- *Corresponding Author:
- Zhaoli Wang
Department of Orthopaedics, Integrated Traditional Chinese and Western Medicine, Tianjin Hospital, China
E-mail: liyuntao66552023@163.com
| Date of Received | 04 October 2022 |
| Date of Revision | 25 May 2023 |
| Date of Acceptance | 11 March 2024 |
| Indian J Pharm Sci 2024;86(2):468-475 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
For probing the influence of kukoamine A on interleukin-1 beta-evoked damage of chondrocytes and its possible mechanism. Interleukin-1 beta was used to induce human knee joint chondrocytes to establish a cell injury model, and different concentrations of kukoamine A were used to treat chondrocytes. Following anti-microRNA-302b-3p introduction into chondrocytes, interleukin-1 beta (10 ng/ml) was employed for treating cells for 24 h. microRNA-NC and microRNA-302b-3p mimics were respectively transfected into chondrocytes and treated with kukoamine A (40 μmol/l) and interleukin-1 beta (10 ng/ml) for 24 h. Enzyme-linked immunosorbent assay method was used for detecting the levels of interleukin-6, tumour necrosis factor alpha, and interferon gamma. Flow cytometry was used for detecting the rate of cell apoptosis. The quantitative reverse transcription polymerase chain reaction was utilized for detecting microRNA-302b-3p level. Western blot was employed for detecting Bcl-2 associated X, apoptosis and B-cell lymphoma 2 protein levels. Kukoamine A could reduce interleukin-6, tumour necrosis factor alpha, and interferon gamma levels in chondrocytes induced by interleukin-1 beta, and could reduce the rate of apoptosis and the protein level of Bcl-2 associated X, apoptosis, and could also reduce the level of micoRNA-302b-3p, whereas it promoted B-cell lymphoma 2 level dose-dependently. After anti-microRNA-302b-3p introduction, interleukin-6, tumour necrosis factor alpha, and interferon gamma were downregulated, the rate of apoptosis and the protein level of Bcl-2 associated X, apoptosis were declined, whereas B-cell lymphoma 2 level was elevated. Transfection of microRNA-302b-3p mimics reversed kukoamine A impact on inflammation and apoptosis in interleukin-1 beta-evoked chondrocytes. Kukoamine A could inhibit cell inflammatory reaction and apoptosis by downregulating microRNA-302b-3p level, thereby reducing interleukin-1 beta induced chondrocyte damage.
Keywords
Interleukin-1 beta, knee chondrocytes, kukoamine A, microRNA-302b-3p, inflammation, apoptosis
Osteoarthritis (OA) is an ordinary joint illness, and its incidence is related to age. Inflammation and apoptosis of articular chondrocytes are important causes of chondrocyte injury[1,2]. Interleukin-1 Beta (IL-1β) is an inflammatory factor released by activated synovial cells and macrophages, which can promote the occurrence of OA. Meanwhile, the inflammatory response can also lead to chondrocyte apoptosis and extracellular matrix degradation, leading to the degeneration of cartilage tissue[3,4]. Kukoamine A (KuA) belongs to the active component of the Solanaceae plant lycii radicis cortex extract, which has a variety of biological activities[5]. Research suggests that KuA could attenuate the Lipopolysaccharide (LPS)-induced apoptosis, inflammation in nucleus pulposus cells[6]. KuA protected mice against OA through restraining inflammation and ferroptosis by regulating Silent Information Regulator 1 (SIRT1)/Glutathione Peroxidase 4 (GPX4) axis in chondrocytes[7]. However, the impact of KuA on IL- 1β induced inflammatory damage in chondrocyte is underreported.
MicroRNA (miRNA) are widely found in eukaryotes, which can regulate cell biological behavior by targeting and inhibiting expression of target gene[8,9]. As reported, miR-302b-3p was upregulated in isoflurane-evoked neuronal damage, and silencing its expression has a protective effect on isoflurane-induced neuronal damage[10]. However, the influence of miR-302b- 3p in inflammatory damage of IL-1β treated chondrocyte was unknown. Therefore, in this study, IL-1β induced human knee cartilage cells were used to establish a cell injury model, and to explore whether KuA affects IL-1β induced inflammatory injury of cartilage cells through regulating miR-302b-3p.
Materials and Methods
Clinical tissues:
10 patients who received knee joint replacement from January 2020 to March 2020 in our hospital were chosen as study subjects, all of whom had knee OA, including 6 males and 4 females among 40 and 60 ages, the mean age of them was (53.26±4.13) y.
Primary isolation and culture of human knee cartilage cells:
The articular cartilage tissue was placed in Dulbecco's Modified Eagle Medium (DMEM) (Solarbio, Beijing, China) plus double antibody and Fetal Bovine Serum (FBS) (Solarbio), washed with Phosphate Buffer Solution (PBS), and the cartilage tissue was chopped to 1 mm3 size, then added with 0.2 % type II collagenase digestion solution, digested at 37° in 5 % Carbon dioxide (CO2) incubator, after 3000 r/min low temperature centrifugation for 5 min, the supernatant was abandoned.
DMEM medium was added for washing precipitate, then centrifuged and discarded supernatant under the same conditions, filtered cell suspension with cell sieve, removed the undigested tissue mass, the filtered cell suspension was inoculated into a 25 cm2 culture bottle and then placed in incubator. DMEM was replaced every 2 d and was discarded when cell growth density achieved to 80 %. After digested cells using trypsin, fresh medium was used for terminating cell digestion, and cells were cultivated after centrifugation and prepared for the subsequent experiment when the cells were passed to the 3rd generation[11].
Primary isolation and culture of human knee cartilage cells:
The articular cartilage tissue was placed in Dulbecco's Modified Eagle Medium (DMEM) (Solarbio, Beijing, China) plus double antibody and Fetal Bovine Serum (FBS) (Solarbio), washed with Phosphate Buffer Solution (PBS), and the cartilage tissue was chopped to 1 mm3 size, then added with 0.2 % type II collagenase digestion solution, digested at 37° in 5 % Carbon dioxide (CO2) incubator, after 3000 r/min low temperature centrifugation for 5 min, the supernatant was abandoned. DMEM medium was added for washing precipitate, then centrifuged and discarded supernatant under the same conditions, filtered cell suspension with cell sieve, removed the undigested tissue mass, the filtered cell suspension was inoculated into a 25 cm2 culture bottle and then placed in incubator. DMEM was replaced every 2 d and was discarded when cell growth density achieved to 80 %. After digested cells using trypsin, fresh medium was used for terminating cell digestion, and cells were cultivated after centrifugation and prepared for the subsequent experiment when the cells were passed to the 3rd generation[11].
Experimental grouping:
The cultured chondrocytes in DMEM plus 10 ng/ml IL-1β (Solarbio) for 24 h[12,13], which was recorded as IL-1β group. The normal cultured chondrocytes were recorded as control group. Chondrocytes cultured with various doses (10, 20 or 40 μmol/l) of KuA (purity ≥98 %; Biyang Biotechnology, Chengdu, China)[7,14] and IL-1β for 24 h, were severally recorded as IL-1β+KuA- low (L), IL- 1β+KuA-Medium (M) or IL-1β+KuA-high (H) group. Chondrocytes were transduced of anti-miR-302b-3p or anti-miR-NC by liposome transfection method following Lipofectamine 2000 (Invitrogen, Carlsbad, California, United States of America (USA)), and later co-cultured with DMEM plus IL-1β for 24 h after successful transfection, which were severally recorded as IL-1β+anti-miR-302b- 3p or IL-1β+anti-miR-NC group. Chondrocytes were transduced of miR-NC or miR-302b-3p mimics by liposome transfection method, and cultivated with DMEM plus 40 μmol/l KuA and IL-1β for 24 h, which were severally recorded as IL-1β+KuA+miR-NC or IL-1β+KuA+miR-302b-3p group. Therein, anti-miR-NC, anti-miR-302b-3p, miR-NC and miR-302b-3p mimics were gained from RiboBio Biotechnology (Guangzhou, China).
Enzyme-Linked Immunosorbent Assay (ELISA):
After the collection of culture supernatant of chondrocytes, IL-6, Tumor Necrosis Factor Alpha (TNF-α) and Interferon Gamma (IFN-γ) levels were tested by ELISA following specifications of corresponding kits (Solarbio).
Flow cytometry:
After digestion, the supernatant of chondrocytes was discarded; the sediment was received abstersion of pre-cooled PBS and added with 500 μl binding buffer. Where after, Annexin V-Fluorescein Isothiocyanate (FITC) and Propidium Iodide (PI) (5 μl, all from cell apoptosis detection kit, Solarbio) were respectively added followed by the shake and incubation for 10 min. FACS Calibur flow cytometer was employed for detecting cell apoptosis.
Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR):
Total Ribonucleic Acid (RNA) was acquired by Trizol (Invitrogen), complementary Deoxyribonucleic Acid (cDNA) synthesis from RNA (2 μg) was proceeded by Reverse transcription kit (Tiangen, Beijing, China). The amplification of qRT-PCR was proceeded using cDNA as template using SYBR Green kit (Tiangen). ABI StepOnePlus fluorescence quantitative PCR was utilized for detecting relative gene expression. miR-302b-3p level was computed using 2-ΔΔCt tactic with U6 serving as the inner reference.
Western blot:
The chondrocytes were lysed with Radioimmunoprecipitation Assay (RIPA) lysate to obtain total protein. 40 μg protein was added to each well for Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis (SDS-PAGE), after its transfer into Polyvinylidene Difluoride (PVDF) membrane, it was closed for 2 h using 5 % skim milk. Rabbit anti-human antibodies against B-Cell Lymphoma Protein 2 (Bcl-2) and Bcl-2-Associated X Protein (BAX) (1:1000, Abcam, Cambridge, United Kingdom) and internal reference Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH) (1:2000, Abcam) were added onto above membrane at 4° for reaction overnight, followed by 2 h-incubation of secondary antibody (1:5000, Abcam) at 37°.
Later, Quantity One software was utilized for quantification of protein bands.
Statistical analysis:
Statistical Package for the Social Sciences (SPSS) 21.0 statistical software was employed for analyzing the data, which were expressed as (x͞ ±s). Analysis of Variance (ANOVA) or t-test was separately preceded for comparing the difference of multiple groups or between two groups. p<0.05 was considered statistically significant.
Results and Discussion
After IL-1β treatment, IL-6, TNF-α and IFN-γ levels were overtly elevated, while the levels of them were dose-dependently decreased after various dosages (10 μmol/l, 20 μmol/l, 40 μmol/l) of KuA, as listed in Table 1.
| Group | IL-6 (ng/l) | TNF-α (ng/l) | IFN-γ (ng/l) |
|---|---|---|---|
| Control | 4.19±0.38 | 3.29±0.39 | 17.23±1.34 |
| IL-1β | 52.05±4.86* | 44.42±4.15* | 97.08±7.39* |
| IL-1β+KuA-L | 37.05±3.08# | 30.13±2.95# | 70.68±6.07# |
| IL-1β+KuA-M | 22.48±2.14#& | 19.12±1.59#& | 48.34±4.11#& |
| IL-1β+KuA-H | 11.96±0.98#&$ | 7.28±0.69#&$ | 29.54±2.66#&$ |
| F | 430.640 | 439.904 | 394.095 |
| p | 0.000 | 0.000 | 0.000 |
Note: *p<0.05 vs. control group; #p<0.05 vs. IL-1β group; &p<0.05 vs. IL-1β+KuA-L group and $p<0.05 vs. IL-1β+KuA-M group
Table 1: Effect of KuA on IL-1β Induced Inflammation in Chondrocytes (x͞ ±s, n=9)
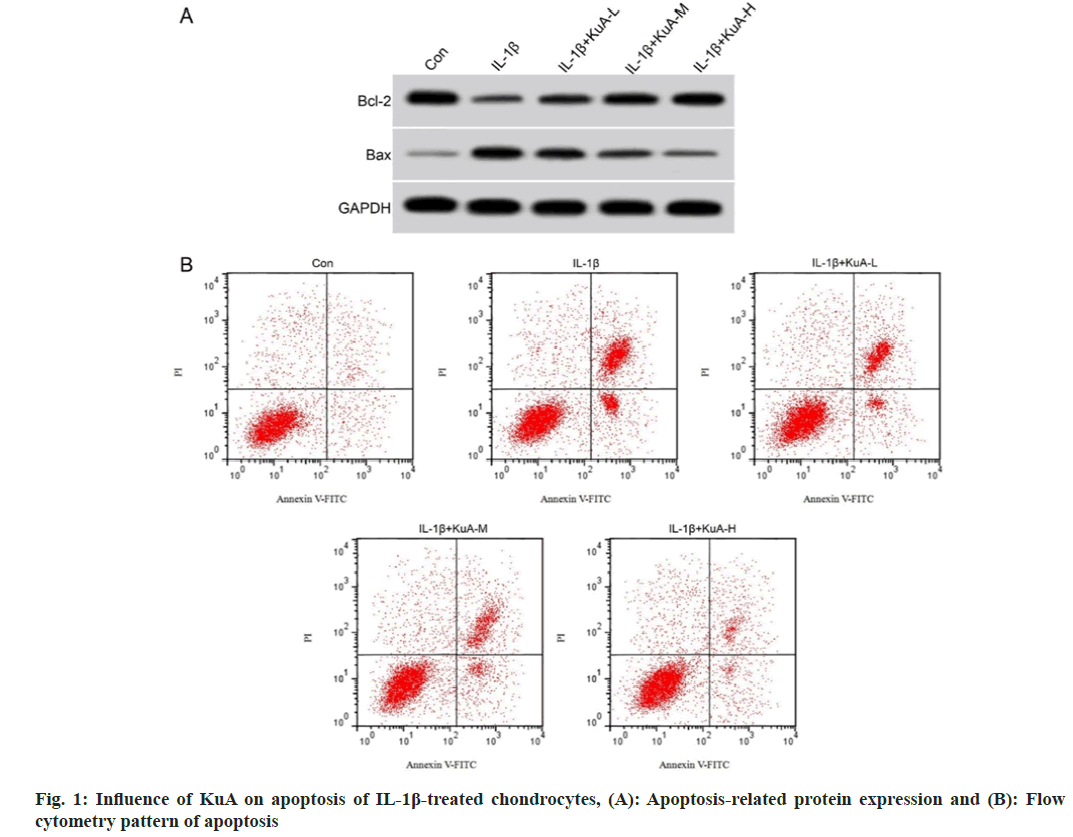
Then, the effect of KuA on apoptosis in IL-1β mediated chondrocytes was further probed. As demonstrated in fig. 1 and Table 2, the apoptosis and the level of BAX protein in IL-1β group were upregulated vs. control group, while Bcl-2 level was declined. Moreover, the co-treatment of IL- 1β and KuA-L, KuA-M or KuA-H had decreased levels of apoptosis rate and BAX protein vs. IL- 1β group, while Bcl-2 protein level was increased dose-dependently.
| Group | Apoptosis rate (%) | Bcl-2/GAPDH | BAX/GAPDH |
|---|---|---|---|
| Control | 5.84±0.55 | 0.83±0.07 | 0.14±0.02 |
| IL-1β | 33.72±3.03* | 0.31±0.03* | 0.67±0.05* |
| IL-1β+KuA-L | 23.21±2.18# | 0.44±0.04# | 0.53±0.05# |
| IL-1β+KuA-M | 15.19±1.48#& | 0.61±0.05#& | 0.37±0.03#& |
| IL-1β+KuA-H | 9.15±0.78#&$ | 0.75±0.05#&$ | 0.25±0.02#&$ |
| F | 334.619 | 167.371 | 303.045 |
| p | 0.000 | 0.000 | 0.000 |
Note: *p<0.05 vs. control group; #p<0.05 vs. IL-1β group; &p<0.05 vs. IL-1β+KuA-L group and $p<0.05 vs. IL-1β+KuA-M group
Table 2: Influence of KuA on Apoptosis of IL-1β Treated Chondrocytes (x͞ ±s, n=9)
The miR-302b-3p level was increased in chondrocytes following IL-1β treatment. However, miR-302b-3p level after KuA-L, KuA-M or KuA-H treatment in IL-1β-evoked chondrocytes was gradually decreased as shown in Table 3.
| Group | miR-302b-3p |
|---|---|
| Control | 1.00±0.00 |
| IL-1β | 3.52±0.27* |
| IL-1β+KuA-L | 2.66±0.21# |
| IL-1β+KuA-M | 1.95±0.14#& |
| IL-1β+KuA-H | 1.31±0.13#&$ |
| F | 306.783 |
| p | 0.000 |
Note: *p<0.05 vs. control group; #p<0.05 vs. IL-1β group; &p<0.05 vs. IL-1β+KuA-L group and $p<0.05 vs. IL-1β+KuA-M group
Table 3: Effect of KuA on Expression of miR-302B-3p in IL-1β Treated Chondrocytes (x͞ ±s, n=9)
Subsequently, miR-302b-3p role in chondrocytes was probed. As the exhibition of Table 4, IL- 6, TNF-α and IFN-γ levels were declined in chondrocytes transduced of anti-miR-302b-3p and added with IL-1β.
| Group | miR-302b-3p | IL-6 (ng/l) | TNF-α (ng/l) | IFN-γ (ng/l) |
|---|---|---|---|---|
| IL-1β+anti-miR-NC | 1.00±0.00 | 56.08±4.62 | 46.64±4.32 | 98.44±6.75 |
| IL-1β+anti-miR-302b-3p | 0.24±0.03* | 18.15±1.42* | 12.98±1.06* | 35.57±3.17* |
| t | 76.000 | 23.543 | 22.702 | 25.292 |
| p | 0.000 | 0.000 | 0.000 | 0.000 |
Note: *p<0.05 vs. IL-1β+anti-miR-NC group
Table 4: Effects of miR-302B-3p Interference on Inflammation in LPS-Evoked Chondrocytes (x͞ ±s, n=9)
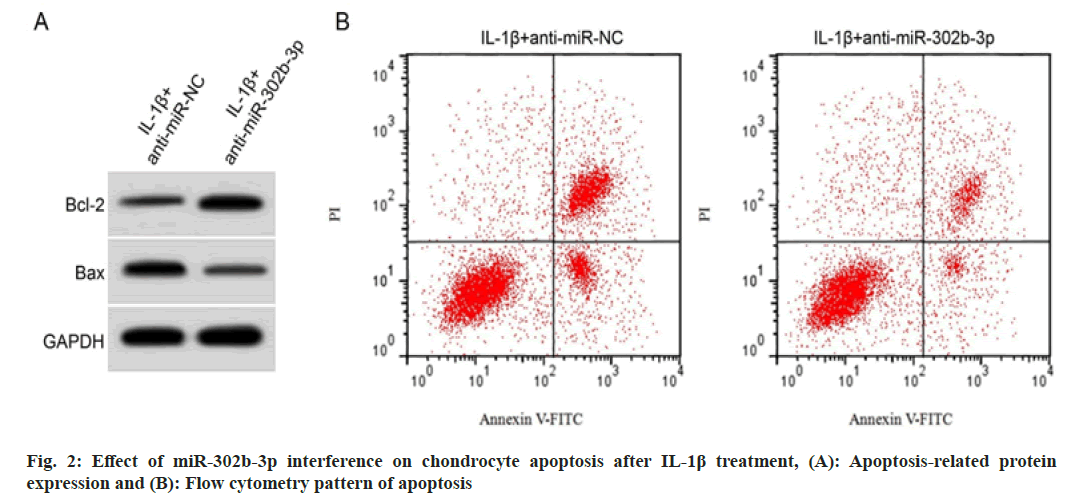
For exploring miR-302b-3p role in IL-1β evoked chondrocytes, miR-302b-3p was downregulated. Fig. 2 and Table 5 suggested that cell apoptosis and the level of BAX protein in IL-1β+anti-miR- 302b-3p group was decreased, whereas Bcl-2 protein expression was elevated in IL-1β evoked chondrocytes with miR-302b-3p silence.
| Group | Apoptosis rate (%) | Bcl-2/GAPDH | BAX/GAPDH |
|---|---|---|---|
| IL-1β+anti-miR-NC | 36.69±3.04 | 0.32±0.03 | 0.69±0.05 |
| IL-1β+anti-miR-302b-3p | 12.08±1.11* | 0.71±0.05* | 0.29±0.02* |
| t | 22.813 | 20.065 | 22.283 |
| p | 0.000 | 0.000 | 0.000 |
Note: *p<0.05 vs. IL-1β+anti-miR-NC group
Table 5: Effects of miR-302B-3p Interference on IL-1β Induced Apoptosis of Chondrocytes (x͞ ±s, n=9)
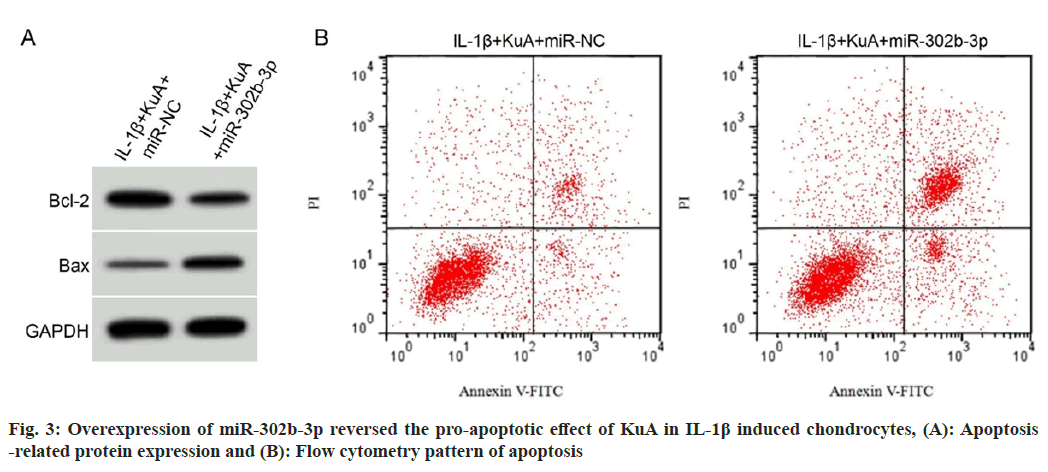
As depicted in fig. 3 and Table 6, miR-302b-3p upregulation could facilitate IL-6, TNF-α and IFN-γ levels, also it could facilitate cell apoptosis and the level of BAX protein, while it could block Bcl-2 level in chondrocytes treated with IL-1β and KuA.
| Group | miR-302b-3p | IL-6 (ng/l) | TNF-α (ng/l) | IFN-γ (ng/l) | Apoptosis rate (%) | Bcl-2/GAPDH | BAX/GAPDH |
|---|---|---|---|---|---|---|---|
| IL-1β+KuA+miR-NC | 1.00±0.00 | 10.19±1.15 | 7.17±0.67 | 28.23±2.73 | 9.85±0.67 | 0.77±0.05 | 0.24±0.02 |
| IL-1β+KuA+miR-302b-3p | 3.08±0.29* | 41.96±4.01* | 34.41±3.37* | 84.65±7.45* | 24.54±2.07* | 0.41±0.04* | 0.56±0.04* |
| t | 21.517 | 22.847 | 23.784 | 21.332 | 20.255 | 16.867 | 21.466 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Note: *p<0.05 vs. IL-1β+KuA+miR-NC group
Table 6: Overexpression of miR-302B-3p Overturned the Influence of KuA on IL-1β Induced Inflammatory Injury of Chondrocytes (x͞ ±s, n=9)
The main feature of OA is the metabolic disorder and matrix degradation of articular cartilage. Currently, non-steroidal anti-inflammatory drugs are often utilized in clinical remedy of OA, but they have great side influence. Natural plants are verified to have low toxic side impacts and can reduce the damage of chondrocytes[15]. Meanwhile, medicinal plants have latent pharmacological action and can serve as the novel drug candidates for OA[16]. miRNA is abnormally expressed in OA and can affect the growth of OA[17]. Nevertheless, whether miRNA can serve as a latent target of Chinese medicine for the treatment of OA needs further investigation.
KuA can reduce nerve cell damage by inhibiting oxidative stress and cell apoptosis[18,19]. KuA can protect neuronal cells[20], and had neuroprotective effects in the animal model of neurotoxin-induced Parkinson[21]. KuA can reduce hippocampal neuron damage through inactivating NF-κB pathway and further inhibiting inflammatory response[22]. This study certified that IL-6, IFN-γ and TNF-α levels in IL-1β mediated chondrocytes were increased, which was similar to reported results in related studies[23], suggesting that the cell damage model was successfully established. Further studies found that with the increase of KuA concentration, IL-6, IFN-γ and TNF-α levels in IL-1β evoked chondrocytes were gradually decreased, suggesting that KuA can inhibit IL-1β induced chondrocyte inflammation. We testified that apoptosis rate and BAX protein level were elevated in IL-1β evoked chondrocytes, whereas Bcl-2 level was lessened, which was similar to the results reported in related studies[24]. KuA can dose-dependently reduce apoptosis rate and BAX level while enhance Bcl-2 level, suggesting that KuA can block the apoptosis of chondrocytes induced by IL-1β.
miR-302b-3p is upregulated in neuronal injury induced by oxygen-glucose deprivation/reoxygenation, and interference with its expression can inhibit apoptosis and oxidative stress[25]. Inhibiting miR-302b-3p can inhibit the inflammatory response of nerve cells and thus reduce cell damage[26]. Here, it was certified that miR-302b-3p had elevated level in IL-1β evoked chondrocytes, and miR-302b-3p in chondrocytes induced by IL-1β after KuA treatment was downregulated, and it was dramatically decreased with the increase of drug concentration. It is suggested that KuA may exert a role in chondrocytes through inhibiting miR-302b-3p level. Besides, inhibiting miR-302b-3p could block the inflammatory reaction and apoptosis of cells, while upregulation of miR-302b-3p could antagonize the repressive impact of KuA on IL-1β evoked inflammatory response and apoptosis of chondrocytes. It is suggested that KuA can slow down the development of OA by inhibiting miR- 302b-3p level.
In summary, KuA can suppress IL-1β evoked chondrocyte inflammatory response and cell apoptosis through inhibiting miR-302b-3p level, thereby reducing cell damage. Thus, we deduced that miR-302b-3p may implement as a latent target of KuA in treating OA. Nevertheless, the particular theory of its action still requires more profound investigation.
Funding:
This work was supported by Tianjin Key Scientific Research Projects in Traditional Chinese Medicine (No: 2023015).
Conflict of interests:
The authors declared no conflict of interests.
References
- Liu J, Meng Q, Jing H, Zhou S. Astragaloside IV protects against apoptosis in human degenerative chondrocytes through autophagy activation. Mol Med Rep 2017;16(3):3269-75.
[Crossref] [Google Scholar] [PubMed]
- Li W, Wang Y, Tang Y, Lu H, Qi Y, Li G, et al. Quercetin alleviates osteoarthritis progression in rats by suppressing inflammation and apoptosis via inhibition of IRAK1/NLRP3 signaling. J Inflamm Res 2021;14:3393-403.
[Crossref] [Google Scholar] [PubMed]
- Zhang Y, Zeng Y. Curcumin reduces inflammation in knee osteoarthritis rats through blocking TLR4 /MyD88/NF-kappaB signal pathway. Drug Dev Res 2019;80(3):353-9.
[Crossref] [Google Scholar] [PubMed]
- Shao Z, Wang B, Shi Y, Xie C, Huang C, Chen B, et al. Senolytic agent quercetin ameliorates intervertebral disc degeneration via the Nrf2/NF-kappaB axis. Osteoarthr Cartil 2021;29(3):413-22.
[Crossref] [Google Scholar] [PubMed]
- Li X, Lin J, Chen B, Xie H, Chen D. Antioxidant and cytoprotective effects of kukoamines a and b: Comparison and positional isomeric effect. Molecules 2018;23(4):973.
[Crossref] [Google Scholar] [PubMed]
- Wang D, Qu H, Kang H, Xu F, Huang W, Cai X. Kukoamine A attenuates lipopolysaccharide-induced apoptosis, extracellular matrix degradation, and inflammation in nucleus pulposus cells by activating the P13K/Akt pathway. Bioengineered 2022;13(4):8772-84.
[Crossref] [Google Scholar] [PubMed]
- Sun J, Zhang Y, Wang C, Ruan Q. Kukoamine A protects mice against osteoarthritis by inhibiting chondrocyte inflammation and ferroptosis via SIRT1/GPX4 signaling pathway. Life Sci 2023;332:122117.
[Crossref] [Google Scholar] [PubMed]
- Li P, Chen Y, Juma CA, Yang C, Huang J, Zhang X, et al. Differential inhibition of target gene expression by human microRNAs. Cells 2019;8(8):791.
[Crossref] [Google Scholar] [PubMed]
- Peng W, Wu R, Dai W, Ning Y, Fu X, Liu L, miRNA-gene network embedding for predicting cancer driver genes. Brief Funct Genomics 2023;22(4):341-50.
[Crossref] [Google Scholar] [PubMed]
- Li L, Lu S, Fan X. Silencing of miR-302b-3p alleviates isoflurane-induced neuronal injury by regulating PTEN expression and AKT pathway. Brain Res Bull 2021;168:89-99.
[Crossref] [Google Scholar] [PubMed]
- Liao FX, Huang F, Ma WG, Qin KP, Xu PF, Wu YF, et al. The new role of sirtuin1 in human osteoarthritis chondrocytes by regulating autophagy. Cartilage 2021;13(2):1237-48.
[Crossref] [Google Scholar] [PubMed]
- Fei J, Liang B, Jiang C, Ni H, Wang L. Luteolin inhibits IL-1 beta induced inflammation in rat chondrocytes and attenuates osteoarthritis progression in a rat model. Biomed Pharmacother 2019;109:1586-92.
- Shi X, Jie L, Wu P, Zhang N, Mao J, Wang P, et al. Calycosin mitigates chondrocyte inflammation and apoptosis by inhibiting the PI3K/AKT and NF-kappaB pathways. J Ethnopharmacol 2022;297:115536.
[Crossref] [Google Scholar] [PubMed]
- Li X, Jiang XW, Chu HX, Zhao QC, Ding HW, Cai CH. Neuroprotective effects of kukoamine A on 6-OHDA-induced Parkinson's model through apoptosis and iron accumulation inhibition. Chin Herb Med 2021;13(1):105-15.
[Crossref] [Google Scholar] [PubMed]
- Wang C, Gao Y, Zhang Z, Chi Q, Liu Y, Yang L, et al. Safflower yellow alleviates osteoarthritis and prevents inflammation by inhibiting PGE2 release and regulating NF-kappaB/SIRT1/AMPK signaling pathways. Phytomedicine 2020;78:153305.
[Crossref] [Google Scholar] [PubMed]
- Kang YH, Lee HJ, Lee CJ, Park JS. Natural products as sources of novel drug candidates for the pharmacological management of osteoarthritis: A narrative review. Biomol Ther (Seoul) 2019;27(6):503-13.
[Crossref] [Google Scholar] [PubMed]
- Zhao G, Gu W. Effects of miR-146a-5p on chondrocyte interleukin-1 beta-induced inflammation and apoptosis involving thioredoxin interacting protein regulation. J Int Med Res 2020;48(11):300060520969550.
- Liu J, Jiang X, Zhang Q, Lin S, Zhu J, Zhang Y, et al. Neuroprotective effects of kukoamine A against cerebral ischemia via antioxidant and inactivation of apoptosis pathway. Neurochem Int 2017;107:191-7.
[Crossref] [Google Scholar] [PubMed]
- Luo L, Guan Z, Jin X, Guan Z, Jiang Y. Identification of kukoamine A as an anti-osteoporosis drug target using network pharmacology and experiment verification. Mol Med 2023;29(1):36.
[Crossref] [Google Scholar] [PubMed]
- Yang Y, Gao L, Niu Y, Li X, Liu W, Jiang X, et al. Kukoamine A protects against NMDA-induced neurotoxicity accompanied with down-regulation of GluN2B-containing NMDA receptors and phosphorylation of PI3K/Akt/GSK-3 beta signaling pathway in cultured primary cortical neurons. Neurochem Res 2020;45(11):2703-11.
[Crossref] [Google Scholar] [PubMed]
- Hu X, Song Q, Li X, Li D, Zhang Q, Meng W, et al. Neuroprotective effects of kukoamine A on neurotoxin-induced parkinson's model through apoptosis inhibition and autophagy enhancement. Neuropharmacology 2017;117:352-63.
[Crossref] [Google Scholar] [PubMed]
- Zhang Y, Gao L, Cheng Z, Cai J, Niu Y, Meng W, et al. Kukoamine a prevents radiation-induced neuroinflammation and preserves hippocampal neurogenesis in rats by inhibiting activation of NF-kappaB and AP-1. Neurotox Res 2017;31(2):259-68.
[Crossref] [Google Scholar] [PubMed]
- Qiao Z, Tang J, Wu W, Tang J, Liu M. Acteoside inhibits inflammatory response via JAK/STAT signaling pathway in osteoarthritic rats. BMC Complement Altern Med 2019;19(1):264.
[Crossref] [Google Scholar] [PubMed]
- Wang BW, Jiang Y, Yao ZL, Chen PS, Yu B, Wang SN. Aucubin protects chondrocytes against IL-1 beta induced apoptosis in vitro and inhibits osteoarthritis in mice model. Drug Des Devel Ther 2019;13:3529-38.
[Crossref] [Google Scholar] [PubMed]
- Zhang Z, Wang N, Zhang Y, Zhao J, Lv J. Downregulation of microRNA-302b-3p relieves oxygen-glucose deprivation/re-oxygenation induced injury in murine hippocampal neurons through up-regulating Nrf2 signaling by targeting fibroblast growth factor 15/19. Chem Biol Interact 2019;309:108705.
[Crossref] [Google Scholar] [PubMed]
- He R, Tang GL, Niu L, Ge C, Zhang XQ, Ji XF, et al. Quietness circ 0000962 promoted nerve cell inflammation through PIK3CA/Akt/NF-kappaB signaling by miR-302b-3p in spinal cord injury. Ann Palliat Med 2020;9(2):190-8.
[Crossref] [Google Scholar] [PubMed]