- *Corresponding Author:
- D. X. Zhao
School of Chemistry and Chemical Engineering, Henan University of Technology, Zhengzhou-450001, China
E-mail: zhaodx798@163.com
| Date of Submission | 15 June 2016 |
| Date of Revision | 10 November 2016 |
| Date of Acceptance | 21 January 2017 |
| Indian J Pharm Sci 2017; 79(1):154-158 |
This is an open access article distributed under terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
Nicotinic acid and niacinamide are used in a variety of therapeutic application as important components of the co-factors, nicotinamide adenine dinucleotide and nicotinamide adenine dinucleotide phosphate. Decreasing the adverse effects will improve the stability and absorption of nicotinic acid. Nicotinic acid aspartic acid dipeptide and tetrapeptide were synthesized via Fmoc solid-phase synthesis, purified via reversed-phase high performance liquid chromatography and characterized via proton and 13C nuclear magnetic resonance and electrospray ionization mass spectrometry to change the property of nicotinic acid for promoting application of niacin derivatives. The interactions of nicotinic acid and nicotinoyl aspartic acid derivatives with calf thymus DNA were investigated by ultraviolet-visible absorption and fluorescence spectroscopy. The hypochromicity in ultraviolet spectra of DNA with increasing of nicotinic acid and derivatives concentration was observed. The fluorescence quenching of nicotinic acid and derivatives to ethidium bromide-DNA was mixture mode according to the Stern-Volmer equation. The results indicated that the interaction modes of nicotinic acid and nicotinoyl derivatives with calf thymus DNA were mixed mode of electrostatic repulsion and embed interaction, and the interaction of derivatives with calf thymus DNA was weakened by the carboxyl number of aspartic acids. Bioavailability and adverse event potential of the synthesized nicotinoyl aspartic acid di and tetrapeptides required to be evaluated.
Keywords
Nicotinoyl derivative, aspartic acid, fluorescence, hypochromicity, solid-phase synthesis
Nicotinic acid (NA) and niacinamide are important components of the co-factors, nicotinamide adenine dinucleotide (NAD) and nicotinamide adenine dinucleotide phosphate (NADP), which widely participate in the lipid metabolism, oxidation and anaerobic decomposition of sugars in the body and also are used in a variety of therapeutic application such as treating pellagra, cardiovascular diseases and Alzheimer's disease [1,2]. A myriad of studies examined the efficacy and safety of niacin and niacinamide, but several studies reported adverse effects of niacin and niacinamide, such as diarrhoea, hyperglycaemia and hepatotoxicity [3]. Many recent studies also focused on improving the stability, absorption and potency of drug by peptidyl modification [4]. Many drugs affect the abnormal physiological metabolic process through interaction with DNA in vivo, and peptidyl derivatives of drugs normally have stronger biological activities [4,5]. NA modified by amino acids as nicotinoyl compounds have been found to be more stable with lower side effects, better bioavailability and activity [6]. Aspartic acid, a usual component of proteins, has special functions for carboxyl side chain in medicines, metalloproteins, and specific enzymes by forming common coordinating ligand or providing acidic surrounding in vivo [7]. Evaluation of the interaction of nicotinoyl peptide with calf thymus DNA (ctDNA) is likely to be helpful to understand the molecular mechanism of drugs, which could assist in drug design and prove to be of significantly important to the field of medicine, pharmacy and biology [8].
Thus, nicotinoyl aspartic acid dipeptide (NA-ASPASP) and tetrapeptide (NA-ASP-ASP-ASP-ASP) are efficiently synthesized in this study via solidphase peptide synthesis, purified via reversed-phase high performance liquid chromatography (HPLC), and characterized via electrospray ionization mass spectrometry (ESI-MS), proton and 13C nuclear magnetic resonance (NMR). Preliminary studies on the DNA-binding properties of niacin and niacin derivatives using UV/Vis absorption and fluorescence spectroscopy are described.
Fmoc-ASP(OtBu)-Wang resin, Fmoc-ASP(OtBu)- OH, N,N-diisopropylethylamine (DIEA), 1-hydroxybenzotriazole hydrate (HOBT), 2-(1H-Benzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HBTU) and piperidine were purchased from GL Biochem (Shanghai) Ltd. (Shanghai, China). Dimethylformamide (DMF), ninhydrin, ct-DNA, NA and ethidium bromide (EB) were purchased from Tiangen Biochemical Reagent Co. Ltd. (Beiging, China). Piperidine, triisopropylsilane (TIS) and trifluoroacetic acid (TFA) were purchased from Shanghai Jingchun Reagent Co. Ltd. (Shanghai, China).
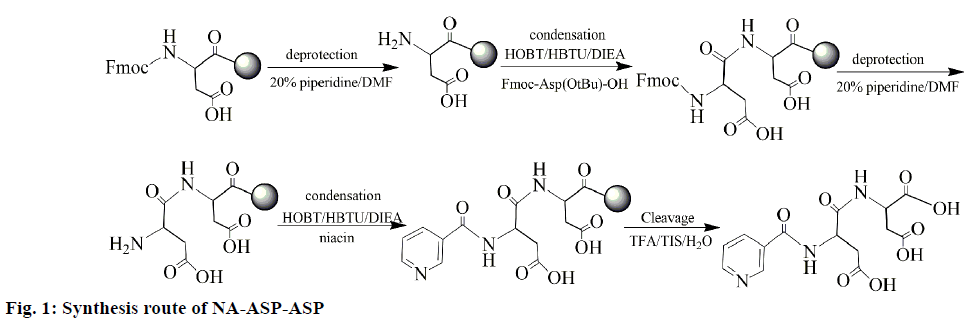
The syntheses of NA aspartic acid derivatives were according to a modification of the literature report [9]. As shown in Figure 1, the NA-ASP-ASP synthesis process is detailed. Fmoc-ASP(OtBu)-Wang resin (512.8 mg, 0.39 mmol/g) was placed in a peptide synthesis vessel and treated with DMF (5 ml, 30 min), 20% piperidine in DMF (5 ml, 3 min), 20% piperidine in DMF (5 ml, 30 min), DMF (5×3 ml, 2 min each), and methanol (5×3 ml, 2 min each). The Kaiser test was performed at this point to identify the presence of free amine available for coupling. In a dried 50 ml round bottom flask, a coupling solution was prepared by adding DIEA (0.135 ml, 0.8169 mmol) to a DMF (5 ml) solution containing Fmoc-ASP(OtBu)-OH (329.2 mg, 0.8000 mmol), HOBT (108.5 g, 0.8000 mmol) and HBTU (303.8 mg, 0.8000 mM) in the ice bath for 2-3 min. The coupling solution was added to the peptide synthesis vessel containing the resin and reacted for 4 h. The resin was then washed with DMF (5×3 ml, 2 min each) and methanol (5×3 ml, 2 min each). The Kaiser test showed the full coupling. A coupling solution was prepared by adding DIEA (0.135 ml, 0.8169 mmol) to a DMF (5 ml) solution containing niacin (98.5 mg, 0.8000 mM), HOBT (108.3 mg, 0.8000 mM) and HBTU (303.4 mg, 0.8000 mM) in an ice bath. The coupling solution was added to the peptide synthesis vessel containing the resin and reacted for 4 h. The coupling solution was added to the peptide synthesis vessel that contains the resin and reacted for 4 h. After the Kaiser Test showed the full coupling, the resin was then washed with DMF (2×10 ml, 2 min each), CH2Cl2 (2×5 ml, 2 min each), methanol (2×5 ml, 2 min each), and DMF (2×10 ml, 2 min each). The cleavage of NA-ASP-ASP from the resin was accomplished using a solution of 95% TFA, 2.5% thioanisole and 2.5% water. Reaction time for cleavage was 3 h. The crude product of NA-ASP-ASP is obtained by precipitation in cold diethyl ether. And the coupling of Fmoc-ASP(OtBu)-OH was repeated three times to obtain NA-ASP-ASP-ASP-ASP. The purification by HPLC afforded pure NA peptides.
The NA derivatives were analysed and purified on an Agilent C18 column (150×4.6 mm) with 5 μm silica as a stationary phase. A gradient elution with eluent A (0.5% TFA in water) and eluent B (acetonitrile, 90:10, v/v) was used at a flow rate of 1 ml×min-1. Peaks were detected at λ=246 nm. 1H NMR and 13C NMR experiments were performed using a Bruker DPX-400 MHz instrument with TMS as the internal standard. Mass spectra were analysed on an Agilent Esquire 3000 mass spectrometer fitted with an ion spray source working in positive ion mode with methanol as a solvent. UV/Vis absorption spectra were recorded in a quartz cell on a UV-2450 spectrometer. The concentration of ctDNA solution was calculated based on the standard ε260=6600 l×mol- 1×cm-1. Then ctDNA solution was titrated with NA or NA derivatives solution using 3 ml corresponding Tris-HCl buffer solution (pH=7.4) as reference, and the UV absorption spectra of ctDNA from 200 nm to 300 nm were measured. The volumes of niacin and niacin derivatives solutions (3.75×10-2 M×l-1) were determined by Rt (Rt = c compound/cDNA). Steady-state fluorescence spectra were recorded in a quartz cell (light path=10 mm) on an F 23010 fluorescence spectrometer. Different volumes of NA and NA derivatives solutions added to a series of 10 ml colorimetric tube at fixed concentration of ctDNA (4.1×10-5 M×l-1) and EB (1.23×10-6 M×l-1), were determined by Rt (Rt = c NA/ cDNA), that were diluted to 10 ml with a Tris-HCl buffer solution (pH=7.4). After heating in a water bath for 1 h at a constant temperature (37°), its fluorescence spectra were measured in the range of 550 nm to 700 nm at an excitation wavelength of 260 nm by using a spectrofluorometer (Varian, America). The entrance and exit slits for all fluorescence measurements were both maintained at 5 nm.
Nicotinoyl aspartic acid peptides were synthesized via solid phase peptide synthesis on Wang resin with good yield, NA-ASP-ASP (83%) and NA-ASP-ASP-ASPASP (83%) analysed by the peak area normalization method of RP-HPLC spectra. The final products were characterized by 1H NMR, 13C NMR and ESI-MS. The purified products isolated via RP-HPLC were judged to be higher than 95% in purity from the ESI-MS results. The characterizations of the synthesized compounds are as following. NA-ASP-ASP 1H NMR (400MHz, D2O): 9.048 (s, 1H, -N=CH-), 8.044 (t, J=1.04 Hz, 1H, =N-CH=CH), 8.529 (d, J=1.12 Hz, 1H, -NH), 4.829-4.892 (m, 1H, NH-CH-CONH), 4.637-4.651 (m, 1H, NH-CH-COOH), 2.924 (d, J=1.25 Hz, 2H, -CH2-CO), 2.843 (d, J=2.88 Hz, 2H, -CH2-CO). 13C NMR (400MHz, D2O): 35.05 (-CH2), 35.26 (-CH2), 49.19 (-CH), 50.80 (-CH), 127.43 (=CH), 132.72 (=C), 141.25 (=CH), 143.82 (=N-CH), 144.94 (-N=CH), 164.28 (-NH-CO), 171.52 (-CH-CO), 173.63-174.29 (-CO). Mass spectrometry calcd for C14H15N3O8: [M+H]+=353.3. Found: 353.8. And mass spectrometry calcd for NA-Asp-Asp-Asp-Asp (C22H25N5O14): [M+H]+=584.2. Found: 583.8. The results of 1H NMR, 13C NMR and ESI-Mass are in accord with the theoretical values of NA derivatives. So the syntheses and purities of nicotinoyl compounds are suitable for further investigating their qualities.
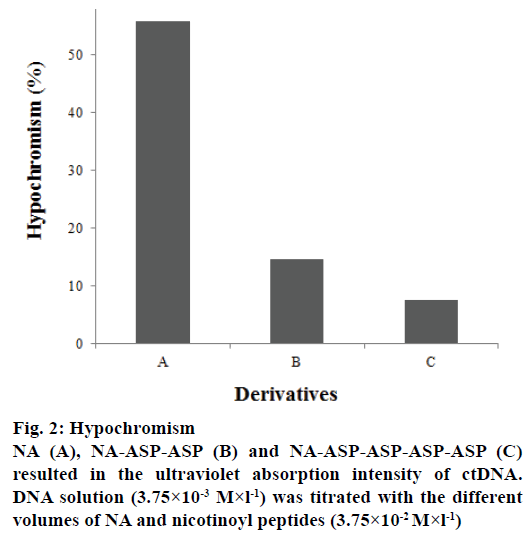
DNA molecular contraction usually induced the hypochromicity in UV absorption intensity of drug- DNA interaction system, and exposed DNA base pair led hyperchromicity[10]. The ctDNA ultraviolet absorption at 260 nm showed a hypochromic effect with the concentration increasing of NA and NA derivatives, and the wavelength of maximum absorption is not changed. The hypochromism of NA and NA derivatives with ctDNA are 55.8% (NA), NAASP- ASP (14.57%), and NA-ASP-ASP-ASP-ASP (7.55%) respectively in Figure 2. With the hypochromism increasing, the derivative-DNA interaction enhanced. Thus, the interaction of nicotinoyl derivatives with ctDNA was weakened because of aspartic acid increasing in number. According to the Eqn., ΔA-1 = (ΔεCD)-1+(ΔεKCD)-1CN-1, where CN is the analysis concentration of NA and NA derivatives, CD is the analysis concentration of ctDNA, Δε is a constant value in a certain measurement conditions, K is the apparent binding constant of ctDNA and NA or NA derivatives [11]. In the experiment, fixed CD and changed CN, then ΔA-1 and CN-1 showed a linear relationship, so the K of compounds with ctDNA obtained from the regression line were 5.81×102 mol-1×l-1 (NA), 1.84×102 mol-1×l-1 (NA-ASP-ASP), and 1.89×102 (NA-ASP-ASP-ASP-ASP), respectively. The binding constants of NA and NA derivatives with ctDNA are below the normal range of 1.1 mol-1×l-1 to 4.8×104 mol-1×l-1 calculated from the intercalation mode of molecular with DNA [12]. Therefore, the interaction of NA and NA derivatives with ctDNA is mainly due to the electrostatic repulsion of molecular with ctDNA phosphate backbone, which caused the conformational contraction of ctDNA and generated a hypochromic effect [10]. The hypochromism of ultraviolet absorption of NA and NA modifier with ctDNA was weakened by carboxyl, which ionized in physiological condition and enhanced the electrostatic repulsion between NA or NA derivatives and the ctDNA phosphate backbone.
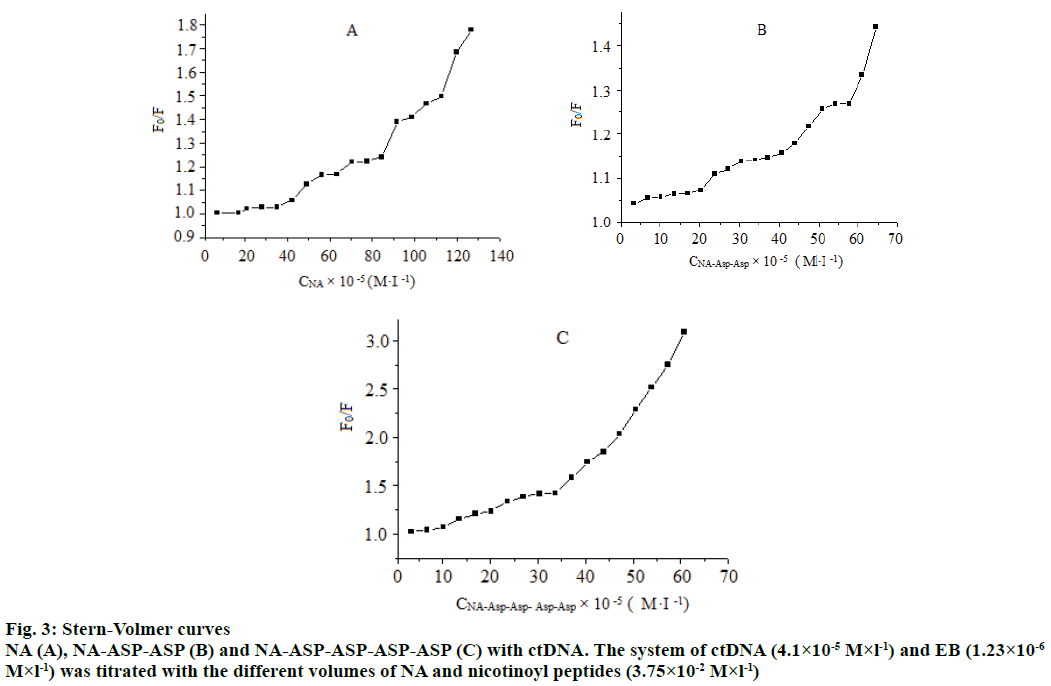
EB was a commonly used fluorescent dye for DNA binding studies because its fluorescence was extremely weak and it could specifically embed in base pairs of DNA double-helical, which caused fluorescence enhancement of the system. When another molecule intercalated with the stacked base pairs of DNA, it would compete with the action site of DNA base pairs with EB and the EB dissociated from the DNA, which finally led to the fluorescence quenching. And the fluorescence quenching extent of EB-DNA could be used to determine the binding extent between the molecule and DNA [13]. So fluorescence spectra studies were performed to investigate the interaction mode of NA or NA derivatives and DNA. The fluorescence quenching is divided into dynamic quenching and static quenching. Static quenching is caused by the non-fluorescent compound produced by the interaction between quenching agent and fluorescent substance in the ground state. Dynamic quenching refers to fluorescent substance molecules in excited state collide with quenching agent molecules, and results in fluorescence intensity quenching [14]. The experiments showed that the fluorescence of EB-DNA system at 610 nm was quenched by increasing the concentrations of NA and NA derivatives. The Stern-Volmer curves of fluorescence intensity and compound concentrations in Figure 3 are non-linear, indicating a complex quenching behaviour between compounds with ctDNA, and this quenching most likely involves both static and dynamic quenching mechanisms because the electrostatic interaction from the charged side-chains of aspartic acids disturbs the weak intercalating of NA pyridyl into ctDNA [15]. This scenario indicates that NA modified with aspartic acid is difficult to intercalate into the stacked base pairs of DNA, and the electrostatic repulsion between NA aspartic acid derivatives and ctDNA is stronger than that of NA and ctDNA.
NA derivatives modified by aspartic acids can be effectively synthesized using Fmoc solid-phase peptide synthesis, and the interaction of NA modifier with ctDNA is smaller than the interaction of NA with DNA. Whether the smaller interaction of derivatives with ctDNA can lead to the controlled release of NA from derivatives, the higher bioavailability and the lower adverse effect, still are required to investigate through further studies.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21301050, 21572046) and the Scientific and Technological Project in Henan Province (162102210197).
Conflict of interest
The authors declare no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Holzhäuser E, Albrecht C, Zhou Q, Buttler A, Preusch MR, Blessing E, et al. Nicotinic acid has antiatherogenic and antiinflammatory properties on advanced atherosclerotic lesions independent of its lipid-modifying capabilities. J Cardiovasc Pharm 2011;57:447-54.
- Green KN, Steffan JS, Martinez-Coria H, Sun X, Schreiber SS, Thompson LM, et al. Nicotinamide restores cognition in Alzheimer's disease transgenic mice via a mechanism involving sirtuin inhibition and selective reduction of Thr231-phosphotau. JNeurosci2008;28:11500-10.
- Pandit A, Sachdeva T, Bafna P. Drug-induced hepatotoxicity:areview. JApplPharmSci2012;2:233-43.
- Liu WJ, Lu X. Research process and application of amino acid fragments used as carrier in targeting delivery system. Asian J Pharm Sci 2012;7:287-92.
- Ezra A, Hoffman A, Breuer E, Alferiev IS, Mönkkönen J, El Hanany-Rozen N, et al. A peptide prodrug approach for improving bisphosphonate oral absorption. JMedChem2000;43:3641-52.
- Kopelevich VM, Gunar VI. Some approaches to the directed search for new drugs on nicotinic acid. Pharm Chem J 1999;33:177-87.
- Xu Z, Li S, Feng XH, Zhan YJ, Xu H. Function of aspartic acid residues in optimum pH control of L-arabinose isomerase from Lactobacillus fermentum. ApplMicrobiol Biotech 2014; 98:3987-96.
- Ning J, Chen W, Li J, Peng Z, Wang J, Ni Z. Structural and energetic insights into sequence-specific interaction in DNA-drug recognition: development of affinity predictor and analysis of binding selectivity. J MolModeling 2013;19:1573-82.
- Zhao DX, Ma L, Lu K, Wu JZ, He J. Syntheses of valpromide dipeptide derivatives and interactions of derivatives with ctDNA. Res ChemIntermediat 2015;41:8591-601.
- Gao E, Zhu M, Yin H, Liu L, Wu Q, Sun Y. Synthesis, characterization, interaction with DNA and cytotoxicity in vitro of dinuclearPd (II) and Pt (II) complexes dibridged by 2,2'-azanediyldibenzoic acid. J InorgBiochem 2008;102:1958-64.
- Liu ZQ, Jiang M, Li YT, Wu ZY, Yang JX. One-dimensional copper (II) polymer with bridging μ-trans-oxamidate and thiocyanate ligands: synthesis, crystal structure and DNA binding studies. InorgChimActa 2009;362:1253-9.
- Zou XH, Ye BH, Li H, Zhang QL, Chao H, Liu JG, et al. The design of new molecular “light switches” for DNA. J BiolInorgChem 2001;6:143-50.
- Zhou CY, Zhao Y, Wu YB, Yin CX, Pin Y. Synthesis, characterization and studies on DNA-binding of a new Cu(II) complex with N1,N8-bis(l-methyl-4-nitropyrrole-2-carbonyl) triethylenetetramine. J InorgBiochem 2007;101:10-8.
- Geng SG, Cui YR, Liu QF, Cui FL, Zhang GS, Chi Y, et al. Spectroscopic and molecular modeling study on the interaction of ctDNA with 3'-deoxy-3'-azido doxorubicin. J Lumin 2013;141:144-9.
- Geng SG, Wu Q, Shi L, Cui FL. Spectroscopic study one thiosemicarbazone derivative with ctDNA using ethidium bromide as a fluorescence probe. Int J BiolMacromol 2013;60:288-94.