- *Corresponding Author:
- Swati Mayur Keny
Department of Pharmaceutical Technology, PES’s Rajaram and Tarabai Bandekar College of Pharmacy, Ponda, Goa 403401, India
E-mail: swatimayur33@gmail.com
| Date of Received | 24 April 2023 |
| Date of Revision | 13 July 2023 |
| Date of Acceptance | 15 May 2024 |
| Indian J Pharm Sci 2024;86(3):1-9 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Ciprofloxacin hydrochloride being a fluoroquinolone antibacterial drug is very frequently preferred in the treatment of bacterial conjunctivitis by the eye specialists. Addition of loteprednol etabonate only adds up to the anti-inflammatory activity of the developed formulation. The objective of the present work was to develop ocular inserts of ciprofloxacin hydrochloride with loteprednol etabonate and further evaluate its potential as a sustained novel ocular drug delivery system. Ophthalmic solutions, suspensions are known for its poor bioavailability and poor therapeutic responses due to many pre-corneal constraints. The researchers usually get triggered with these constrains and strive to formulate controlled and sustained drug delivery system. Ocular inserts based on solvent cast technique were formulated and characterized for in vitro drug release studies using a flow through apparatus that simulated the eye conditions. Compatibility of ciprofloxacin hydrochloride, loteprednol etabonate, polymer and excipients were checked as preliminary preformulation studies. Different combinations of ciprofloxacin hydrochloride, loteprednol etabonate, carbopol 974, 980, 981, polyethylene glycol 400 and glycerine were formulated by solvent cast method and optimized on was evaluated. Fabricated ocuserts were evaluated for its clarity, smoothness, surface pH, drug content and in vitro drug release study to find the optimized film. Formula ciprofloxacin hydrochloride with loteprednol etabonate and carbopol 980 was found to be the best formulation fulfilling the needs of all organoleptic parameters and also the in vitro release study. Based on in vitro correlation and stability studies, it was concluded that this ocular insert formulation could be a promising controlled release formulation for the researchers.
Keywords
Ciprofloxacin hydrochloride, loteprednol etabonate, carbopol 974, 980, 981, ocular inserts and betacyclodextrin complex
Instilling medicaments in eye is a complex process and has emphasized scientists to work in multidisciplinary areas related to the eye including chemical, biochemical, pharmaceutical, medical, clinical and toxicological sciences. In the recent years, attention has been increased on two main objectives. Aiming at finding an effective and a safe drug molecule for various ocular conditions and diseases that are poorly controlled and aiming at improving the existing ocular dosage forms and to develop newer delivery systems for improving the ocular bioavailability of existing molecules[1,2].
Ocular dosage forms are specialized dosage forms especially designed to be instilled onto the external surface of the eye (topical), administered inside (intraocular) or adjacent (periocular) to the eye or used in conjunction with an ophthalmic device.
In terms of sterility ocular preparations are equal with the parental dosage forms. Not only sterility but also in regards with osmotic pressure (tonicity), preservative quantity, tissue compatibility, pyrogen free intraocular dosage forms, particulate matter and suitable packaging[3]. Numerous physiological anatomical constraints are imposed by human eye and hence only a small fraction of the administered drug, effectively 1 % or even less of the instilled dose is ocularly absorbed. The above factors are the driving force for the clinician to recommend a frequent dosing at an extremely high concentration which may results in several side effects of ophthalmic products. Newer and novel ophthalmic delivery systems are being explored just to overcome the problems of conventional ocular therapy and to overall improve the ocular bioavailability of the drug.
Conventional ocular dosage forms are customarily been restricted to solutions, suspensions and ointments. With the recent advancements in material science, the range of ophthalmic dosage forms has expanded significantly which includes gels, either preformed or spontaneous gels responsive to the ocular environment and ocular inserts both forms reducing dosage frequency[4,5].
The aim of the present work was to formulate ocusert with a definite concentration of ciprofloxacin hydrochloride and loteprednol etabonate for the treatment of ocular conjunctivitis and compared for the sustained release of active based United States patents filed for ocular suspension of ciprofloxacin hydrochloride and loteprednol etabonate and methods developed for its analysis[6,7]. The dosage form was fabricated with the objective of increasing the residence time of the drug, reducing the dosing frequency by combining with carbopol 974, 980, 981, Polyethylene Glycol (PEG) 400, Polyvinyl Alcohol (PVA) and glycerine[8,9].
Materials and Methods
Ciprofloxacin hydrochloride was obtained as a gift sample from Indoco Remedies, Verna Industrial Estate, Verna, Goa and loteprednol etabonate was from Ajanta Pharma Pvt. Ltd., carbopol 974, 980 and 981 were gifted by Lubrizol Pvt. Ltd., Mumbai. PVA, PEG 400, Beta-cyclodextrins (β-cyclodextrins) used were procured from Hi- Media. Analytical grade chemicals were used for the analytical purpose.
Preformulation studies:
Preformulation studies were performed on the procured drug samples and excipients with respect to description, melting point, solubility, Infrared (IR) spectra, Ultraviolet-Visible (UV-Vis) spectroscopic studies and Differential Scanning Calorimetry (DSC)[10].
UV spectroscopy study:
Determination of wavelength of maximum absorption: Pure drug sample of ciprofloxacin hydrochloride and loteprednol etabonate were weighed separately and diluted in distilled water. The prepared solutions were scanned in the wavelength region of 200-400 nm. UV-Vis spectrophotometer (UV-Vis Shimadzu) was used for the scanning purpose.
Determination of linearity and range:
Ciprofloxacin hydrochloride (25 mg) and loteprednol etabonate (25 mg) were weighed separately and transferred in two separate (25 ml) volumetric flask. It was further dissolved and diluted up to mark with methanol and water to get a stock solution having strength of 1 mg/ml and further diluted to get 0.1 mg/ml[11,12].
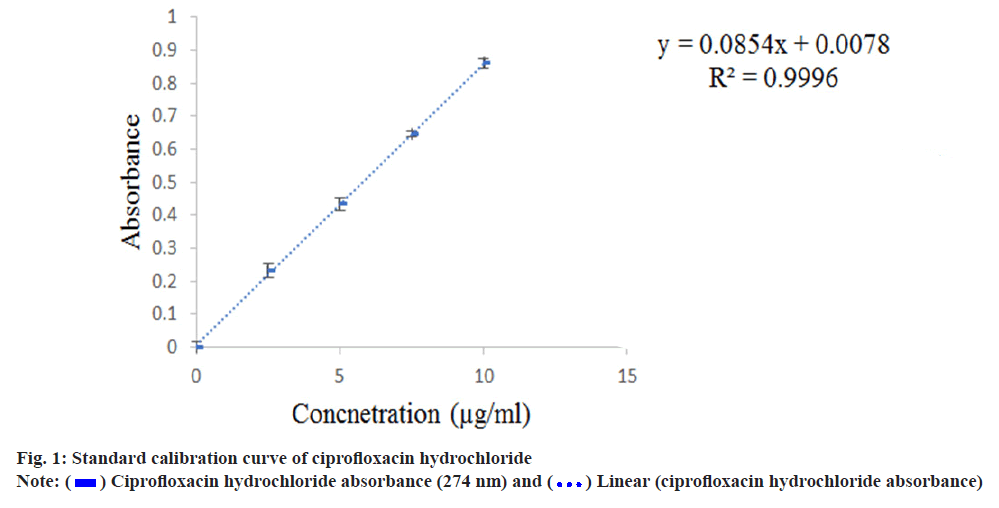
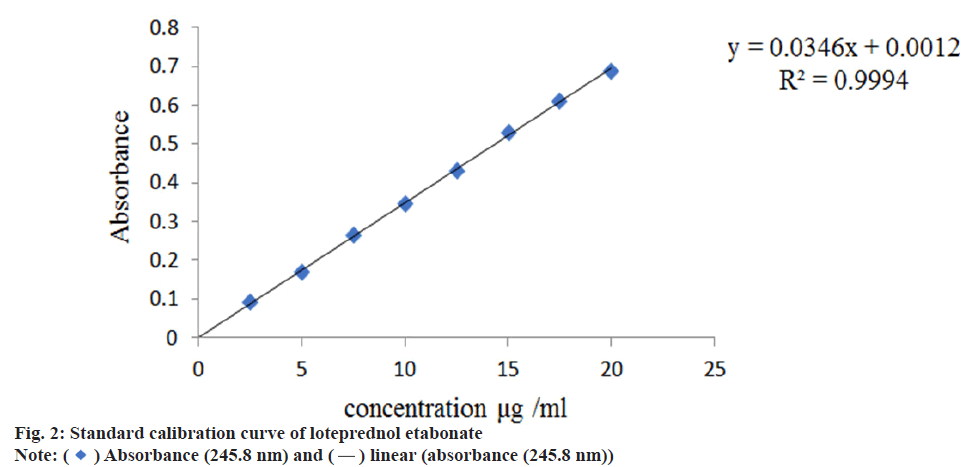
Aliquots of 0.25 ml, 0.5 ml, 0.75 ml, 1.0 ml, 1.25 ml, 1.5 ml, 1.75 ml, 2.0 ml, 2.25 ml and 2.5 ml of working standard solution of individual drugs were transferred to a series of 10 ml standard volumetric flask and diluted with phosphate buffer pH 6.8 to get (2.5 μg/ml) till (15 μg/ml) of ciprofloxacin hydrochloride and (2.5 μg/ml) till (20 μg/ml) loteprednol etabonate respectively. The resulting solutions were estimated in a UVVis spectrophotometer at 274 nm and 245.8 nm respectively for ciprofloxacin hydrochloride and loteprednol etabonate. Beer-Lambert law was verified by plotting a graph of concentration against absorbance. The absorbances measured were tabulated in Table 1 and the standard curves were shown in fig. 1 and fig. 2.
| Concentration | Ciprofloxacin hydrochloride | Loteprednol etabonate |
|---|---|---|
| (µg/ml) | Absorbance at 274 nm | Absorbance at 245.8 nm |
| 0 | 0 | 0 |
| 2.5 | 0.29±0.015 | 0.09±0.015 |
| 5 | 0.54±0.02 | 0.171±0.01 |
| 7.5 | 0.872±0.02 | 0.263±0.015 |
| 10 | 1.169±0.01 | 0.345±0.015 |
| 12.5 | 1.462±0.015 | 0.429±0.01 |
| 15 | 0.29±0.015 | 0.53±0.015 |
| 17.5 | -- | 0.622±0.02 |
| 20 | -- | 0.688±0.015 |
Note: Each value is the mean of three observations. Mean bearing at least one common superscript within a row do not differ significantly (p<0.05)
Table 1: Absorbance of Ciprofloxacin Hydrochloride and Loteprednol Etabonate
DSC:
DSC-60 Shimadzu, TA-60 WS collection software was employed to study the thermal property of drug and excipients alone and in combination. Endothermic and exothermic parameters of the drug and polymer were subsequently obtained.
IR radiation:
Fourier transform infrared spectroscopy spectrums of the obtained sample were compared with the reference standard Fourier transform infrared spectroscopy spectrum of ciprofloxacin hydrochloride and loteprednol etabonate by potassium bromide method.
Preparation of ocusert:
Preparation of β-cyclodextrin and loteprednol etabonate complex: Poorly water-soluble drug like loteprednol etabonate needs to be complexed with β-cyclodextrin to enhance its solubility. Six different molar ratios (1:0.5 to 1:3) were prepared and evaluated. The solubility profile of the drug was checked and the ratio of 1:0.5 to be used (drug: β-cyclodextrin) was finalized based on percentage cumulative drug release[13].
Preparation of ocusert of ciprofloxacin hydrochloride and loteprednol etabonate: Area of the chosen (9 cm) petri dish was calculated. Depending on the area of the petri dish the required drug quantity was calculated. Proportion of 1: 9 (carbopol: PVA cold water soluble) was kept for soaking in 20 ml of distilled water the previous night, followed by incorporation of drug dissolved in 2 ml of phosphate buffer pH 6.8 and PEG 400 and glycerin with stirring on magnetic stirrer for 6 h. At the end of 6 h, the preparation was poured in the mentioned perti dishes and was dried at 50° in a hot air oven for 4 h. 1 cm2×1 cm2 areas of the prepared films were used for the evaluation purpose. CLE 74 represents ciprofloxacin hydrochloride and loteprednol etabonate in carbopol 974, CLE 80 represents ciprofloxacin hydrochloride and loteprednol etabonate in carbopol 980 and CLE 81 represents ciprofloxacin hydrochloride and loteprednol etabonate in carbopol 981[14,15]. Composition of each ocusert is shown in Table 2.
| Ingredients | Quantity | ||
|---|---|---|---|
| CLE 74 | CLE 80 | CLE 81 | |
| Ciprofloxacin | 11.5 mg equivalent to 0.18 mg ciprofloxacin | ||
| Loteprednol etabonate: 1:0.5 β CD | 40 mg equivalent to 0.3 mg loteprednol etabonate | ||
| Carbopol 974 | 60 mg | -- | -- |
| Carbopol 980 | -- | 60 mg | -- |
| Carbopol 981 | -- | -- | 60 mg |
| Poly vinyl alcohol | 540 mg | 540 mg | 540 mg |
| PEG 400 | 0.5 ml | 0.5 ml | 0.5 ml |
| Glycerin | 25 mg | 25 mg | 25 mg |
| Distilled water | 20 ml | 20 ml | 20 ml |
Table 2: Master Formula for Ciprofloxacin Loteprednol Etabonate Ocusert
Surface pH:
Ocular insert should be non-irritating to eye and should be compatible with lachrymal fluid. 0.1 ml double distilled water was taken and the prepared films were allowed to swell in it at room temperature for 30 min. The swollen films were placed under a digital pH meter and the surface pH was determined[16,17].
Drug content:
Fabricated film was cut in dimensions of 1 cm2×1 cm2 and dissolved in 10 ml phosphate buffer pH 6.8. 1 ml was further diluted to 10 ml and analyzed using UV-Vis spectrophotometer at the absorbance value of 274 nm and 245.8 nm respectively[18,19].
In vitro drug release study:
In vitro studies were performed using Franz diffusion. The set up was placed on the magnetic stirrer with a minimum stirring rpm closely relating to eye blinking movement. Room temperature was maintained during the experiment. Semi-permeable membrane (dialysis membrane 50, Hi-Media) was used at the receptor site. 1 ml sample was withdrawn at periodic intervals and subsequently replaced with 1 ml phosphate buffer. Cumulative drug release was calculated from the withdrawn sample[20,21].
Antimicrobial activity:
The cup-plate technique with agar diffusion medium was used. The cup was bored at the center of the plate. The developed film and standard solution of pure drug were taken separately into soyabean casein digest medium earlier seeded with Staphylococcus aureus (S. aureus) organism. On placing the film and standard solution in the plate, they were incubated for a day at 37°. Compared with the standard the Zone of Inhibition (ZOI) was calculated[22].
Sterility testing:
Indian Pharmacopoeia 1996 standard procedure was employed to perform this test. Two media employed were fluid thioglycolate and soyabean casein digest media. The formulated films were cut into two equal halves under laminar air flow and dropped in the two test tubes simultaneously. Both the media were checked for microbial growth by incubating at 37° for 7 d. Positive and negative control samples were used for the comparison studies[23,24].
Antibacterial activity:
Serial dilution method was employed to carry out microbiological assay. S. aureus test organism was employed for the study. Two samples for testing were coded as A (film) and B (pure sample) for Minimum Inhibitory Concentration (MIC). The concentration of pure drug taken was 5 mg/ml. Drug solution of 51 μl contains 256 μg of the drug. Series of 14 test tubes were taken and numbered as 1-14. To the 1st test tube, 2000 μl of broth was added while 1000 μl broth was added to the rest of the test tubes numbered as 2nd till 14th. 51 μl of broth from the 1st test tube were withdrawn and discarded and replaced with drug solution corresponding to 128 μg of drug. 1 ml of the content from 1st test tube was transferred to 2nd and so on. This procedure is repeated till second last test tube corresponding to 128, 64, 32, 16, 8, 4, 2, 1, 0.5 and 0.25 μg/ml. The last test tube serves as negative control. 10 μl of S. aureus broth was added in each tube except negative and kept for incubation at 37° for 24 h. Further MIC was calculated[25].
Results and Discussion
Results of preformulation studies which confirm compatibility between active and excipients are represented in Table 3. IR spectra of pure drugs and excipients were plotted and compared with standard samples and it was confirmed that actives used were compatible with the excipients.
| Observed parameters | Ciprofloxacin hydrochloride | Loteprednol etabonate |
|---|---|---|
| Description | Ciprofloxacin is white in color, powder form | It is white to off white amorphous powder |
| Melting point | 254.5° | 221° |
| Solubility | Sparingly soluble in distilled water, freely soluble in DMSO, DMF, Ethanol and Methanol | Insoluble in water, freely soluble in DMSO, DMF, Methanol and sparingly soluble in Ethanol |
Table 3: Preformulation Study on Drug and Excipients
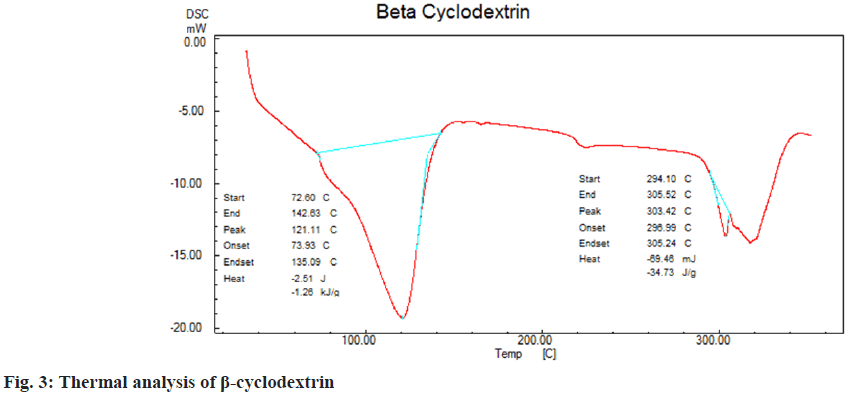
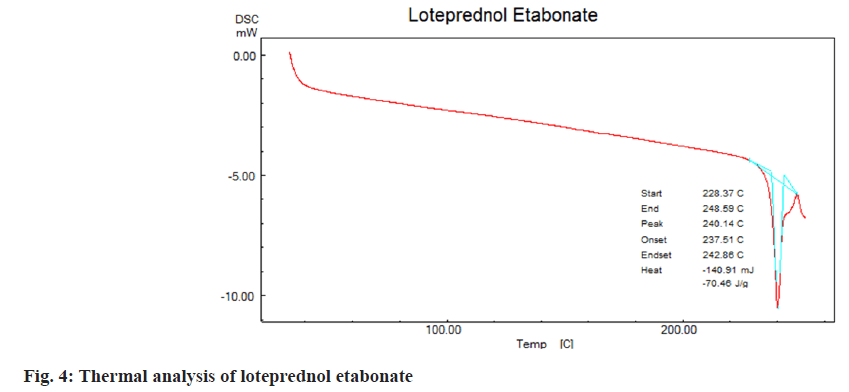
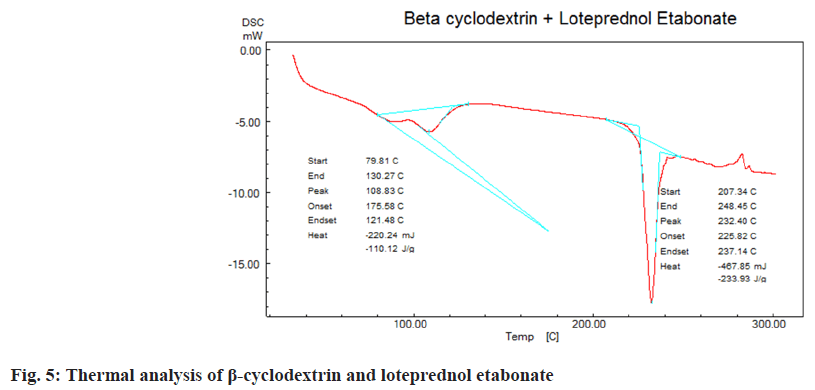
DSC was employed to understand thermal properties of loteprednol etabonate complexed with β-cyclodextrins. Endothermic peaks were observed due to glass transition for loteprednol etabonate at 240°, β-cyclodextrins at 121.1° and complex of loteprednol etabonate with β-cyclodextrins at 232°, 108° respectively (fig. 3-fig. 5) which indicates the drug profile of loteprednol etabonate is intact.
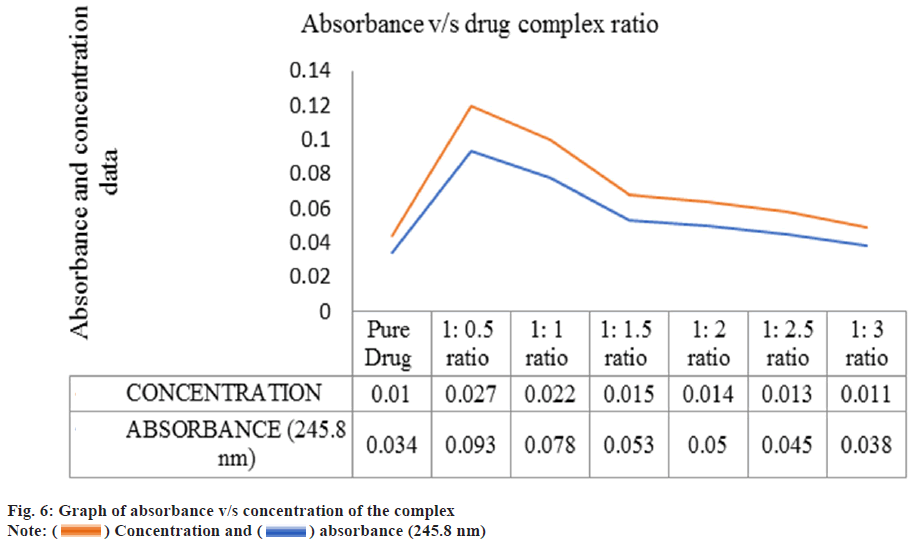
Linear calibration curve was obtained in the concentration range of 3-15 μg/ml at λmax 274 nm for ciprofloxacin hydrochloride and (2-25 μg/ml) at λmax 245.8 nm for loteprednol etabonate. It followed Beer lamberts law with Regression coefficient (R2) value of 0.999 for both ciprofloxacin hydrochloride and loteprednol etabonate. Due to poor water solubility, loteprednol etabonate was complexed with β-cyclodextrins in six different molar ratios ranging from 1:0.5-1:0.3 (drug: β-cyclodextrin) before incorporating in the ocusert. The solubility profile of the drug was checked in terms of high absorbance value and the ratio 1:0.5 (drug: β-cyclodextrin) was finalized based on percentage cumulative drug release as depicted in Table 4 and fig. 6.
| Ratio | Absorbance | Concentration |
|---|---|---|
| Pure drug | 0.034 | 0.01 |
| 1:0.5 ratio | 0.093 | 0.027 |
| 1:1 ratio | 0.078 | 0.022 |
| 1:1.5 ratio | 0.053 | 0.015 |
| 1:2 ratio | 0.05 | 0.014 |
| 1:2.5 ratio | 0.045 | 0.013 |
| 1:3 ratio | 0.038 | 0.011 |
Table 4: Absorbance vs. Concentration Plot for Loteprednol Etabonate Beta Cyclodextrin Complex
UV-Vis simultaneous estimation method was employed for evaluating the drug content from the combined dosage form. Other evaluated parameters of the prepared ocuserts with respect to surface pH, tensile strength, thickness are recorded in tabular column (Table 5).
| Formulation code | CLE 74 | CLE 80 | CLE 81 | |
|---|---|---|---|---|
| Surface texture | Smooth | Smooth | Smooth | |
| Thickness (mm) | 0.112±0.04 | 0.109±0.02 | 0.117±0.01 | |
| Weight (mg) | 198±0.05 | 185±0.03 | 192±0.08 | |
| Tensile strength (g/cm2) | 415±0.05 | 425±0.08 | 430±0.03 | |
| % Drug content (±SD*) | Drug A | 70 | 73.33 | 76.66 |
| Drug B | 83.33 | 77.78 | 66.67 |
Table 5: Evaluated Parameters with Drug Content
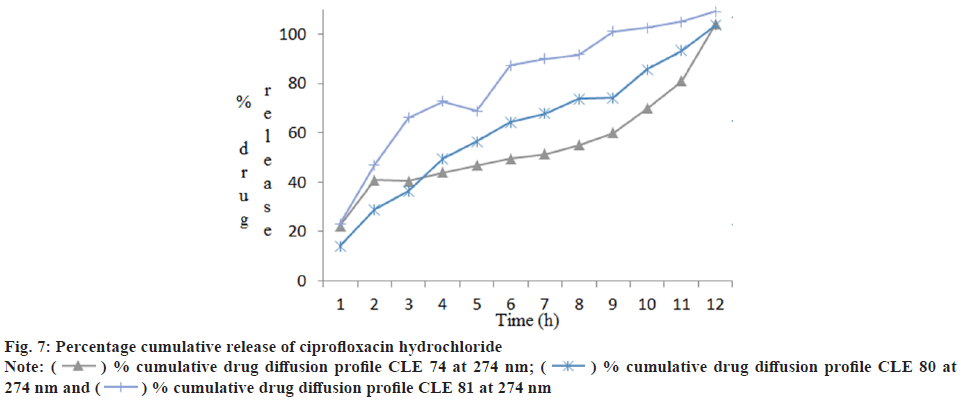
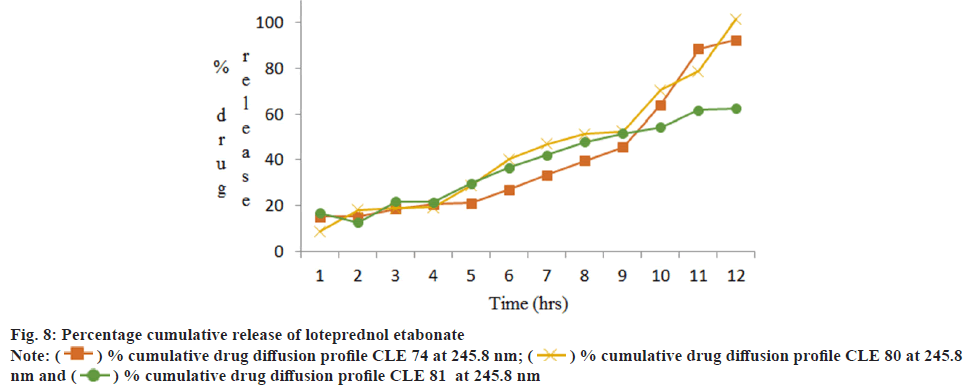
Franz diffusion cell was used to study the cumulative drug release and it was found that formulation CLE 80 gave best results compared to other two formulations. The values are shown in Table 6 and graphical representation in fig. 7 and fig. 8.
| Time (h) | Percentage cumulative drug release | |||||
|---|---|---|---|---|---|---|
| CLE 74 | CLE 80 | CLE 81 | ||||
| 245.8 nm | 274 nm | 245.8 nm | 274 nm | 245.8 nm | 274nm | |
| 1 | 15.16±0.02 | 22.35±0.02 | 8.88±0.02 | 14.16±0.015 | 16.85±0.015 | 23.18±0.01 |
| 2 | 15.19±0.01 | 40.89±0.015 | 18.21±0.015 | 29.02±0.015 | 12.60±0.02 | 47.00±0.01 |
| 3 | 18.54±0.02 | 40.35±0.02 | 18.87±0.02 | 36.24±0.02 | 21.72±0.01 | 66.18±0.01 |
| 4 | 20.59±0.01 | 43.85±0.01 | 19.27±0.01 | 49.56±0.01 | 21.70±0.02 | 72.79±0.02 |
| 5 | 21.14±0.02 | 46.84±0.015 | 28.79±0.02 | 56.47±0.02 | 29.94±0.015 | 68.83±0.01 |
| 6 | 27.05±0.015 | 49.58±0.02 | 40.23±0.01 | 64.22±0.01 | 36.80±0.02 | 87.33±0.01 |
| 7 | 33.58±0.02 | 51.33±0.01 | 46.88±0.015 | 67.75±0.02 | 42.10±0.015 | 90.07±0.01 |
| 8 | 39.65±0.01 | 55.14±0.02 | 51.22±0.01 | 73.84±0.015 | 47.93±0.02 | 91.60±0.02 |
| 9 | 45.60±0.02 | 60.08±0.01 | 52.57±0.015 | 74.14±0.015 | 51.54±0.015 | 101.13±0.015 |
| 10 | 64.18±0.015 | 69.95±0.01 | 70.50±0.015 | 85.64±0.015 | 54.30±0.015 | 102.68±0.015 |
| 11 | 88.62±0.02 | 80.94±0.01 | 78.71±0.02 | 93.40±0.015 | 61.95±0.015 | 104.95±0.02 |
| 12 | 92.52±0.015 | 104.1±0.015 | 101.74±0.015 | 103.66±0.015 | 62.49±0.02 | 109.12±0.015 |
Note: Each value is the mean of three observations. Mean bearing at least one common superscript within a row do not differ significantly (p<0.05)
Table 6: Percentage Cumulative Drug Diffusion Profile of Ciprofloxacin Hydrochloride and Loteprednol Etabonate
ZOI of the formulated films were compared with that of pure drug against a positive and negative control by using ZOI measurement by cup plate method. Readings of this study is tabulated and images on ZOI’s are depicted in Table 7.
| Formula | Zone of inhibition | |
|---|---|---|
| Negative control | -- | -- |
| Positive control | -- | -- |
| Ciprofloxacin | Present | 3.2 cm |
| Loteprednol etabonate | Absent | 0 cm |
| CLE 74 | Present | 3.9 cm |
| CLE 80 | Present | 4.1 cm |
| CLE 81 | Present | 3.6 cm |
Table 7: Zone of Inhibition Values
MIC concentration was found to be 0.5 μg/ml for the film and 4 μg/ml for the pure drug. Turbidity below the mentioned concentration indicates growth of organism. The formulated ocular films prove to be a novel drug delivery system with a promising approach in achieving greater drug absorption in comparison to the conventional ocular drops. The fabricated film CLE 80 proved to be the best amongst the three formulations in terms of drug content, and sustained release of medicament across 12 h obtained through in vitro drug release activity. The optimized film shows anti-microbial activity with high ZOI. No interactions between drug and excipients and also β-cyclodextrins were found when characterized with IR and DSC studies. Hence ocular film with ciprofloxacin hydrochloride and loteprednol etabonate serves as a boost for the researchers and boon to the patients in the future over the conventional ocular dosage forms with 12 h release profile.
Acknowledgements:
We are thankful to Indoco Remedies, Verna, Goa, Ajanta Pharma Pvt. Ltd. Limited and Lubrizol Pvt. Ltd., Mumbai for providing with ciprofloxacin hydrochloride, loteprednol etabonate and carbopol as gift samples for my work. I am also grateful to KLE’s College of Pharmacy for providing laboratory services to carry out DSC studies.
Conflict of interests:
The authors declared no conflict of interests.
References
- Tabbara KF, El-Sheikh HF, MonowarulIslam SM, Hammouda E. Treatment of acute bacterial conjunctivitis with topical lomefloxacin 0.3% compared to topical ofloxacin 0.3%. Eur J Ophthalmol 1999;9(4):269-75.
[Crossref] [Google Scholar] [PubMed]
- Valarmathi S, Shanmugam S, Kumar SS, Shanmugasundaram P. In vivo studies of ophthalmic ocular insert containing aciclovir. Res J Pharm Technol 2017;10(7):2139-42.
- Kaur IP, Garg A, Singla AK, Aggarwal D. Vesicular systems in ocular drug delivery: An overview. Int J Pharm 2004;269(1):1-4.
[Crossref] [Google Scholar] [PubMed]
- HK K. Novel polymeric in situ gels for ophthalmic drug delivery system. Int J Res Pharm Sci 2012;2(1):67-8.
- Nair RV, Nair SC, Anoop KR. Current trends in ocular drug delivery systems and its applications. Res J Pharm Technol 2015;8(5):629-36.
- Dewani A P, Vekariya H J. Optimization of high-performance liquid chromatographic method using central composite design for the simultaneous estimation of ciprofloxacin and loteprednol. J Chromatogr Sci 2023;61(3):279-87.
[Crossref] [Google Scholar] [PubMed]
- Patent Scope. An ophthalmic formulation in suspension of loteprednol etabonate and ciprofloxacin hydrochloride; 2006.
- Nayak S, Jadhav M, Bhaskar V. Recent advances in ocular drug delivery systems. Res J Pharm Technol 2016;9(7):995-1006.
[Crossref] [Google Scholar] [PubMed]
- Suresh PK, Barsa G, Sah AK, Daharwal SJ. Ocular implants as drug delivery device in opthalmic therapeutics: An overview. Res J Pharm Technol 2014;7(6):665-76.
- Jyothirmai KS, Manasa M, Sravani D, Devi GT, Mounika M, Priya NS, et al. Formulation and Evaluation of Ocular in situ hydrogels of Acyclovir. Int J Res Pharm Chem 2017;7:162-70.
- Nayak NS, Bharani SS, Thakur RS. Formulation and evaluation of pH triggered in situ ophthalmic gel of moxifloxacin hydrochloride. Int J Pharm Pharm Sci 2012;4(2):452-9.
- Nithiyananthan TS, Shankarananth V, Rajasekhar KK, Jyothikrishna K, Mukesh O, Vikram KE. Preparation and evaluation of ciprofloxacin ocuserts. J Pharm Res 2009;2(9):1496-9.
- Fialho SL, da Silva‐Cunha A. New vehicle based on a microemulsion for topical ocular administration of dexamethasone. Clin Exp Ophthalmol 2004;32(6):626-32.
[Crossref] [Google Scholar] [PubMed]
- Rajalakshmi R, Padmaja C, Radhika N, Kumuda P, Pavan Kumar P, Ujjwala B, et al. Formulation and assessment of gemifloxacin mesylate ocular in situ gelling system. Int Res J Pharm 2013;4(10):33-8.
- Sultana Y, Aqil M, Ali A. Ocular inserts for controlled delivery of pefloxacin mesylate: Preparation and evaluation. Acta Pharm 2005;55(3):305-14.
[Google Scholar] [PubMed]
- Parmar RB, Tank HM. Design formulation and evaluation of reservoir type controlled released moxifloxacin hydrochloride ocular insert. Asian J Res Pharm Sci 2013;3(1):19-24.
- Patel UL, Chotai NP, Nagda CD, Patel MP, Patel KN. Formulation and in vitro evaluation of moxifloxacin hydrochloride ophthalmic inserts. Int J Pharm Res 2009;1(1):23-30.
- Pawar PK, Katara R, Majumdar DK. Design and evaluation of moxifloxacin hydrochloride ocular insert. Acta Pharm 2012;62(1):93-104.
[Crossref] [Google Scholar] [PubMed]
- Arnab S, Saroj KG. Prolonged delivery of ciprofloxacin hydrochloride from hydrophilic ocular inserts. Acta Pol Pharm 2004; 61(5): 343-349.
- Harpinder KG, Shweta B. A novel in situ gel for sustained ophthalmic delivery of ciprofloxacin hydrochloride and diclofenac sodium: Design and characterization. World J Pharm Pharm Sci 2015;4:1347-56.
- Sharma J, Sharma S, Kaura A, Durani S. Formulation and in vitro evaluation of moxifloxacin ocular inserts. Int J Pharm Pharm Sci 2014;6(10):177-9.
- Mishra DN, Gilhotra RM. Development studies on gel-forming erodible ocular polymeric films of gatifloxacin sesquehydrate. Acta Pharm Sci 2008;50(2):145-52.
- Indian Pharmacopoeial Commission, Gaziabad, Government of India Ministry of Health and Welfare; Indian Pharmacopoeia 2018;1(2):59-66. [Crossref] [Google Scholar] [PubMed]
- Keny SM, Shah K. Formulation and development of extended release ocusert for gemifloxacin mesylate with dexamethsaone. Res J Pharm Technol 2020;13(2):697-702.
- Gadad AP, Wadklar PD, Dandghi P, Patil A. Thermosensitive in situ gel for ocular delivery of lomefloxacin. Indian J Pharm Educ Res 2016;50(2):S96-105.