- *Corresponding Author:
- Lixia Zhang
Department of Drug Dispensing, The Second Affiliated Hospital of Xingtai Medical College, Xingtai, Hebei Pr ovince 054000, China
E-mail: zjfhao001@163.com
| Date of Received | 10 February 2023 |
| Date of Revision | 18 December 2023 |
| Date of Accepted | 12 August 2024 |
| Indian J Pharm Sci 2024;86(4):1510-1516 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To study the mechanism of long-non-coding RNA metastasis associated lung adenocarcinoma transcript 1 affecting apoptosis and invasion of meningioma cells through phosphoinositide 3-kinase/protein kinase B signal pathway. Meningioma CH157 cell lines were cultured and divided into control (group A), long-non-coding RNA metastasis associated lung adenocarcinoma transcript 1 knockout (group B) and low expression of long non-coding RNA metastasis associated lung adenocarcinoma transcript 1+phosphoinositide 3-kinase activator (740Y-P) (group C). The apoptosis rate of meningioma cells CH157 was detected by flow cytometry and the invasion of meningioma cells were observed by Transwell test. The expression of messenger ribonucleic acid and protein were detected by reverse transcription-quantitative polymerase chain reaction and Western blot method. The apoptosis rate of meningioma cells in group B was increased than group A, while that in group C was lower than group B. The invasion number of meningioma cells in group B was increased than group A, and that in group C was reduced than group B. The expression of B-cell lymphoma 2 was decreased and the B-cell lymphoma 2-associated protein X was increased in group B than group A, and the B-cell lymphoma 2 and B-cell lymphoma 2-associated protein X in group C was raised than group B. The long-non-coding RNA metastasis associated lung adenocarcinoma transcript 1 in group B was reduced than group A. The protein p-phosphoinositide 3-kinase and p-protein kinase B in group B were decreased than group A, while these in group C were raised than group B. Long-non-coding RNA metastasis associated lung adenocarcinoma transcript 1 knockout promotes apoptosis and inhibits cell invasion by activating phosphoinositide 3-kinase/ protein kinase B signaling pathway. The activation of phosphoinositide 3-kinase/protein kinase B signal pathway can partially reverse the inhibitory effect of low expression of long-non-coding RNA metastasis associated lung adenocarcinoma transcript 1 on apoptosis and invasion of meningioma cells.
Keywords
Long-non-coding RNA, metastasis, lung adenocarcinoma, meningioma, phosphoinositide 3-kinase/ protein kinase B, apoptosis
Meningioma originate from arachnoid cap cells, accounting for 20 %-36 % of all primary intracranial tumors, with the highest incidence in old age, and the incidence in women is higher than that in men, while high-grade meningioma are more common in men[1]. According to the pathological characteristics of meningioma, the World Health Organization (WHO) divides meningioma into three grades (WHO I, WHO II and WHO III). Among them, WHO I meningioma is the most common, with growth retardation and low recurrence rate after operation[2]. Compared with WHO I meningioma, WHO II meningioma has higher malignant degree and invasiveness, such as distant metastasis and local recurrence after operation. The remaining 1 % to 3 % are grade WHO III meningioma, which have the highest probability of local invasion, recurrence and distant metastasis[3]. At present, the first choice for the treatment of meningioma is surgical resection, but for meningioma, the effect of surgery, radiotherapy and chemotherapy, and drug treatment is not good, there is an urgent need to explore the pathogenesis of meningioma in order to obtain effective treatment targets. Metastasis Associated Lung Adenocarcinoma Transcript 1 (MALAT1) is a long-non-coding RNA (lncRNA). It is first reported that, it is highly expressed in lung adenocarcinoma and plays an important role in lung adenocarcinoma metastasis[4]. The nuclear speckle structure located in the nucleus can activate abnormal signal pathways in tumor cells by interacting with nuclear speckle related proteins or through competitive inhibition of targeted microRNA (miRNA) expression, it can regulate the malignant biological behavior of tumor cells[5,6]. MALAT1 is not only involved in regulating the processing of precursor messenger Ribonucleic Acid (mRNA), but also considered to be one of the promoters of cancer. However, the mechanism of metastasis in meningioma is not clear. Therefore, this study explored the effect of lncRNA MALAT1 knockout on apoptosis and invasion of meningioma cells, and determined whether it could be achieved through Phosphoinositide 3-Kinase (PI3K)/Protein Kinase B (AKT) signal pathway.
Materials and Methods
Experimental cell:
The meningioma CH157 cell line was purchased from the Institute of Neurology, Tongji Hospital, Huazhong University of Science and Technology, and stored in a liquid nitrogen tank and a refrigerator at -80°. They were divided into three groups; control (group A), si-MALAT1 (group B) and si-MALAT1+740Y-P (group C) group. Group A without any treatment; group B, construct si-MALAT1+GV367 vector and transfect into meningioma CH157 cells and group C, add 740YP (50 g/l) treatment on the basis of si-MALAT1 group.
Experimental instruments and reagents:
Phosphate Buffered Saline (PBS) buffer, Dulbecco's Modified Eagle Medium (DMEM) medium, Bicinchoninic Acid (BCA) profigtein quantitative kit, primary antibody diluent and secondary antibody diluent were all purchased from Solarbio company; RNA extraction kit was purchased from Nanjing Novozan Co. Ltd.; reverse transcription kit was purchased from Axygen company; Radioimmunoprecipitation Assay (RIPA) lysate was purchased from Shanghai Biyuntian Biotechnology Co. Ltd.; Cell Counting Kit-8 (CCK-8) kit and apoptosis detection kit were purchased from Kaiji Biotechnology Company; Transwell chamber enough from Corning Company of the United States of America (USA). High-speed centrifuges and Carbon dioxide (CO2) constant temperature incubators were purchased from Semefeld, while Polymerase Chain Reaction (PCR) amplifiers, spectrophotometers and multifunction enzyme markers were purchased from StepOne company of the USA.
Detection of apoptosis by flow cytometry:
The supernatant of meningioma CH157 cells in logarithmic phase was centrifuged and the supernatant was added successively with binding buffer; 7-ADD prepared according to the proportion of 10:1.5~15 min was cultured at room temperature without light, and apoptosis was detected by flow cytometry.
Detection of invasive ability of meningioma cells by Transwell chamber test:
Three groups of cells were collected and CH157 cells were inoculated in 12-well plate at the density of 1×105 cells/ml. Scratch test was carried out 48 h after transfection. 10 μl gun head was used and washed with precooled PBS for 3 times. Continue to culture for 24 h, and then at 0 h and 24 h, 5 visual fields were randomly selected under fluorescence inverted microscope for recording and statistics.
Detection of lncRNA MALAT1 expression in meningioma cells by RT-PCR:
RNA was extracted by Trizol reagent and complementary Deoxyribonucleic Acid (cDNA) was synthesized by Primescript reverse transcription kit. ABIPrism7500 fluorescent quantitative PCR instrument was used for amplification, and 20 μl reaction system (2 μl cDNA, 10 μl protease, 0.5 μl upstream and downstream products, 7 μl sterilized double distilled water) was added to each well. The experiment was repeated for 3 times, and 3 multiple holes were set up in each group. The relative expression level of lncRNA MALAT1 was calculated by 2-??Ct method, and the primer sequence was shown in Table 1.
| Primer name | Primer sequence?5'-3'? |
|---|---|
| LncRNA MALAT1 | F: GGACAGGTCAGAGGGTTTC |
| R: CTCGTAACTCTTCTCTGTGCC | |
| GAPDH | ACCCAGAAGACTGTGGATGG |
| R: ACACATTGGGGGTAGGAACA |
Table 1: Primer Sequence
Detection of protein expression of p-PI3K and p-AKT by Western blot method:
Three groups of cell samples were collected, the lytic of protease inhibitor was added, and the cell lytic was transferred to the centrifuge tube for centrifugation (4°, 12 000 revolutions per minute (rpm) for 15 min). The supernatant after centrifugation was stored in the refrigerator at -20°. BCA protein quantitative kit was used to determine the protein content and calculate the protein concentration. Prepare 10 % separation gel, perform Sodium Dodecyl Sulphate- Polyacrylamide Gel Electrophoresis (SDS-PAGE) gel electrophoresis, then transfer to Nitrocellulose (NC) membrane, add the sealing liquid of 5 % Bovine Serum Albumin (BSA) at room temperature and seal for 1.5 h.
The first antibody was added and sealed at 4° for 24 h. Wash the NC film with buffer solution for 3 times, add secondary antibody, incubate at room temperature for 1 h, add chromogenic solution and develop.
Statistical analysis:
Statistical Package for the Social Sciences (SPSS) 22.0 software was used for statistical analysis, and the measurement data were expressed by mean±standard deviation (x? ±s). Single factor analysis of variance was used for comparison among groups, and Least Significant Difference (LSD)-test method was used for pairwise comparison. ap<0.05 compared with control group and bp<0.05 compared with si-MALAT1 group.
Results and Discussion
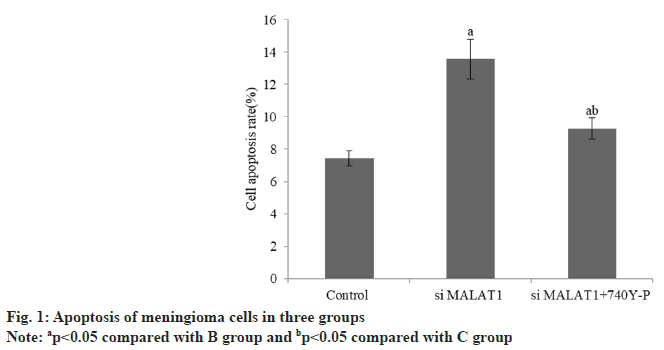
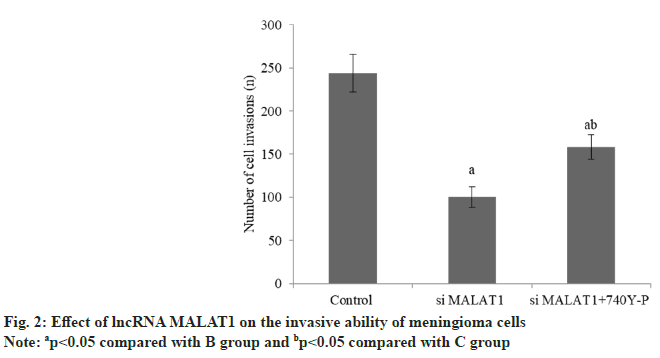
The apoptosis rate of meningioma cells in group B was increased than group A, while that in group C decreased than group B (Table 2 and fig. 1). The invasion number of meningioma cells in group B was reduced than group A, and that group C was raised than group B (Table 3 and fig. 2).
| Group | n | Apoptosis rate (%) |
|---|---|---|
| A | 3 | 7.42±0.48 |
| B | 3 | 13.57±1.22a |
| C | 3 | 9.28±0.65ab |
| F | 41.81 | |
| p | <0.001 |
Note: ap<0.05 compared with B group and bp<0.05 compared with C group
Table 2: Apoptosis of Meningioma Cells in Three Groups
| Group | n | Number of cell invasion (unit) |
|---|---|---|
| A | 3 | 244.17±21.54 |
| B | 3 | 100.34±12.05a |
| C | 3 | 158.46±14.27ab |
| F | 9262.2 | |
| p | <0.001 |
Note: ap<0.05 compared with B group and bp<0.05 compared with C group
Table 3: Effect of lncRNA MALAT1 on the Invasive Ability of Meningioma Cells
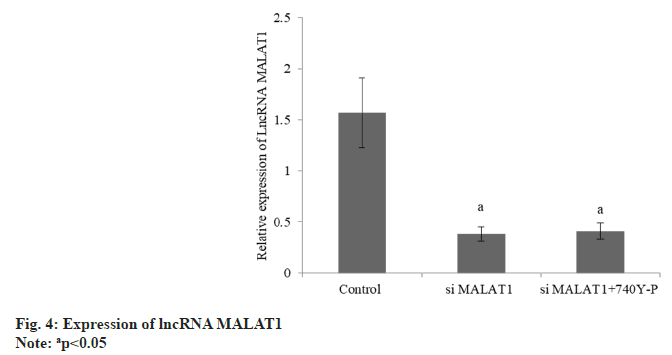
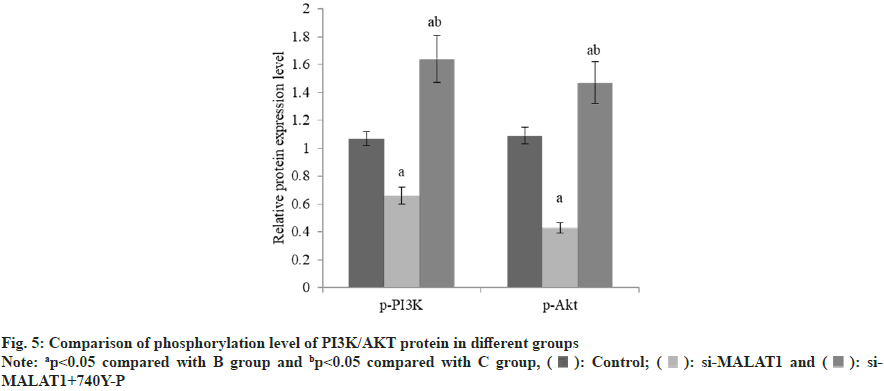
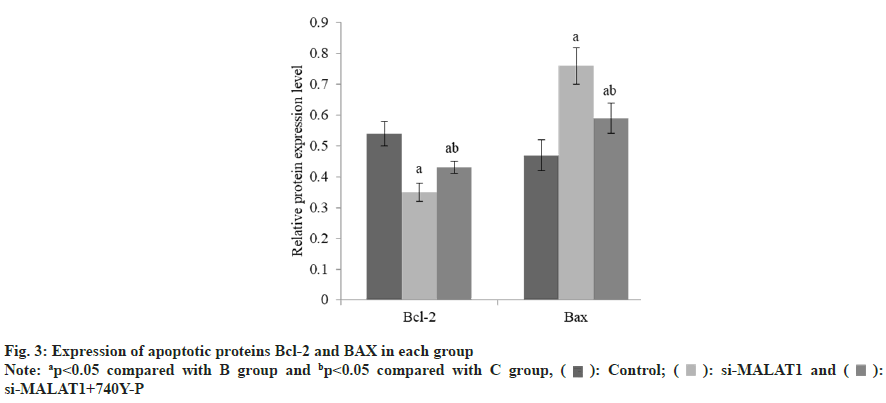
The B-cell lymphoma-2 (Bcl-2) was decreased and the BCL-2-Associated protein X (BAX) was increased in group B than group A, and these in group C was raised than group B (Table 4 and fig. 3). The lncRNA MALAT1 in group B was reduced than group A, but there was no difference between group B and C (Table 5 and fig. 4). The expression of p-PI3K and p-Akt in group B were decreased than group A, while these in group C were raised than group B (Table 6 and fig. 5).
| Group | n | Bcl-2 | BAX |
|---|---|---|---|
| A | 3 | 0.54±0.04 | 0.47±0.03 |
| B | 3 | 0.35±0.05a | 0.76±0.06a |
| C | 3 | 0.43±0.02ab | 0.59±0.08ab |
| F | 76.47 | 38.17 | |
| p | <0.001 | <0.001 |
Note: ap<0.05 compared with B group and bp<0.05 compared with C group
Table 4: Expression of BCL-2 and BAX in Each Group
| Group | n | Expression of mRNA |
|---|---|---|
| A | 3 | 1.57±0.34 |
| B | 3 | 0.38±0.07a |
| C | 3 | 0.41±0.08a |
| F | 32.65 | |
| p | <0.001 |
Note: ap<0.05 compared with B group and bp<0.05 compared with C group
Table 5: Expression of lncRNA MALAT1
| Group | n | p-PI3K | p-Akt |
|---|---|---|---|
| A | 3 | 1.07±0.05 | 1.09±0.06 |
| B | 3 | 0.66±0.06a | 0.43±0.04a |
| C | 3 | 1.64±0.17ab | 1.47±0.15ab |
| F | 543.8 | 78.1 | |
| p | <0.001 | <0.001 |
Note: ap<0.05 compared with B group and bp<0.05 compared with C group
Table 6: Effects of lncRNA MALAT1 on Proteins Related to PI3K/Akt Pathway
Meningioma is one of the most common central nervous system tumors. According to the 2016 WHO classification of central nervous system tumors, meningioma’s are classified into WHO I grade, WHO II grade, WHO III grade and 15 pathological subtypes[7]. The biological characteristics of meningioma with different grades and pathological subtypes were also different. The higher the WHO grade, the higher the invasiveness and recurrence rate. The most common genetic factor for meningioma is Neurofibromatosis type 2 (NF2), which may also be related to exposure to high doses of radiation, external trauma, virus infection, etc.,[8]. Although the existing comprehensive treatment strategies including surgery, radiotherapy, chemotherapy and drug therapy have limited effect on malignant meningioma, exploring its pathological mechanism and developing new treatment methods are of great significance to improve the therapeutic effect of meningioma.
LncRNA MALAT1 is reported to be overexpressed in lung adenocarcinoma, breast cancer, cervical cancer, meningioma and other cancers. Studies have shown that lncRNA MALAT1 can promote the proliferation[9], regeneration, invasion and metastasis of cervical cancer cells, affect vascular remodeling and inhibit apoptosis. It regulates the alternative splicing of mRNA by regulating the activity of serine/arginine splicing factor, which further promotes the process of malignant proliferation of tumors[10]. However, the role of lncRNA MALAT1 in meningioma is not clear.
This study showed that the lncRNA MALAT1 in group B was decreased, while the apoptosis rate was raised than group A. It is suggested that knockout of lncRNA MALAT1 can inhibit the invasion of meningioma cells and promote apoptosis. LncRNA MALAT1 may be an effective target for the treatment of meningioma, but the mechanism is not clear.
Phosphoinositide 3-Kinase/Protein Kinase B (PI3K/AKT) pathway is closely related to the progression of lung adenocarcinoma, breast cancer and cervical cancer. This pathway is overexpressed in many kinds of meningioma and plays critical role in the proliferation, invasion and apoptosis of meningioma[11]. It is activated in invasive meningioma, and mTOR inhibitors can inhibit the proliferation of meningioma tumor cells[12]. Therefore, PI3K/Akt signal pathway has become the focus of cancer therapy and targeted drug development.
PI3K can Phosphorylate Phosphatidylinositol 4,5-Bisphosphate (PIP2) and produce PIP3 after activation. When the concentration of PIP3 increases, Akt/PKB and Phosphatidylinositide- Dependent protein Kinase 1 (PDK1) are recruited to the cell membrane, which activates Akt and phosphorylates the downstream substrate. This process can regulate cell migration, metabolism and cycle progression[13,14]. In meningioma, matrix metalloproteinases downstream of PI3K/Akt pathway can degrade extracellular matrix and basement membrane, thus promoting tumor spread and metastasis[15]. The protein expression levels of p-PI3K and p-Akt in group B were reduced than group A, while these in group C were raised than group B. It is suggested that lncRNA MALAT1 knockout can inhibit the phosphorylation of PI3K/ Akt pathway, and the phosphorylation level of PI3K/Akt pathway is increased by adding PI3K activator 740Y lncRNA MALAT1. The activation of PI3K/AKT signal pathway can partially reverse the inhibitory effect of low expression of lncRNA MALAT1 on apoptosis and invasion of meningioma cells. It is further confirmed that lncRNA MALAT1 regulates the level of phosphorylation of PI3K/Akt pathway on apoptosis and invasion of meningioma cells.
To sum up, lncRNA MALAT1 knockout promotes meningioma cell apoptosis and inhibits tumor cell invasion by reducing the level of phosphorylation of PI3K/AKT signaling pathway. The activation of PI3K/AKT signal pathway can partially reverse the inhibitory effect of low expression of lncRNA MALAT1 on apoptosis and invasion of meningioma cells.
Conflict of interests:
The authors declared no conflict of interests.
References
- Filippidis AS, Phillips KR, Lopez-Rivera V, Enriquez-Marulanda A, Mackel CE, Alterman RL, et al. Surgery in octogenarians with intracranial meningiomas improves functional outcome at 1 year. Acta Neurochirurgica 2023;165(12):4183-9.
[Crossref] [Google Scholar] [PubMed]
- Bagcchi S. WHO's global tuberculosis report 2022. Lancet Microb 2023;4(1):e20.
[Crossref] [Google Scholar] [PubMed]
- Oya S, Ikawa F, Ichihara N, Wanibuchi M, Akiyama Y, Nakatomi H, et al. Male sex and presence of preoperative symptoms are associated with early recurrence of WHO grade I meningiomas after surgical resection: Analysis from the nationwide brain tumor registry of Japan. Neurosurg Rev 2022;46(1):10.
[Crossref] [Google Scholar] [PubMed]
- Chen Y, Li Z, Chen X, Zhang S. Long non-coding RNAs: From disease code to drug role. Acta Pharm Sin B 2021;11(2):340-54.
[Crossref] [Google Scholar] [PubMed]
- Kashyap R, Jain A, Nalesnik M, Carr B, Barnes J, Vargas HE, et al. Clinical significance of elevated α-fetoprotein in adults and children. Dig Dis Sci 2001;46:1709-13.
[Crossref] [Google Scholar] [PubMed]
- Shi J, Yang C, Zhang J, Zhao K, Li P, Kong C, et al. NAT10 is involved in cardiac remodeling through ac4C-mediated transcriptomic regulation. Circ Res 2023;133(12):989-1002.
[Crossref] [Google Scholar] [PubMed]
- Komori T. Grading of adult diffuse gliomas according to the 2021 WHO classification of tumors of the central nervous system. Lab Invest 2022;102(2):126-33.
[Crossref] [Google Scholar] [PubMed]
- Luo Q, Yang L, Zhou X. The value of multimodal magnetic resonance imaging in breast cancer and its correlation with pathological features and prognosis. Eur Rev Med Pharmacol Sci 2023;27(18):8397-403.
[Crossref] [Google Scholar] [PubMed]
- Liu B, Zhan X, Liu C. Long noncoding RNA MALAT1 interacts with miR-124-3p to modulate osteosarcoma progression by targeting SphK1. J Oncol 2021;2021;8390165.
[Crossref] [Google Scholar] [PubMed]
- Xu Y, Zhang X, Hu X, Zhou W, Zhang P, Zhang J, et al. The effects of lncRNA MALAT1 on proliferation, invasion and migration in colorectal cancer through regulating SOX9. Mol Med 2018;24(1):52.
[Crossref] [Google Scholar] [PubMed]
- Guo T, Gu C, Li B, Xu C. PLODs are overexpressed in ovarian cancer and are associated with gap junctions via connexin 43. Lab Invest 2021;101(5):564-9.
[Crossref] [Google Scholar] [PubMed]
- Graillon T, Sanson M, Campello C, Idbaih A, Peyre M, Peyriere H, et al. Everolimus and octreotide for patients with recurrent meningioma: Results from the phase II CEVOREM trial. Clin Cancer Res 2020;26(3):552-7.
[Crossref] [Google Scholar] [PubMed]
- Sharma P, Khan MA, Najmi AK, Chaturvedi S, Akhtar M. Myricetin-induced apoptosis in triple-negative breast cancer cells through inhibition of the PI3K/Akt/mTOR pathway. Med Oncol 2022;39(12):248.
[Crossref] [Google Scholar] [PubMed]
- Xu W, Li B, Xu M, Yang T, Hao X. Traditional Chinese medicine for precancerous lesions of gastric cancer: A review. Biomed Pharmacother 2022;146:112542.
[Crossref] [Google Scholar] [PubMed]
- Zhu X, Bu J, Zhu T, Jiang Y. Targeting KK-LC-1 inhibits malignant biological behaviors of triple-negative breast cancer. J Transl Med 2023;21(1):184.
 ): Control; (
): Control; ( ): si-MALAT1 and (
): si-MALAT1 and ( ): si-MALAT1+740Y-P
): si-MALAT1+740Y-P