nifedipine type [7]. Several methods have been described for the synthesis of 1,4-dihydropyridine [8-12]. Recently, some new 3,5-substituted 1,4-dihydropyridine derivatives were synthesized which exhibit pharmacological activities [13-16]. Thosemicarbazone also has significant biological activities such as antitumour, fungicide, bactereocide, antiinflammatory, and antiviral activities [17-20]. Keeping these observations in mind, the present study worked on the synthesis of a new series of 1,4-dihydropyridine derivatives and screened their level of anticonvulsant activity.
Materials and Methods
Melting points were recorded in open capillary tubes and are uncorrected. The IR spectra were recorded in KBr on a FT - IR Shimadzu 8201pc (4000-400 cm-1) and 1H NMR and 13CNMR were recorded on a Broker DRX-300 MHz. Mass spectra (EI) were obtained on a Joel JMS D-300 spectrometer operating at 70eV. Elemental analyses (C, H, N, and S) were undertaken using an Elementer analyser model vario EL III. The purity of the compounds was checked by thin layer chromatography (TLC) with silica gel plates.
Synthesis of diethyl 4-(furan-2-yl)-2,6-dimethyl-1,4- dihydropyridine-3,5-dicarboxy late (1a)
A reaction mixture was made up of ethyl acetoacetate (2 mol), furualdehyde (1 mol) and ammonium hydroxide (1 mol) in methanol (20 ml). It was then heated and refluxed for 4 h. The obtained solid was filtered off, the solid was washed with water and recrystallized using absolute ethanol. The above procedure was followed for the synthesis of compounds (1b–g). Yield 75%, mp: 158, IR (KBr, cm-1) ν: 3349 (N-H str), 3030 (Ar-H), 2940 (C-H str of CH3), 1745 (C=O, ester), 812 (Ar-H). 1H NMR (DMSO-d6): δ 8.20 (s, 1H, NH of pyridine ring), 6.27-6.10 (d, 3H, furylring), 4.72 (s, 2H, C4-H), 4.20 (q, 4H, C3-OCH2CH3 and C5-OCH2CH3), 2.31 (s, 6H, C2-CH3 and C6-CH3), 1.34 (t, 6H, C2-OCH2CH3 and C6-OCH2CH3). Elemental analysis calculated for C17H12NO5: C 63.94, H 6.63, N 4.39. Found: C 63.98, H 6.67, N 4.35.
Diethyl 2,6-dimethyl-4-phenyl-1,4-dihydropyridine- 3,5-dicarboxylate(1b):
Yield 66%, mp: 253, IR (KBr, cm-1) ν: 3350 (N-H str), 3034 (Ar-H), 2953 (C-Hstr of CH3), 1755 (C=O, ester), 802 (Ar-H). 1H NMR (DMSO-d6): δ 8.25 (s, 1H, NH of pyridine ring), 7.33-7.27 (m, 5H, Ph-ring), 4.70 (s, 1H, C4-H), 4.22 (q, 4H, C3-OCH2CH3 and C5-OCH2CH3), 2.28 (s, 6H, C2-CH3 and C6-CH3), 1.32 (t, 6H, C2-OCH2CH3 and C6-OCH2CH3). Elemental analysis calculated for C19H23NO4 : C 69.28, H 7.04, N 19.43. Found: C 69.24, H 7.07, N 19.41.
Diethyl 4-(4-chlorophenyl)-2,6-dimethyl-1,4- dihydropyridine-3,5-dicarboxylate (1c)
Yield 57%, mp: 240, IR(KBr, cm-1) ν: 3332 (N-H sYield 57%, mp: 240, IR (KBr, cm-1) ν: 3332 (N-H str), 3074 (Ar-H), 2942 (C-Hstr of CH3), 1741 (C=O, ester), 837 (C-Cl), 787 (Ar-H). 1H NMR (DMSO-d6): δ 8.31 (s, 1H, NH of pyridine ring), 7.36-7.19 (dd, 4H, Ph-ring), 4.76 (s, 1H, C4-H), 4.18 (q, 4H, C3-OCH2CH3 and C5-OCH2CH3), 2.21 (s, 6H, C2-CH3 and C6-CH3), 1.34 (t, 6H, C2-OCH2CH3 and C6-OCH2CH3). Elemental analysis calculated for C19H22ClNO4: C 62.72, H 6.09, N 3.85. Found: C 62.75, H 6.07, N 3.81.
Diethyl 4-(4-hydroxyphenyl)-2,6-dimethyl-1,4- dihydropyridine-3,5-dicarboxylate (1d)
Yield 56%, mp: 240, IR (KBr, cm-1) ν: 3342 (N-H str), 3024 (Ar-H), 2922 (C-H str of CH3), 1764 (C=O, ester), 1447 (C-OH), 814 (Ar-H). 1H NMR (DMSO-d6) : δ 9.47 (s, 1H, C-OH), 8.41 (s, 1H, NH of pyridine ring), 7.34-7.07 (dd , 4H, Ph-ring), 4.67 (s, 1H, C4-H), 4.28 (q, 4H, C3-OCH2CH3 and C5- OCH2CH3), 2.12 (s, 6H, C2-CH3 and C6-CH3), 1.28 (t, 6H, C2-OCH2CH3 and C6-OCH2CH3). Elemental analysis calculated for C19H23NO5 : C 69.07, H 6.71, N 4.06. Found: C 69.O3, H 6.75, N 4.01.
Diethyl 2,6-dimethyl-4-(4-nitrophenyl)-1,4- dihydropyridine-3,5-dicarboxylate (1e)
Yield (69%), mp: 197, IR (KBr, cm-1) ν: 3354 (N-H str), 3037 (Ar-H), 2973 (C-H str of CH3), 1762 (C=O, ester), 1536 (C-NO2), 812 (Ar-H). 1HNMR (DMSO-d6) : δ 7.13-7.47 (dd , 4H, Ph-ring), 8.11 (s, 1H, NH of pyridine ring), 4.79 (s,1H,C4-H), 4.25 (q, 4H, C3-OCH2CH3 and C5-OCH2CH3), 2.31 (s, 6H, C2-CH3 and C6-CH3), 1.37 (t, 6H, C2-OCH2CH3 and C6-OCH2CH3 ). Elemental analysis calculated for C19H22N2O6 : C 60.95, H 7.48, N 7.48. Found: C 60.91, H 7.42, N 7.41.
Diethyl 4-(4-methoxyphenyl)-2,6-dimethyl-1,4- dihydropyridine-3,5-dicarboxylate(1f)
Yield (72%), mp: 197, IR ( KBr, cm-1) ν: 3352 (N-H str), 3026 (Ar-H), 2961 (C-H str of CH3 ), 1742 (C=O, ester), 823 (Ar-H). 1H NMR (DMSO-d6): δ 8.21 (s, 1H, NH of pyridine ring), 6.86-7.17 (dd, 4H, Ph-ring), 4.69 (s, 1H, C4-H), 4.23 (q, 4H, C3- OCH2CH3 and C5-OCH2CH3), 3.84 (s, 3H, -OCH3), 2.23 (s, 6H, C2-CH3 and C6-CH3), 1.30 (t, 6H, C2- OCH2CH3 and C6-OCH2CH3). Elemental analysis calculated for. C20H25NO5: C 66.83, H 7.01, N 3.90. Found: C 66.87, H 7.07, N 3.97.
Diethyl 4-(4-(dimethylamino)phenyl)-2,6-dimethyl- 1,4-dihydropyridine-3,5-dicarboxylate (1g)
Yield (56%), mp: 227, IR (KBr, cm-1) ν: 3348 (N-H str), 3027 (Ar-H), 2956 (C-Hstr of CH3), 1761 (C=O, ester), 808 (Ar-H). 1H NMR (DMSO-d6): δ 8.37 (s, 1H, NH of pyridine ring), 7.28-7.21 (dd, 4H, Phring), 4.70 (s, 2H, C4-H), 4.22 (q, 4H, C3-OCH2CH3 and C5-OCH2CH3), 3.12 (s, 6H, -N(CH3)2), 2.28 (s, 6H, C2-CH3 and C6-CH3), 1.32 (t, 6H, C2-OCH2CH3 and C6-OCH2CH3). Elemental analysis calculated for C19H23NO4: C 67.72, H 7.58 ,N 7.52. Found: C 67.77, H 7.52, N 7.55.
Synthesis of 2,2'-{ [4-(furan-2-yl)-2,6-dimethyl- 1,4-dihydropyridine-3,5-diyl]dicarbonyl} dihydrazinecarbothioamide (2a)
A reaction mixture was made up of compound (1a) (0.1 mol), thiosemicarbazide dissolved in ethanol (30 ml) and a few drops DMSO. It was then heated under reflux for 10 h. The obtained solid was allowed to cool and then poured in to crushed ice. The solid was collected by filtration, washed with water and recrystallised using ethanol. The above procedure was followed for the synthesis of compounds (2b–g). Yield (70%). mp:197
IR (KBr, cm-1) ν: 3370 (NH), 3221 (NH2), 3192 (NHC=O), 3037(ArH), 1721 (C=O), 1263 (C=S), 1095 (N-C-N), 811 (Ar-H). 1H NMR (CDCl3): δ 9.64 (s, 2H, NH2), 8.46 (s, 1H, NH of pyridine ring), 8.12 (d, 1H, C3- CONH and C5- CONH), 7.22 (s, 5H, Ph-ring), 6.14-6.32 (d, 2H, furyl ring), 5.15 (s, 2H, C4-H), 2.33 (s, 6H, C2-CH3 and C6-CH3), 2.14 (d, 1H, -NHCS). 13C NMR (CDCl3): δ 111.8, 108.3, 143.2, 152.8 (4C in furyl ring), 105.3 (3,5 C in pyridine ring), 166.2 (3,5 C=O), 182.1 (3,5 C=S), 148.9 (2,6-C in pyridine ring), 35.3 (4C in pyridine ring), 18.2 (2,6-CH3 in pyridine ring). MS (m/z, relative abundance, %): 410 (M++1, 30.2), 291.30, 161.27, 175.22, 147.12, 81.11. Elemental analysis calculated for C17H21N7O2S2: C 48.67, H 5.50, N 23.37, S 15.29. Found: C 48.64, H 5.57, N 23.31, S 15.34.
2,2'- [(2,6-dimethyl-4-phenyl-1,4-dihydropyridine- 3,5-diyl)dicarbonyl]dihydrazinecarbothioamide (2b)
Yield (53%), mp: 192, IR (KBr, cm-1) ν: 3372 (NH), 3200 (NH-C=O), 3218 (NH2), 3034 (Ar-H), 1718 (C=O), 1260 (C=S), 1091 (N-C-N); 808 (Ar-H). 1H NMR (CDCl3): δ= 9.62 (s, 2H, NH2), 8.43 (s, 1H, NH of pyridine ring ), 8.09 (d, 1H, C3-CONH and C5-CONH ), 7.39-7.22 (m, 5H, Ph-ring), 5.17 (s, 2H, C4-H), 2.37 (s, 6H, C2-CH3 and C6-CH3), 2.12 (d, 1H, -NHCS). 13CNMR (CDCl3): δ= 131.3, 128.5, 130.9, 141.8 (4C in furyl ring), 106.8 (3,5 C in pyridine ring), 164.6 (3,5 C=O), 182.8 (3,5 C=S), 147.9 (2,6-C in pyridine ring), 34.6 (4C in pyridine ring), 18.9 (2,6-CH3 in pyridine ring). MS (m/z, relative abundance, %) : 420.20 ( M++1, 20.1), 301.34, 241.28, 185.2, 157.21, 81.11. Elemental analysis calculated for C17H21N7O2S2: C 48.67, H 5.50, N 23.37, S 15.29. Found: C 48.64, H 5.55, N 23.33,S 15.33.
2 , 2 ' - { [ 4 - ( 4 - c h l o rophenyl)-2,6-dimethyl- 1,4-dihydropyridine-3,5-diyl]dicarbonyl} dihydrazinecarbothioamide (2c)
Yield (68%), mp: 194, IR(KBr, cm-1) ν: 3325 (NH), 3231 (NH2), 3198 (NH-C=O), 3024 (Ar-H), 1707 (C=O), 1265 (C=S), 1097 (N-C-N), 827 (C-Cl). 1HNMR (CDCl3): δ 9.41 (s, 2H, NH2), 8.41 (bs, 1H, NH of pyridine ring), 8.11 (d, 1H, C3 - CONH and C5- CONH), 7.38- 7.14 (m, 5H, Ph-ring), 5.10 (s, 2H, C4-H), 2.45 (s, 6H, C2-CH3 and C6-CH3), 2.08 (d, 1H, -NHCS).13C NMR (CDCl3): δ 128.7, 108.3, 143.2, 152.8 (4C in furyl ring), 105.3 (3,5 C in pyridine ring), 166.2 (3,5 C=O), 182.1 (3,5 C=S), 148.9 (2,6-C in pyridine ring), 39.3 (4C in pyridine ring), 18.2 (2,6-CH3 in pyridine ring). MS (m/z, relative abundance, %) : 454.12 (M++1, 12.3), 335.78, 275.73, 219.70, 157.21, 81.11. Elemental analysis calculated for C17H20ClN7O2S2: C 44.98, H 4.40, N 21.60, S14.14. Found: C 44.94, H 4.44, N 21.64, S14.18.
2,2'-{ [4-(4-hydroxyphenyl)-2,6-dimethyl- 1,4-dihydropyridine-3,5-diyl]dicarbonyl}di hydrazinecarbothioamide(2d)
Yield (74%), mp: 201, IR (KBr, cm-1) ν: 3342 (NH), 3220 (NH2), 3192 (NH-C=O), 3028 (Ar-H), 1717 (C=O), 1472 (C-OH), 1242 (C=S), 1091 (N-C-N). 1H NMR(CDCl3): δ 9.71 (s, 2H, NH2), 9.41 (s, 1H, OH), 8.64 (bs, 1H, NH of pyridine ring), 8.01 (d, 1H, C3-CONH and C5-CONH), 7.33-7.27 (m, 5H, Ph-ring), 5.11 (s, 2H, C4-H), 2.25 (s, 6H, C2-CH3 and C6-CH3), 2.02 (d, 1H, -NHCS).13C NMR (CDCl3): δ 155.8, 137.1, 130.3, 114.2 (4C in furyl ring), 102.9 (3,5 C in pyridine ring), 164.9 (3,5 C=O), 184.6 (3,5 C=S), 148.1 (2,6-C in pyridine ring), 43.8 (4C in pyridine ring), 19.2 (2,6-CH3 in pyridine ring). MS (m/z, relative abundance, %): 435.52 (M++1, 27.2), 257.28, 201.26, 173.21, 157.21, 81.11. Elemental analysis calculated for C17H21N7O2S2: C 46.88, H 22.51, N 4.86, S 14.72. Found: C 46.84, H 22.54, N 4.84, S 14.76.
2 , 2 ' - { [ 4 - ( 4 - n i t ro p h e n y l ) - 2 , 6 - d i m e t h y l - 1,4-dihydropyridine-3,5-diyl]dicerbonyl} dihydrazinecarbothioamide (2e)
Yield (76%), mp: 195, IR (KBr, cm-1) ν: 3310 (NH), 3241 (NH2), 3218 (NH-C=O), 3041 (Ar-H), 1530 (C-NO2), 1272 (C=S), 1710 (C=O), 1091 (N-C-N). 1H NMR (CDCl3): δ 9.77 (s, 2H, NH2), 8.60 (bs, 1H, NH of pyridine ring ), 8.15 (d, 1H, C3-CONH and C5-CONH ), 7.42-7.18 (m, 5H, Ph-ring), 5.17 (s, 2H, C4-H), 2.31 (s, 6H, C2-CH3 and C6-CH3), 2.08 (d, 1H, -NHCS). ¹³C NMR (CDCl3): δ 143.2, 123.7, 126.7 (4C in furyl ring), 102.9 (3,5 C in pyridine ring), 164.9 (3,5 C=O), 181.9 (3,5 C=S), 149.9 (2,6 C in pyridine ring), 44.5 (4C in pyridine ring), 19.7 (2,6- CH3 in pyridine ring). MS ( m/z, relative abundance %): 465.52 (M++1, 12.78), 346.34, 286.20, 258.23, 230.21, 202.20, 81.11. Elemental analysis calculated for C17H20N8O4S2: C43.96, H4.34, N24.12, S13.81. Found: C43.91, H4.38, N24.17, S15.87.
2 , 2 '-{ [4-(4-methoxyhenyl) - 2 , 6 - d imethyl- 1,4-dihydropyridine-3,5-diyl]dicarbonyl} dihydrazinecarbothioamide(2f)
Yield (66%), mp: 210, IR (KBr, cm-1) ν: 3323 (NH), 3251 (NH-C=O), 3231 (NH2), 3034 (Ar-H), 1717 (C=O), 1251 (C=S), 1091 (N-C-N), 801 (Ar-H). 1H NMR(DMSO-d6): δ 9.82 (s, 2H, NH2), 8.57 (bs, 1H, NH of pyridine ring ), 8.05 (d, 1H, C3-CONH and C5-CONH ), 7.33-7.27 (m, 5H, Ph-ring), 5.21 (s, 2H, C4-H), 3.81 (s, 3H, -OCH3), 2.10 (d, 1H, -NHCS), 2.25 (s, 6H, C2-CH3 and C6-CH3). 13C NMR (CDCl3): δ 111.8, 108.3,143.2, 152.8 (4C in furyl ring), 105.3 (3,5 C in pyridine ring), 166.2 (3,5 C=O), 181.7 (3,5 C=S), 147.7 (2,6-C in pyridine ring), 44.7 (4C in pyridine ring), 18.8 ( 2,6-CH3 in pyridine ring), 55.9 (-OCH3). MS (m/z, relative abundance, %): 450.21 ( M++1, 29.12), 331.36, 271.31, 243.25, 215.29, 185.26, 157.21. Elemental analysis calculated for C18H23N7O3S2: C 48.09, H 5.16, N 21.81, S 14.27. Found: C 48.08, H 5.19, N 21.83, S,14.25.
2,2'-{ [4-(4-dimethylnitrophenyl)-2,6-dimethyl- 1,4-dihydropyridine-3,5-diyl]dicarbonyl} dihydrazinecarbothioamide(2g)
Yield (61%), mp: 205, IR (KBr, cm-1) ν: 3321 (NH), 3211 (NH2), 3118 (NH-C=O), 3021 (Ar-H), 1712 (C=O), 1248 (C=S), 1091 (N-C-N), 808 (Ar-H). 1H NMR (DMSO-d6): δ 9.66 (s, 2H, NH2), 8.52 (s, 1H, NH of pyridine ring), 8.03 (d, 1H, C3-CONH and C5-CONH), 6.62-7.07 (m, 4H, Ph-ring), 5.13 (s, 2H,C4-H), 2.07 (d, 1H, -NHCS), 3.06 (s, 1H, -N(CH3)2) 2.19 (s, 6H, C2-CH3 and C6-CH3). 13C NMR (CDCl3): δ 112.8, 134.8, 128.3, 148.2, 152.8 (4C in furyl ring), 106.3 (3,5 C in pyridine ring), 165.2 (3,5 C=O), 181.1 (3,5 C=S), 147.9 (2,6-C in pyridine ring), 39.3 (4C in pyridine ring), 40.8 (N(CH3)2, 46.5 (4C in pyridine ring), 18.2 (2,6-CH3 in pyridine ring). MS (m/z, relative abundance, %) : 463.22 (M++1, 16.24), 432.56, 344.41, 284.35, 256.29, 213.23, 199.24, 185.26. Elemental analysis calculated for C19H26N8O2S2: C 49.33, H 5.67, N 24.22, S 13.86. Found: C 49.34, H 5.69, N 24.24, S 13.84.
Anticonvulsant activity
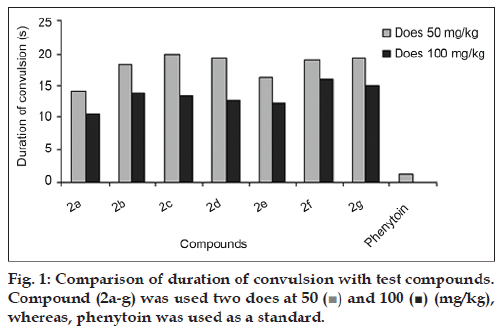
Anticonvulsant activity method described in the anticonvulsant drug development (ADD) program protocol [21,22]. Compounds (2a-g) were screened for their anticonvulsant activity against the pentyleneteterzole induced convulsions. The Swiss albino-rats are weighing 150 g divided into 9 groups containing 5 animals in each group, the test compounds are dissolved in DMSO and doses at (50 and 100 mg/kg). Normal saline solution was intraperitoneally administered, followed 15 min later by an intravenous 48.7 mg dose of pentamethylenetrazole dissolved in physiological saline. Convulsions reports are presented in [Table 1].
| C. No | Dose (mg/Kg) | Duration of convulsion (s) | Percentage of activity (%) |
|---|---|---|---|
| Normal saline | - | 63.6 | 0 |
| 2a | 50 | 14.2* | 78 |
| 100 | 10.4* | 84 | |
| 2b | 50 | 18.2* | 71 |
| 100 | 14.0* | 77 | |
| 2c | 50 | 20.0* | 68 |
| 100 | 13.4* | 78 | |
| 2d | 50 | 19.2* | 69 |
| 100 | 12.8* | 79 | |
| 2e | 50 | 16.0* | 74 |
| 100 | 12.5* | 80 | |
| 2f | 50 | 19.0* | 70 |
| 100 | 16.0* | 74 | |
| 2g | 50 | 19.2* | 69 |
| 100 | 15.1* | 76 | |
| Phenytoin | 50 | 1.7* | 98 |
Table 1: Effect of compounds (2a-g) on the Duration of convulsions
Assay group
A solution of the compound being tested in physiological saline was intrapertioneally administered after 15 min a time that was considered sufficient for complete absorption, that same dose of pentamethyleneteterzole was administered.
Reference group
Phenytoin (50 mg/kg) was dissolved in physiological saline. After 15 min the same dose of pentamethylenetetrazole was applied. The test was evaluated by maximal electrode induced convulsion method. The maximal electroshock seizure (MES) convulsions electroshock is applied through the corneal electrodes.
Results and Discussion
A series diethyl 2,6-dimethyl-4-substituted phenyl- 1,4-dihydropyridine-3,5-dicarboxylate derivatives (1a-g) were prepared as base by following the method previously described literature [23]. 2,6-dimethyl-4-substitutedphenyl-1,4-dihydropyridine-3,5- dicarboxylate (1a-g) reacted with thiosemicarbazide to give 2,2'-{ [4-(4-substituted aromatic alcohols)- 2,6-dimethyl-1,4-dihydropyridine-3,5-diyl]dicarbonyl} dihydrazinecarbothio amide (2a-g) by hydrazinolysis method [24,25] (Scheme 1). The Physical constants and percentage yields of all compounds are summarized in Table 2. The IR spectrum of the compounds (1a-g) showed an absorption band at 3332 to 3354 cm-1 due to the NH stretching, and another absorption band at 1741-1764 cm-1 due to the carbonyl group present in the ester function. The compound 1b showed an absorption band for the Cl-C group at 837 cm-1 and compound 1c showed an absorption band for the OH-C group at 1447 cm-1, the compound 1d showed an absorption bands at 1536 cm-1 corresponding to (NO2–C). The 1H NMR spectrum of compound (1a-g), showed a singlet at δ 8.11 to 8.41, attributable to NH protons present in 1,4-dihydropyridine ring, and another important singlet at δ 4.67 to 4.79 which was attributable to the 4-CH present in the 1,4-dihydropyridine ring. The IR spectrum of compounds (2a-g), showed an absorption band at 3320 to 3372 cm-1 due to NH group present in the 1,4-dihydropyridine ring and, another absorption band at 3118- 3198 cm-1 which is due to the NH-C=O stretch. An absorption band for C=S group was observed at 1245 to 1272 cm-1.
| Compounds | Ar | Yield % | mp(°) |
|---|---|---|---|
| 1a | -Furan | 75 | 158 |
| 1b | -Ph | 66 | 253 |
| 1c | 4-ClC6H4 | 57 | 240 |
| 1d | 4-OHC6H4 | 56 | 246 |
| 1e | 4-NO2C6H4 | 69 | 197 |
| 1f | 4-CH3OC6H4 | 72 | 188 |
| 1g | 4-(CH3)2N-C6H4 | 56 | 227 |
| 2a | -Furan | 70 | 197 |
| 2b | Ph | 53 | 192 |
| 2c | 4-ClC6H4 | 68 | 194 |
| 2d | 4-OHC6H4 | 74 | 201 |
| 2e | 4-NO2C6H4 | 76 | 195 |
| 2f | 4-CH3OC6H4 | 66 | 210 |
| 2g | 4-(CH3)2N-C6H4 | 61 | 205 |
Table 2: Physical constants of synthesized Compounds
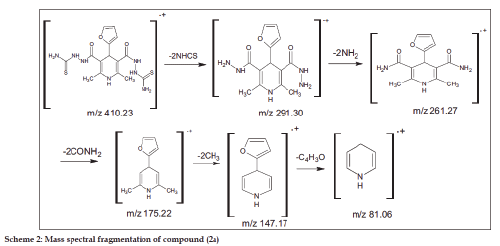
The 1HNMR spectrum of (2a-g) showed a singlet at δ 8.41 - 8.64 attributable to NH protons, present in the 1,4-dihydropyridinering. The NHCS and NH2 groups showed a singlet at δ 2.02–2.12 and 9.14–9.82, respectively. The 13C NMR spectrum of compounds (2a-g) showed peaks at δ 163.1-166.2, corresponding to the 3,5- position of CONH in the pyridine ring, 181.1–184.6 corresponding to the 3,5-position of CS in the pyridine ring, 34.6–46.5 corresponding to 4- position of carbon in the pyridine ring and 18.2- 19.7 corresponding to the 2,6- position of CH3 in the pyridine ring, respectively. The mass spectrum of compound (2a) showed that the molecular ion peak at m/z 410.23 and base peak of the compound m/z 261.25. The mass spectral fragmentation of compound (2a) showed the Scheme 2. Fig. 1 indicates that effect of compounds (2a-g) on the duration of convulsions. Compounds (1a-g) were inactive at the doses tested while compounds (2a-g) have significant activity at 100 mg/kg concentration. The effect of compounds (2a-g) on neuronal excitability as measured by their influence on the percentage of animals affected by convulsions is shown in Table 1. The compound (2a) had highly active compared with other compounds (2b-g) at both doses (50 and 100 mg / kg). Since a dose of 150 mg/kg caused no signs of toxicity during the 24 h following its administration to a group of animals, this can be beneficial for further studies. The compound (2a) has highly active due to the presence of furan ring in 4-position of 1,4-dihydropyridine ring. Pharmacological and further preclinical investigations are currently underway.
Acknowledgments
We wish to thank for state Government of Tamil Nadu, India. They are providing state government fellowship for financial support. We sincerely thank, Principal of Jamal Mohamed College, for providing Laboratory facilities.
References
- Gaudio AC, Korolkovas A, TakahataY. Quantitative structure relationships for 1,4-DHP calcium channel. J Pharm Sci 1994;83:1110-5.
- Schleifer KJ. Stereoselective characterization of the 1,4-dihydropyridine binding site at L-type calcium channels in the resting state and the opened inactivated state. J Med Chem 1999;42:2204-11.
- Visentin S, Amiel P, Frittero R, Boschi D, Roussel C, Giusta L, et al. Synthesis and Voltage-Clamp Studies of Methyl 1,4-dihydro-2,6- dimethyl-5-nitro-4-(benzofurazanyl)pyridine-3-carboxylate Racemates and Enantiomers and of their Benzofuroxanyl Analogues. J Med Chem 1999;42:1422-7.
- Jiang JL, Li AH, Jang SY, Chang L, Melman N, Moro S, et al. Chiral Resolution and Stereo specificity of 6-Phenyl-4-phenylethynyl-1,4- dihydropyridines as Selective Adenosine Receptor Antagonists. J Med Chem 1999;42:3055-65.
- Godfraid T, Miller R, Wibo M. Calcium antagonism and calcium entry Blockade. Pharmocol Rev 1986;38:321-416.
- Khadilkar B, Borkar S. Silica gel supported ferric nitrate a convenient oxidizing reagent. Synth Commun 1998;28:207-12.
- Schnell B, Krenn W, Faber K, Kappe CO. Synthesis and reactions of Biginelli compounds part 23. Chemoenzymatic synthesis of enantionmericallypur 4-aryl, 3,4-dihydropyrimdin-2(1H)-ones. J Chem Soc Perkin Trans1 2000;24:4382-9.
- Sabitha G, Reddy GS, Reddy CS. A novel TMSI-mediated synthesis of Hantzsch 1,4-dihydropyridines at ambient temperature. Tetrahedron Lett 2003;44:4129-31.
- Stout DM, Meyers AI. Recent Advances in the Chemistry of Dihydropyridines. Chem Rev 1982;82:223-43.
- Ji SJ, Jiang ZQ, Lu J, Loh TP. Facile ionic liquids-promoted onepot synthesis of polyhydroquinoline derivatives under solvent free conditions. Synlett 2004;5:831-35.
- Suarez M, Ochoa E, Verdecia Y. A joint experimental and theoretical structural study of novel substituted 2,5-dioxo-1,2,3,4,5,6,7,8- octahydroquinolines. Tetrahedron 1999;55:875-84.
- Tu S, Wei Q, Ma H. The synthesis of novel substituted 2,5-dioxo- 1,2,3,4,5,6,7,8-octahydroquinolines without solvent under microwave irradiation. Synth Commun 2001;31:2657-61.
- Pattan SR, Rasal VB, Venkatramana N, Khade AB, Butle SR, Jadhav SG, et al. Synthesis and evaluation of some 1,4-dihydropyridine and their derivatives as antihypertensive agents. Indian J Chem 2007;46B:698-701.
- Suresh T, Swamy SK, Reddy VM. Synthesis and bronchodilatory activity of new 4-aryl-3,5-bis(2-chlorophenyl)-carbamoyl-2,6-dimethyl- 1,4-dihydropyridnes and their 1-substituted analogues. Indian J chem 2007;46B:115-21.
- Bhavik D, Sureja D, Naliapara Y, Shah A, Saxena AK. Synthesis and QSAR Studies of 4-Substituted phenyl-2,6-dimethyl-3,5-bis- N-(substitutedphenyl) carbamoyl-1,4-dihydropyridines as potential antituercular agents. Bioorg Med Chem 2001;9:1993-8.
- Amini M, Navidpour L, Shafiee A. Synthesis and antitubercular Activity of new N,N-diaryl-4-(4,5-dichloroimidazole-2-yl)-1,4-dihydro2,6-dimethyl-3,5-pyridine dicarboxamides. Daru 2008;16:9-12.
- Nandi AK, Chaudhri S, Mazumdah SK, Ghosh S. Effect of chlorine substitution on the structure and activity of 4-phenylthiosemicarbazide: Crystal and molecular structure of 4-(4-chlorophenyl)thiosemicarbazide J Chem Soc Perkin Trans 2 1984;11:1729-33.
- Ali MA, Chowdhary MA, Naziruddin M. Four- and five-coordinate copper(II) complexes containing mixed ligands. Polyhedron 1984;3:595-8.
- Scovill JP, Klayman DL, Franchino CF. 2-Acetylpyridine thiosemicarbazones. 4. Complexes with transition metals as antimalarial and antileukemic agents. J Med Chem 1982;25:1261-4.
- Bindu P, Kurup MR, Satyakeerty TR. Epr cyclic voltammetric and biological activities of copper(II) complexes of salicylaldehyde N(4)- substituted thiosemicarbazone and heterocyclic bases. Polyhedron 1999;18:321-31.
- Krall RJ, Penry JK, White BG, Kupferberg HJ, Swinyard EA. Antiepileptic drug development II anticonvulsant drug screening. Epilepsia 1978;19:409-28.
- Poter RJ, Cereghino JJ, Gladding GD, Hessie BJ, Kupferberg HJ, Scoville B, et al. Antiepileptic Drug Development Program. Cleve Clin Q 1984;51:293-9.
- Srivastava SK, Srivastava S, Srivastava SD. Synthesis of new 1,2,4-triazolo-thiadiazoles and 2-oxoazetidines as antimicrobial, anticonvulsant and antiinflammatory agents. Indian J Chem 2002;41B:2357-63.
- Ojha S, Ameta U, Dhakar N, Talesara GL. Synthesis and characterization of some alkoxyphthalimide derivatives of benzotriazolylthiadiazoles and benzotriazolylthiazolidinones. Indian J Chem 2007;46B:860-5.
- Hadizadeh F, Shaficee A, Kazemi R, Mohammadi M. Synthesis of 4-(1-Phenylmethyl-5-imidazolyl)-1,4-dihydropyridines as calcium channel Antagonists. Indian J Chem 2002;41B:2679-82.