- *Corresponding Author:
- S. J. Yang
National Traditional Chinese Medicine Clinical Research Base and Drug Research Center, Affiliated Traditional Chinese Medicine Hospital, Southwest Medical University, Luzhou, Sichuan 646000, China
E-mail: ysjimn@sina.com
| This article was originally published in a special issue, “Modern Applications in Biomedical Research and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2022:84(3) Spl Issue “234-246” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
This study aimed to elucidate the potential pharmacological mechanism of Zhilong Huoxue Tongyu capsule on ischemic stroke through network pharmacology and molecular docking technology. Traditional Chinese medicine system pharmacology analysis platform and bioinformatics analysis tool for molecular mechanism of traditional Chinese medicine databases were used to screen the active ingredients and targets of Zhilong Huoxue Tongyu capsule and ischemic stroke-related targets were screened by GeneCards, online human Mendelian inheritance database, PharmGKB and therapeutic target databases. By taking the intersection of drugs and disease targets, the possible targets of Zhilong Huoxue Tongyu capsule in the treatment of ischemic stroke were obtained, and the regulatory network diagram of Zhilong Huoxue Tongyu capsule drugs-active ingredients-targets was drawn and the screened intersection genes were imported into search tool for the retrieval of interacting genes/proteins and Cytoscape databases to draw protein-protein interaction network diagram; gene ontology and Kyoto encyclopedia of genes and genomes enrichment analysis was performed on the intersecting genes to predict related biological functions and signaling pathways. Finally, the key compounds and key genes obtained by screening were used for molecular docking. Quercetin, crocetin, luteolin and ursolic acid have the highest degree value and they are the five most important active ingredients. The top 5 targets were albumin, tumor necrosis factor, AKT serine/threonine kinase 1, insulin and interleukin-1 beta. Zhilong Huoxue Tongyu capsule could treat ischemic stroke through interleukin-17 signaling pathway, tumor necrosis factor signaling pathway, mitogen-activated protein kinase signaling pathway, toll-like receptor pathway, calcitonin receptor-like receptor pathway and other pathways. Our study uses network pharmacology and molecular docking methods to predict the basic pharmacological effects and related mechanisms of Zhilong Huoxue Tongyu capsule in the treatment of ischemic stroke, which provides a certain basis for the next experimental verification research of the research group.
Keywords
Zhilong Huoxue Tongyu capsule, traditional Chinese medicine, network pharmacology, molecular docking, ischemic stroke
Stroke is the leading cause of disability and death worldwide[1]. Among them, ischemic stroke accounts for 80 %-90 % of the total number of patients[2], mostly caused by focal brain occlusion or cerebral artery stenosis. Ischemic stroke has complex pathological features, including calcium overload, blood-brain barrier disruption, neuronal apoptosis, brain inflammation, etc. It often causes a series of syndromes such as local blood circulation obstruction, brain tissue necrosis, brain edema, neurological dysfunction and cognitive dysfunction[3]. Western medicine treatment focuses on improving cerebral circulation, brain protection, anti-cerebral edema, lowering intracranial pressure, arteriovenous thrombolysis and interventional therapy. Clinically, intravenous thrombolysis is often used for acute ischemic stroke, but the increased risk of intracranial hemorrhage and the possibility of reperfusion injury due to activation of the fibrinolytic system limit its clinical application[4]. Therefore, effective new treatments for ischemic stroke should be explored. Traditional Chinese medicine has a protective effect on the central nervous system of acute and subacute ischemic stroke and it can effectively improve the degree of neurological deficit in patients. The combined application of multiple traditional Chinese medicines can also reduce ischemic pathological damage and promote the recovery of symptoms and signs of patients. Traditional Chinese medicine has a unique and diverse method for the treatment of ischemic stroke, which can effectively make up for the disadvantages of Western medicine selection. It has good clinical efficacy and small adverse reactions, and has been widely used in the treatment of ischemic stroke.
Ischemic stroke belongs to the category of “stroke” in traditional Chinese medicine. The pathogenesis is mainly caused by breath deficiency and blood stasis, internal stasis of blood, blocking of collaterals, abnormal breath and blood perfusion, the brain is deprived of nourishment. Governing law is to nourish breath, activate blood and dredge collaterals.
Zhilong Huoxue Tongyu Capsule (ZHTC) is a compound preparation of traditional Chinese medicine. Clinical experiments and in vivo and in vitro experiments have confirmed that it has a good effect on ischemic stroke. However, the pharmacological mechanism is not clear. ZHTC formula reuses Astragalus to replenish the breath in the chest and help breath rise to the brain. Leeches, Dilong, Daxueteng promote blood circulation and remove blood stasis, clear meridians and collaterals accompanied by cinnamon sticks to warm the meridians and regulate breath. The whole party played together the effects of invigorating breath, dispelling wind, promoting blood circulation and dredging collaterals. The previous research of our group has confirmed that ZHTC can effectively reduce nerve damage in ischemic stroke rat models by increasing mitochondrial Adenosine Triphosphate (ATP) content and maintaining membrane potential stability[5]. It can promote post-stroke immunosuppression by reducing the immune inflammatory response in stroke patients[6]. It can improve cerebral edema in patients with cerebral infarction, protect brain tissue, promote the recovery of patients neurological function and the improvement of daily living ability, and improve the prognosis of patients[7]. However, although the therapeutic effects of ZHTC are known, their pharmacological and molecular mechanisms of action have not been fully elucidated.
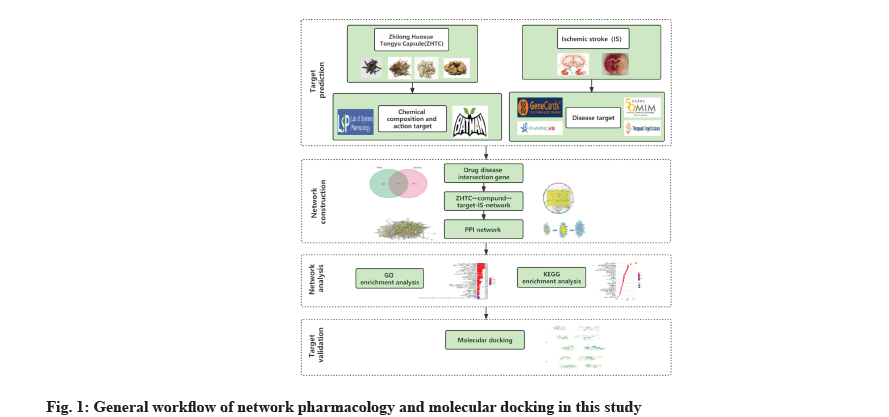
Network pharmacology is a new research model based on high-throughput omics data analysis and computer virtual computing. The mechanism of disease, its research ideas are consistent with the understanding of the “holistic view” of traditional Chinese medicine[8]. Under the guidance of the holistic view and the theory of syndrome differentiation and treatment, traditional Chinese medicine has unique advantages in delaying ischemic stroke. Combining with network pharmacology to conduct data mining of classical prescriptions, researching the complex mechanism of classical prescriptions is the key to the modernization of traditional Chinese medicine. In this study, based on network pharmacology and molecular docking methods, through multi-database mining, a ZHTC-compound-target-ischemic stroke multi-level network was constructed to explore the relationship between ZHTC and ischemic stroke, and to analyze its mechanism of action (fig. 1).
Materials and Methods
Chemical composition, collection and target acquisition of ZHTC:
In order to obtain the chemical constituents and action targets of ZHTC, the Traditional Chinese Medicine System Pharmacology Analysis Platform (TCMSP: https://tcmsp-e.com/tcmsp.php) and Bioinformatics Analysis Tool for Molecular Mechanism of Traditional Chinese Medicine (BATMAN-TCM) database (http://bionet.ncpsb.org/batman-tcm/) are used to screen the active ingredients and their corresponding targets of ZHTC. The screening conditions were set as Oral Bioavailability (OB)≥30 %, Drug-Likeness (DL) index≥0.18 and the potential active ingredients of ZHTC were screened.
Ischemic stroke disease target source:
In Human Gene database (GeneCards, https://genealacart.genecards.org/), Online Human Mendelian Inheritance database (OMIM, https://www.omim.org/), PharmGKB database (https://www.pharmgkb.org/), Therapeutic Target Database (TTD, http://db.idrblab.net/ttd/) to obtain ischemic stroke-related targets. Using “ischemic stroke” as the key word to screen, set the species as human, obtain disease targets, merge and deduplicate, and use them as the final source of disease targets. In this study, the UniProt database was used to unify the names of the obtained genes. In this study, the UniProt database was used to unify the names of the obtained genes. That is to get “Gene Symbol”.
The acquisition of compound-disease intersection genes:
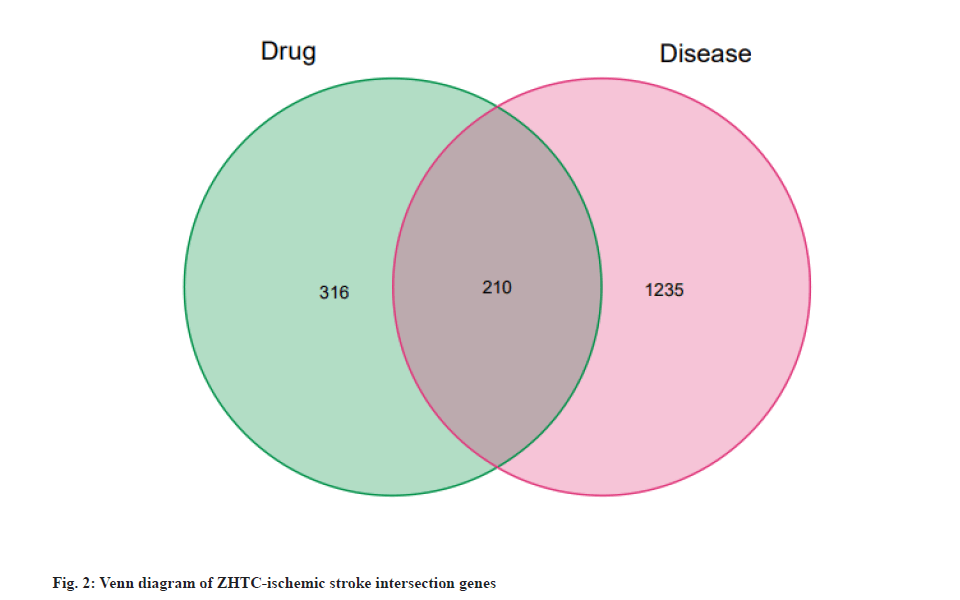
The obtained drug targets and disease targets were subjected to a Wayne analysis to obtain the intersection genes, which are potential targets of ZHTC in the treatment of ischemic stroke, and a Venn diagram was drawn.
Construction of chemical composition-target network diagram:
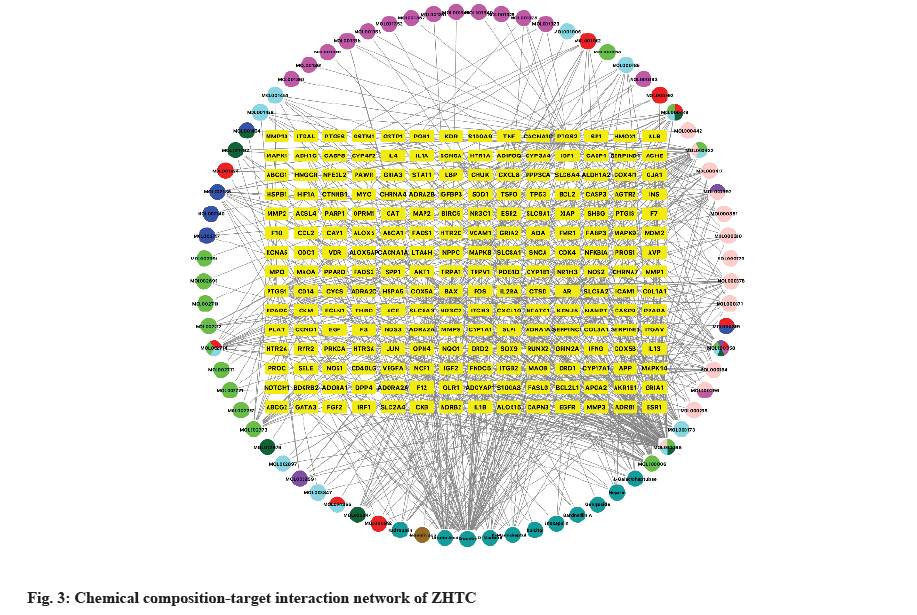
The obtained intersection genes were imported into the network visualization software Cytoscape 3.9.1 and the chemical composition-target interaction network was constructed. The network is analyzed and the degree value parameter is used as the criterion for evaluating the importance of nodes in the network.
Protein-Protein Interaction (PPI) analysis:
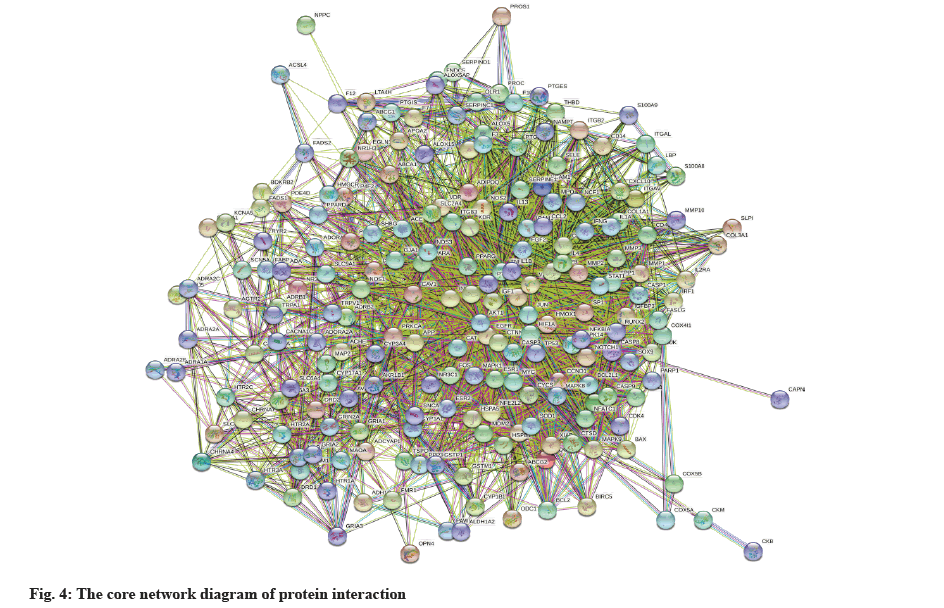
Log into Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) database (https://string-db.org/), select multiple proteins, enter the obtained intersection genes under the search item, limit the species to Homo sapiens and keep the rest of the settings unchanged, construct the PPI functional protein interaction network, and draw the network diagram. To screen core proteins, the generated protein interaction entries Node 1, Node 2 and Combined-score were imported into Cytoscape 3.9.1, the network analyzer plugin was used to analyze the network, and the CytoNCA plugin under the APP entry was selected for network topology analysis. The component-disease-target network map was imported into Cytoscape 3.9.1 for topological analysis. The components are sorted by degree. The higher the degree value, the more important the components are.
Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis:
Through the Database for Annotation, Visualization and Integrated Discovery (DAVID), GO functional annotation and KEGG pathway enrichment analysis were performed on the targets in the molecule-target protein network and visualized. GO analysis includes Cellular Component (CC), Biological Process (BP), Molecular Function (MF). The enrichment conditions were p value cutoff=0.05 and q value cutoff=0.05, and the rest defaulted to the original settings. The KEGG enrichment analysis was used to obtain the signal pathways enriched by ZHTC acting on ischemic stroke and a bar graph or bubble chart was drawn.
Molecular docking:
The molecular docking software Autodock was used to verify the molecular docking between the top 5 components of the predicted target and the top 5 targets, and the three-dimensional structure of the active component was obtained through the PubChem database (https://www.rcsb.org/). Download the receptor protein from the Protein Data Bank (PDB) website (https://pubchem.ncbi.nlm.nih.gov/), pre-process the protein receptor and small molecule with Autodock molecular docking software, and then perform molecular docking to obtain the docking binding energy and docking map.
Results and Discussion
In the TCMSP and BATMAN-TCM databases, leech, Dilong, Panax notoginseng, Chuanxiong, Qishe, Astragalus, peach kernel, safflower, red peony root, Pueraria lobata and Achyranthes sichuanensis were used as keywords for retrieval. A total of 1037 chemical components were obtained in ZHTC, including 13 leeches, 2 earthworms, 1 Qishen, 112 Astragalus, 91 peach kernels, 214 safflower, 144 red peony, 36 Pueraria, 66 Sichuan Achyranthes, 214 Chuanxiong, 144 notoginseng. There are 526 targets with corresponding gene names in the Uniprot database. Taking OB≥30 % and DL≥0.18 as compound screening conditions, 133 potential active ingredients were obtained, including 13 leech, 2 earthworm, 1 Cymbidium, 20 Astragalus, 23 peach kernel, 22 safflower, 29 red peony, 4 Pueraria root, 4 Sichuan Achyranthes, 7 Chuanxiong and 8 Panax notoginseng.
A total of 1445 ischemic stroke-related targets were screened from the GeneCards, OMIM, PharmGKB and TTD databases, and the disease target genes were corrected by the UniProt database as candidate target sources.
The intersection of ZHTC targets and ischemic stroke-related targets genes was drawn to draw a Venn diagram (fig. 2). The intersection part is the possible target of ZHTC in the treatment of ischemic stroke, with a total of 210 targets.
Import the active ingredients of ZHTC, the common targets of drugs and diseases into Cytoscape, and obtain the relationship diagram of “ZHTC drugs-active ingredients-targets” (fig. 3). The network has a total of 210 nodes and 3865 edges. The core targets are shown as yellow rectangles, the active chemical components are shown as circles and different drugs are shown as different colors (dark yellow: Cymbidium, light pink: Astragalus, light purple: Peach kernel, light green; dark red: Red peony; dark purple: Pueraria; sky blue: Achyranthes Sichuan, etc.). The relationship between compounds and targets in the network is shown with each edge. It can be seen from the figure that quercetin also exists in Achyranthes chinensis, safflower, Panax notoginseng, and Astragalus, and beta-sitosterol exists in peach kernel, Achyranthes chinensis, red peony, safflower, notoginseng, and Pueraria. Quercetin, crocetin, luteolin, ursolic acid and kaempferol were associated with more target genes.
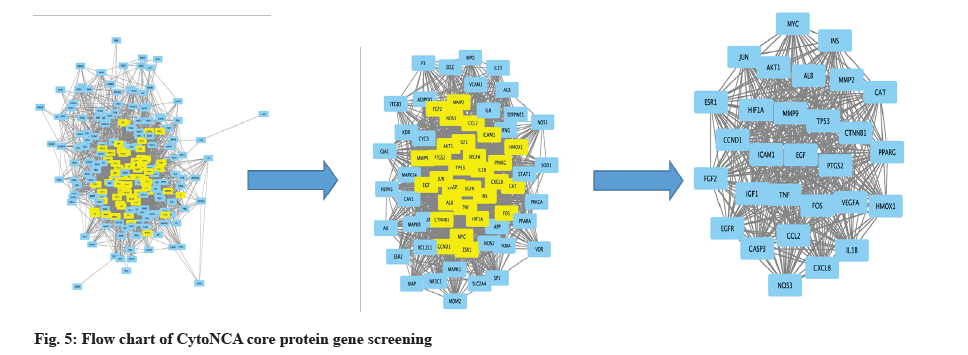
The STRING database was used to initially construct a protein action network for the intersecting genes, as shown in fig. 4. Nodes represent proteins and edges represent interactions between proteins, the higher the degree of network connection, the closer the relationship between proteins. Import the STRING-interactions.txt file obtained from the STRING database into Cytoscape 3.9.1, use the network analyzer plug-in to construct the network, select the CytoNCA plug-in to analyze the network topology and filter the key nodes in the network. Core protein screening parameters are betweenness centrality, closeness centrality, degree centrality, eigenvector centrality, Local Average Connectivity (LAC)-based method. Screening conditions-The online programming package of R software is used to measure the values of between centrality, closeness centrality, degree centrality, eigenvector centrality, and LAC-based method under each intersecting gene, and the values in each group are greater than the median value in this group. The genes with values greater than the median value in each group were retained and the core protein genes were screened. The core proteins are shown in Table 1 (top 20 proteins by degree value). The screening process is shown in fig. 5.
| Serial number | Protein name | Betweenness | Closeness | Degree | Eigenvector | LAC |
|---|---|---|---|---|---|---|
| 1 | ALB | 3430.37 | 0.74377 | 138 | 0.15964 | 42 |
| 2 | TNF | 2374.75 | 0.73852 | 136 | 0.16188 | 43.5 |
| 3 | AKT1 | 2348.89 | 0.72318 | 132 | 0.15982 | 43.7273 |
| 4 | INS | 2696.04 | 0.71821 | 128 | 0.15359 | 42.0938 |
| 5 | IL-1β | 1481.59 | 0.70847 | 124 | 0.15592 | 44.3548 |
| 6 | VEGFA | 627.811 | 0.66349 | 107 | 0.15019 | 48.0187 |
| 7 | TP53 | 524.33 | 0.65313 | 103 | 0.14614 | 47.8641 |
| 8 | CASP3 | 635.183 | 0.65313 | 101 | 0.14516 | 47.9604 |
| 9 | PTGS2 | 882.274 | 0.65109 | 101 | 0.14138 | 45.5644 |
| 10 | MMP9 | 492.149 | 0.6372 | 97 | 0.1403 | 47.0309 |
| 11 | JUN | 487.406 | 0.6411 | 97 | 0.14291 | 48.5361 |
| 12 | PPARG | 769.36 | 0.63914 | 96 | 0.13179 | 41.625 |
| 13 | EGFR | 561.729 | 0.63914 | 94 | 0.13437 | 44.2766 |
| 14 | HIF1A | 341.542 | 0.63142 | 92 | 0.13836 | 48.2391 |
| 15 | CTNNB1 | 785.582 | 0.6372 | 92 | 0.13109 | 43.6304 |
| 16 | CXCL8 | 482.351 | 0.62575 | 91 | 0.13038 | 43.8242 |
| 17 | CCL2 | 397.562 | 0.61652 | 89 | 0.12886 | 44.0449 |
| 18 | EGF | 392.403 | 0.62388 | 89 | 0.13354 | 46.5169 |
| 19 | FOS | 1403.32 | 0.62763 | 89 | 0.12085 | 39.1011 |
| 20 | MYC | 545.415 | 0.62018 | 86 | 0.12585 | 43.6279 |
Table 1: Main Targets of ZHTC in the Treatment of Ischemic Stroke (Top 20).
The component-disease-target network map was imported into Cytoscape 3.9.1 for topological analysis. The ingredients are sorted by degree and the top 15 drug ingredients are screened out. The higher the degree value, the more important the ingredients are (Table 2).
| Name | Average shortest path length | Betweenness centrality | Closeness centrality | Degree |
|---|---|---|---|---|
| Quercetin | 2.29474 | 0.33085 | 0.43578 | 85 |
| Crocetin | 2.46316 | 0.33182 | 0.40598 | 64 |
| Luteolin | 2.72982 | 0.07233 | 0.36632 | 34 |
| Ursolic acid | 2.6386 | 0.16907 | 0.37899 | 33 |
| Kaempferol | 2.6386 | 0.06842 | 0.37899 | 31 |
| Wogonin | 2.83509 | 0.02542 | 0.35272 | 22 |
| Baicalein | 2.87018 | 0.03256 | 0.34841 | 18 |
| Beta-carotene | 2.91228 | 0.02237 | 0.34337 | 18 |
| Beta-sitosterol | 2.91228 | 0.02186 | 0.34337 | 17 |
| Formononetin | 2.80702 | 0.01647 | 0.35625 | 17 |
| Isorhamnetin | 2.75088 | 0.03286 | 0.36352 | 17 |
| 7-O-methylisomucronulatol | 2.8 | 0.02062 | 0.35714 | 17 |
| Stigmasterol | 2.83509 | 0.04015 | 0.35272 | 15 |
| Myricanone | 2.83509 | 0.00909 | 0.35272 | 13 |
| 3,9-di-O-methylnissolin | 2.82807 | 0.01569 | 0.3536 | 11 |
Table 2: Key Ingredient Information Table (Top 15).
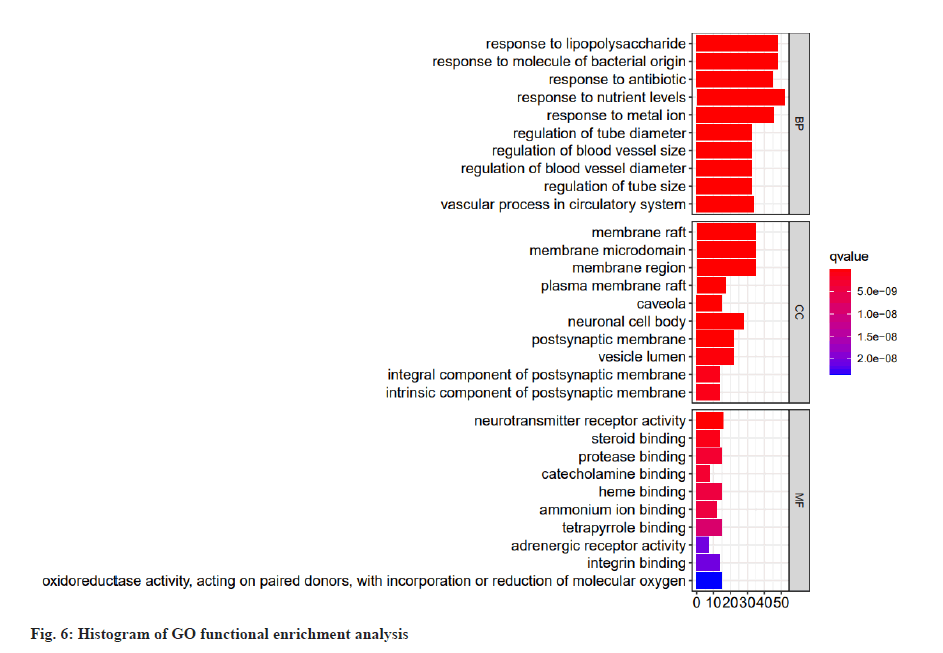
Through analysis, 3391 GO entries were enriched. There are 840 CCs, mainly including membrane rafts, membrane micro domains, membrane domains, plasma membrane rafts, pits, neuronal cell bodies, postsynaptic membranes, vesicle lumen, components of postsynaptic membranes and postsynaptic membranes intrinsic components of synaptic membranes, etc. There are 2293 BPs, mainly including the response to lipopolysaccharide, the response to bacteria derived molecules, the response to antibiotics, the response to nutrient levels, the response to metal ions, the regulation of blood vessel diameter, the regulation of blood vessel size, the regulation of blood vessel diameter and the regulation of vascular processes in the circulatory system, etc. There are 258 MFs, mainly including neurotransmitter receptor activity, steroid binding, protease binding, catecholamine binding, heme binding, ammonium ion binding, tetrapyrrole binding, adrenergic receptor activity, integrin binding, oxidoreductase activity, acts on paired donors, binds or reduces molecular oxygen, steroid hormone receptor activity, G protein-coupled amine receptor activity, organic acid binding, receptor ligand activity, nuclear receptor activity, transcription factor activity, direct ligand regulation sequence-specific Deoxyribonucleic Acid (DNA) binding, fatty acid binding, long-chain fatty acid binding, cytokine activity, antioxidant activity, etc. Select the top 10 enriched items and draw a histogram according to the p value, q value of each item and the number of genes enriched on it, is shown in fig. 6. As shown in fig. 6, the abscissa represents the number of target points, the left side represents BP, CC, MF and the color represents the p value. The smaller the p value, the more red the color is, and the larger the p value, the more blue it is.
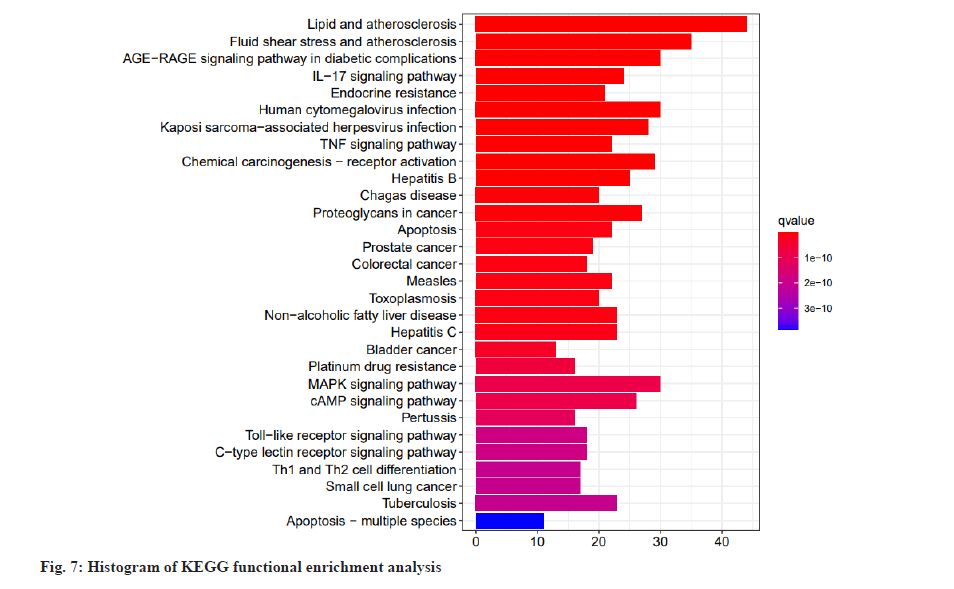
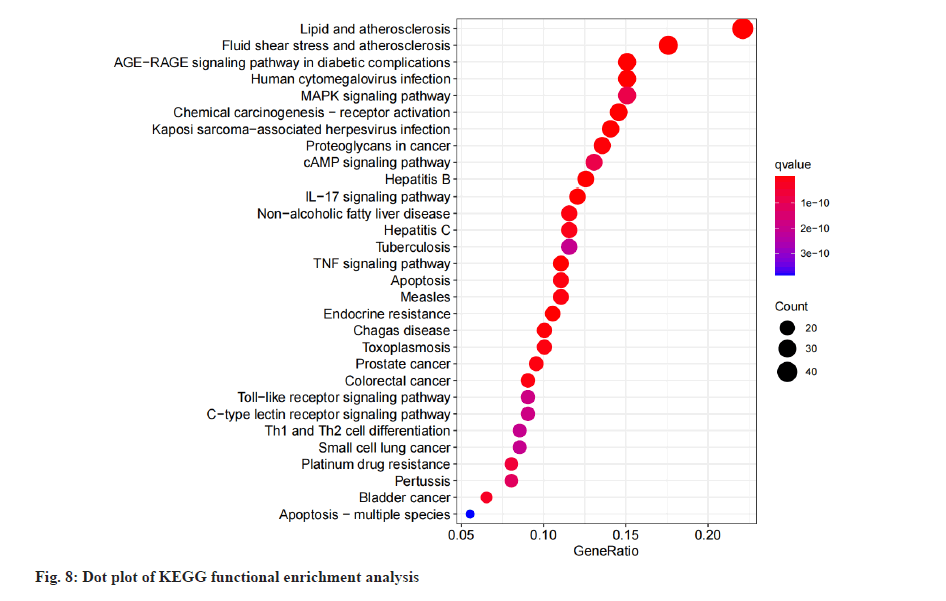
Based on the “Pathview” online editing package of R language, KEGG pathway enrichment analysis was performed on the intersection genes. The analysis results showed that a total of 149 pathways were enriched (p<0.05). The top 20 enriched pathways were selected and a histogram was drawn according to the p value, q value of each pathway and the number of genes enriched on it, is shown in fig. 7. As shown in fig. 7, the abscissa represents the number of enriched genes, the left side represents the pathway name, and the color represents the p value. The smaller the p value, the more red the color is, and the larger the p value, the more blue it is. It mainly involves Interleukin-17 (IL-17) signaling pathway, Tumor Necrosis Factor (TNF) signaling pathway, Mitogen-Activated Protein Kinase (MAPK) signaling pathway, cyclic Adenosine 3,5-Monophosphate (cAMP) signaling pathway, Toll-Like Receptor (TLR) pathway, Calcitonin Receptor-Like Receptor (CLR) pathway and other pathways. Dot plot of KEGG functional enrichment analysis is shown in fig. 8.
In order to further verify the binding ability of active compounds to key targets and improve the accuracy of the target network, molecular docking was performed by AutoDock. The main 5 active ingredients in the obtained ZHTC: Quercetin, crocetin, luteolin, ursolic acid, kaempferol and the top 5 key targets Albumin (ALB), TNF, AKT Serine/Threonine Kinase 1 (AKT1), Insulin (INS) and IL-1 beta (β) were verified by molecular docking. A total of 25 dockings were conducted and the docking success rate was 100 %. The results showed that they all had a good degree of binding. The smaller the binding energy, the better the docking effect, among which luteolin has the best binding degree to the target AKT1B (Table 3 and Table 4).
| Compounds | ALB (5OKL) | TNF (6CGI) | AKT1 (2F7Z) | INS (5W2H) | IL-1β (5G9N) |
|---|---|---|---|---|---|
| Quercetin | -8.7 | -9.4 | -9.7 | -7.9 | -7.3 |
| Crocetin | -8.7 | -7.4 | -8.7 | -7 | -6 |
| Luteolin | -9 | -9.9 | -10.3 | -7.7 | -7.3 |
| Ursolic acid | -8.1 | -7.8 | -9.1 | -9.6 | -8.5 |
| Kaempferol | -8.9 | 8.9 | -9 | -7.4 | -6.2 |
Table 3: Molecular Docking Results.
| Protein | Database ID | Chemical compound | Molecular formula | Structure | 3D molecular docking diagram | Hydrogen bond | Binding energy (kcal/mol) |
|---|---|---|---|---|---|---|---|
| TNF | 6CGI | Quercetin | C15H10O7 | 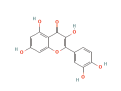 |
 |
PHE-53 HIS-187 ASP-325 ASP-228 TYR-224 ARG-194 | -9.4 |
| TNF | 6CGI | Luteolin | C15H10O6 | 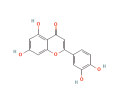 |
 |
TYR-224 ARG-194 GLU-51 PHE-53 ARG-55 | -9.9 |
| AKT1 | 2F7Z | Quercetin | C15H10O7 | 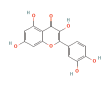 |
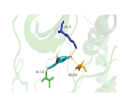 |
LYS-72 VAL-123 ASP-184 | -9.7 |
| AKT1 | 2F7Z | Luteolin | C15H10O6 | 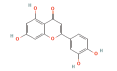 |
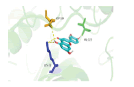 |
ASP-184 LYS-72 VAL-123 | -10.3 |
| INS | 5W2H | Ursolic acid | C30H48O3 | 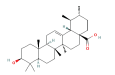 |
 |
THR-134 | -9.6 |
Table 4: Molecular Docking Results of the Active Ingredients of ZHTC and their Targets (Top 5 Binding Energy) (Kcal/mol).
Ischemic stroke is a localized ischemic necrosis of brain tissue or encephalomalacia caused by cerebral blood supply disorder, ischemia and hypoxia. It has a high morbidity rate, a high disability rate and a higher recurrence rate[9]. There are few effective Western medicine treatments for acute ischemic stroke. The advantage of traditional Chinese medicine compounds lies in their overall efficacy. However, the complex chemical components and pharmacological effects of traditional Chinese medicines bring great challenges to the research of traditional Chinese medicine, which to some extent also limits the international promotion and application of Chinese medicine compounds[10]. In recent years, the application of network pharmacology in the field of traditional Chinese medicine has gradually attracted people’s attention, providing a new way of thinking for the research of traditional Chinese medicine. This study systematically explored the mechanism of ZHTC in the treatment of ischemic stroke by means of network pharmacology. The study found that ZHTC has the characteristics of “multi-component, multi-target and multi-channel” in the treatment of ischemic stroke.
In this study, according to the analysis of network topology value, the main chemical components of ZHTC with large degree value are quercetin, crocetin, luteolin, ursolic acid, which may be the main active components of ZHTC to prevent ischemic stroke. The pathogenesis of ischemic stroke mainly includes oxidative stress, inflammation, apoptosis and so on. One of the flavonoids with antioxidant capacity is quercetin, which can promote the behavioral recovery and biomolecular changes of melanocortin 4 receptors in ischemic stroke mice[11]. The pharmacological effects of saffron are attributed to the presence of crocin and saffron aldehyde. Saffron and its components exert their effects as antioxidant, anti-inflammatory and anti-apoptotic agents by inhibiting biochemical, inflammatory and oxidative stress markers, resulting in neuroprotective effects on cerebral ischemic stroke[12]. Luteolin is a natural plant flavonoid compound. It may have a therapeutic effect on stroke through TNF signaling. Rholin has been shown to combat ischemia and reperfusion injury, reducing the size of the infarcted area in experimental models[13]. Ursolic acid regulates TLR signaling and exhibits significant anti-inflammatory properties, which can reduce the production of inflammatory cytokines through the High Mobility Group Box 1 (HMGB1)/TLR4/Nuclear Factor Kappa B (NF-κB) signaling pathway to protect the brain from cerebral ischemia and reperfusion injury[14]. Previous studies have confirmed the reliability of the network pharmacological predictions, showing that the main components of ZHTC are effective in the treatment of ischemic stroke.
Key targets ALB, TNF, AKT1, INS, IL-1β were obtained from the protein interaction PPI network. Specific analysis of key targets shows that plasma ALB is a non-specific transport protein that maintains plasma colloid osmotic pressure in vivo and transports metabolites. When the body is stressed and reacted, the level of ALB decreases significantly. Low ALB level is associated with poor neurological prognosis in acute ischemic stroke. Therefore, serum ALB is known for its neuroprotective effect and is a marker for improving the prognosis of patients with acute ischemic stroke[15]. TNF is an important mediator of inflammatory response, involved in every stage of stroke-related neuronal damage, such as inflammation and prothrombotic events. TNF-alpha (α) has been shown to have an important role in ischemic stroke, with properties including activation of microglia and astrocytes, effects on blood-brain barrier permeability, and effects on glutamatergic transmission and synaptic plasticity[16]. AKT1 is a serine-threonine protein kinase with significantly high expression of phosphorylated AKT during ischemic reperfusion. After being phosphorylated by Phosphatidylinositol 3-Kinase (PI3K), the PI3K/AKT1 signaling pathway is the main way to protect the hippocampus from ischemic injury in stroke rats[17]. INS is the only hormone in the body that can lower blood sugar and is secreted by pancreatic beta cells. Elevated blood glucose after stroke is related to increased levels of INS and other hormones in the blood, suggesting that stroke patients have insulin resistance at the same time, and has an impact on regional Cerebral Blood Flow (rCBF) in overweight healthy men[18]. Inflammation and pyroptosis mediated by the inflammatory factor IL-1β may contribute to acute and chronic neuronal death after stroke[19].
KEGG pathway analysis showed that IL-17 signaling pathway, TNF signaling pathway, MAPK signaling pathway, cAMP signaling pathway, TLR pathway, CLR receptor pathway and other signaling pathways may be the key signaling pathways for ZHTC to treat ischemic stroke. Among these pathways, after stroke, the IL-17 signaling pathway induces the secretion of inflammatory factors (such as TNF-α, IL-6 and Chemokine C-X-C motif Ligand 1 (CXCL1)), recruits neutrophils to infiltrate the central nervous system, impairs the integrity of the blood-brain barrier, promote the development of stroke and it will activate the inflammatory signaling pathways of CCAAT-Enhancer-Binding Proteins (C/EBP), NF-κB and MAPK, thereby promoting the expression of pro-inflammatory cytokines and amplifying the inflammatory response[20]. The MAPK signaling pathway regulates gene expression and fundamental cellular processes such as cell proliferation, differentiation, migration, metabolism and apoptosis in eukaryotic cells, and is considered a therapeutic target for many diseases. So far, more and more evidence shows that the MAPK signaling pathway is involved in the regulation of cytokine expression and apoptosis after stroke, and has become a new therapeutic target. After stroke, all kinds of brain cells are damaged and various inflammatory factors (such as TNF-α, IL-1β) and oxidative stress factors are released, which participate in the damage and repair of the nervous system through the regulation of MAPK signaling pathway, and p38/MAPK/Serine/Threonine-Protein Kinase (SGK1) dependent pathway is involved in the disruption of the blood-brain barrier in permanent ischemic stroke[21]. cAMP-mediated signaling pathways are involved in brain plasticity and promote behavioral recovery induced by forced limb use following stroke in adult rats[22]. Among them, the cAMP/Protein Kinase A (PKA)/cAMP Response Element-Binding Protein (CREB) pathway can mediate motor function recovery and axonal regeneration in mice after stroke[23]. TLRs play a key role in cerebral ischemia and reperfusion injury by inducing the production of inflammatory mediators such as ILs and TNF-α. All major central nervous system cell types express TLR 4. This receptor is critical for myriad immune functions, such as cytokine secretion and the phagocytic activity of microglia. An imbalance in TLR4 activation leads to the progression of neurodegenerative diseases and TLR4-specific antagonists can suppress neuroinflammation by reducing the overproduction of inflammatory mediators[24]. Activation of TNF and TLR triggers necroptosis and is involved in the pathogenesis of ischemic brain injury. Genetic variants of the CLR have been found to be associated with stroke[25]. TNF signaling pathway is the upstream pathway of MAPK, NF-κB, PI3K-AKT and other signaling pathways. In humans, there are two isoforms of TNF: TNF-α and TNF-β. At present, the research on the role of TNF in cerebral infarction at home and abroad mainly focuses on the research on TNF-α and the research on the correlation between TNF-β and central nervous system diseases is relatively small. The levels of TNF-α and TNF-β in serum can reflect the severity of cerebral infarction patients. Microglia-derived TNF-α mediates endothelial cell necrosis and aggravates the destruction of the blood-brain barrier after ischemic stroke[26].
Molecular docking is one of the most important and commonly used methods to compare the biological activities of molecules to enzymes, and the most important parameter of molecular docking is affinity. Molecules with the lowest value of this parameter have the highest biological activity. To further screen the core bioactive components of ZHTC affecting ischemic stroke, we tested the affinity of the top 5 potential bioactive components in ZHTC to the top 5 core target proteins. Based on the relationship between potential components, the core bioactive components and core target proteins that may affect ischemic stroke were further analyzed. The results showed that the five potential bioactive components had good binding activity to the top 5 core target, among which luteolin had the best binding to the target AKT1B.
Taken together, the results indicate that the bioactive components of ZHTC may affect ischemic stroke through important signaling pathways in BPs such as “oxidative stress”, “inflammatory response” and “vascular endothelial function regulation”, such as IL-17 signaling pathway, TNF signaling pathway, MAPK signaling pathway, cAMP signaling pathway, TLR pathway, CLR receptor pathway and other pathways. And core targets such as ALB, TNF, AKT1, INS and IL-1β were determined to be involved in these processes by PPI network analysis. The five potential bioactive components were verified by docking with the top 5 core target by molecular docking software. This study revealed the potential mechanism of ZHTC in the treatment of ischemic stroke to a certain extent, but the information on the network data platform is constantly being updated, so the results of this study cannot fully reflect all the potential targets of ZHTC in the treatment of ischemic stroke. More targets remain to be explored further. At the same time, limited by the research conditions, the conclusions of this study are only obtained from the network pharmacology platform and have not been verified by relevant experiments at the level of cells and animals. Therefore, relevant experiments should be improved and verified in the future.
Author’s contributions:
Raoqiong Wang, Wei Ren, Pan Liang and Mengnan Liu are co-first authors and contributed equally to this work.
Funding:
This study was supported by National Traditional Chinese Medicine Inheritance and Innovation Team (Number: ZYYCXTD-C-202207).
Conflict of interests:
The authors declared no conflict of interest.
References
- Mendelson SJ, Prabhakaran S. Diagnosis and management of transient ischemic attack and acute ischemic stroke: A review. JAMA 2021;325(11):1088-98.
[Crossref] [Google scholar] [PubMed]
- Cui Q, Zhang YL, Ma YH, Yu HY, Zhao XZ, Zhang LH, et al. A network pharmacology approach to investigate the mechanism of Shuxuening injection in the treatment of ischemic stroke. J Ethnopharmacol 2020;257:112891.
[Crossref] [Google scholar] [PubMed]
- Maida CD, Norrito RL, Daidone M, Tuttolomondo A, Pinto A. Neuroinflammatory mechanisms in ischemic stroke: Focus on cardioembolic stroke, background, and therapeutic approaches. Int J Mol Sci 2020;21(18):6454.
[Crossref] [Google scholar] [PubMed]
- Rabinstein AA. Update on treatment of acute ischemic stroke. Continuum 2020;26(2):268-86.
[Crossref] [Google scholar] [PubMed]
- Li YQ, Ren W, Luo G, Liu MN, Mao LS, Han M, et al. Effect of Zhilong Huoxue Tongyu capsule on ischemic stroke model rats and its effect on mitochondria of cerebral cortex neurons. Chin Med Pharmacol Clin 2021;37(5):119-24.
- Jian XL. Effects of Zhilong Huoxue Tongyu capsules on the immune function of patients with acute ischemic stroke (wind-phlegm stasis type). Southwest Medical University; 2020.
- Gao B, Wang BL. Study on the curative effect and neuroprotective effect of Zhilong Huoxue Tongyu capsule combined with Dengzhan Xixin on patients with cerebral infarction. Chin J Chin Med 2019;47(6):86-9.
- Luo TT, Lu Y, Yan SK, Xiao X, Rong XL, Guo J. Network pharmacology in research of Chinese medicine formula: Methodology, application and prospective. Chin J Integr Med 2020;26(1):72-80.
[Crossref] [Google scholar] [PubMed]
- Feske SK. Ischemic stroke. Am J Med 2021;134(12):1457-64.
[Crossref] [Google scholar] [PubMed]
- Zhu JB, Wang YH, Hu ZD, Li J. Research progress on pathogenesis of ischemic stroke and traditional Chinese medicine commonly used for treatment of ischemic stroke. Zhongguo Zhong Yao Za Zhi 2019;44(3):422-32.
[Crossref] [Google scholar] [PubMed]
- Ulya T, Ardianto C, Anggreini P, Budiatin AS, Setyawan D, Khotib J. Quercetin promotes behavioral recovery and biomolecular changes of melanocortin-4 receptor in mice with ischemic stroke. J Basic Clin Physiol Pharmacol 2021;32(4):349-55.
[Crossref] [Google scholar] [PubMed]
- Azami S, Shahriari Z, Asgharzade S, Farkhondeh T, Sadeghi M, Ahmadi F, et al. Therapeutic potential of saffron (Crocus sativus L.) in ischemia stroke. Evid Based Complement Alternat Med 2021;2021:1-8.
[Crossref] [Google scholar] [PubMed]
- Dong R, Huang R, Shi X, Xu Z, Mang J. Exploration of the mechanism of luteolin against ischemic stroke based on network pharmacology, molecular docking and experimental verification. Bioengineered 2021;12(2):12274-93.
[Crossref] [Google scholar] [PubMed]
- Wang Y, Li L, Deng S, Liu F, He Z. Ursolic acid ameliorates inflammation in cerebral ischemia and reperfusion injury possibly via high mobility group box 1/Toll-like receptor 4/NFκB pathway. Front Neurol 2018;9:253.
[Crossref] [Google scholar] [PubMed]
- Wang C, Deng L, Qiu S, Bian H, Wang L, Li Y, et al. Serum albumin is negatively associated with hemorrhagic transformation in acute ischemic stroke patients. Cerebrovasc Dis 2019;47(1-2):88-94.
[Crossref] [Google scholar] [PubMed]
- Tuttolomondo A, Pecoraro R, Pinto A. Studies of selective TNF inhibitors in the treatment of brain injury from stroke and trauma: A review of the evidence to date. Drug Des Devel Ther 2014;8:2221-38.
[Crossref] [Google scholar] [PubMed]
- Tao J, Cui Y, Duan Y, Zhang N, Wang C, Zhang F. Puerarin attenuates locomotor and cognitive deficits as well as hippocampal neuronal injury through the PI3K/Akt1/GSK-3β signaling pathway in an in vivo model of cerebral ischemia. Oncotarget 2017;8(63):106283.
[Crossref] [Google scholar] [PubMed]
- Wingrove JO, O'Daly O, Forbes B, Swedrowska M, Amiel SA, Zelaya FO. Intranasal insulin administration decreases cerebral blood flow in cortico‐limbic regions: A neuropharmacological imaging study in normal and overweight males. Diabetes Obes Metab 2021;23(1):175-85.
[Crossref] [Google scholar] [PubMed]
- Kim H, Seo JS, Lee SY, Ha KT, Choi BT, Shin YI, et al. AIM2 inflammasome contributes to brain injury and chronic post-stroke cognitive impairment in mice. Brain Behav Immun 2020;87:765-76.
[Crossref] [Google scholar] [PubMed]
- Zhang Q, Liao Y, Liu Z, Dai Y, Li Y, Li Y, et al. Interleukin‐17 and ischaemic stroke. Immunology 2021;162(2):179-93.
[Crossref] [Google scholar] [PubMed]
- Zheng Y, Han Z, Zhao H, Luo Y. MAPK: A key player in the development and progression of stroke. CNS Neurol Disord Drug Targets 2020;19(4):248-56.
[Crossref] [Google scholar] [PubMed]
- Qu H, Zhao M, Zhao S, Xiao T, Tang X, Zhao D, et al. Forced limb-use enhances brain plasticity through the cAMP/PKA/CREB signal transduction pathway after stroke in adult rats. Restor Neurol Neurosci 2014;32(5):597-609.
[Crossref] [Google scholar] [PubMed]
- Gao X, Zhang X, Cui L, Chen R, Zhang C, Xue J, et al. Ginsenoside Rb1 promotes motor functional recovery and axonal regeneration in post-stroke mice through cAMP/PKA/CREB signaling pathway. Brain Res Bull 2020;154:51-60.
[Crossref] [Google scholar] [PubMed]
- Leitner GR, Wenzel TJ, Marshall N, Gates EJ, Klegeris A. Targeting toll-like receptor 4 to modulate neuroinflammation in central nervous system disorders. Expert Opin Ther Targets 2019;23(10):865-82.
[Crossref] [Google scholar] [PubMed]
- Koyama T, Kuriyama N, Ozaki E, Matsui D, Watanabe I, Takeshita W, et al. Genetic Variants of RAMP2 and CLR are associated with stroke. J Atheroscler Thromb 2017;24(12):1267-81.
[Crossref] [Google scholar] [PubMed]
- Chen AQ, Fang Z, Chen XL, Yang S, Zhou YF, Mao L, et al. Microglia-derived TNF-α mediates endothelial necroptosis aggravating blood brain-barrier disruption after ischemic stroke. Cell Death Dis 2019;10(7):1-8.
[Crossref] [Google scholar] [PubMed]