- *Corresponding Author:
- A. Swargiary
Department of Zoology, Bodoland University, Rangalikhata, Debargaon, 783370, Assam, India
E-mail: ananbuzoo101@gmail.com
| Date of Received | 21 December 2020 |
| Date of Revision | 04 May 2020 |
| Date of Acceptance | 25 July 2020 |
| Indian J Pharm Sci 2020;82(4):707-712 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Musa balbisiana colla is an important plant native to India and many other Asian countries. Parts of this plant such as seeds, fruit pulp, inflorescence, pseudo-stem, and corm have been known to possess several medicinal values. In Kokrajhar district of Assam, the decoction of corm part of the plant is traditionally used as antidiabetic medicine. The present study was aimed to investigate the phytochemicals, antioxidants, trace element, and major compounds of the corm part of Musa balbisiana. Phenolic and flavonoid contents were estimated following standard protocols. The antioxidant activity of the plant was studied by ferric reducing antioxidant power assay, total antioxidant capacity, 1,1-diphenyl-2-picryl-hydrazyl, and lipid peroxidation scavenging assay. The phytochemical study revealed that the corm extract is rich in protein, carbohydrates, phenolics, and flavonoid content. Antioxidant study revealed strong free radical scavenging property of the crude corm extract of Musa balbisiana. The elemental analysis showed highest Zn content (0.2993 ppm) followed by Ni, Cu, and Mn. Pb, Cd, and Cr were not detected in the extract. GC-MS analysis showed difluroisocyanotophosphine to be the major compound of Musa balbisiana corm extract
Keywords
Phytochemicals, antioxidants, trace elements, gc-ms, Musa balbisiana, kokrajhar
Natural products play an important role in the treatment of various diseases and drug discovery processes. Plants have been used in ethnomedicine system since ancient times to cure many diseases including diabetes[1]. Many therapeutically active plants are known to be used in the preparation of herbal medicine. Plants are rich sources of pharmacologically active substances which can be helpful in designing therapeutically active medicines for treating various ailments. Phytochemical content such as phenols or their oxygen substituted derivatives such as tannins while some may contain nitrogen or sulfur that are biologically active and useful for the treatment of diseases and preserve well-being in humans and animals[2]. It is estimated that about 70- 80% population of tropical countries rely on medicinal plant as the source of medicine, and the tendency to use ethnomedicine is also gradually increasing in other developed countries because of its healthy effects[3]. In many developing countries like India, several plants and its derivatives have been used traditionally for the treatment of many diseases[4,5]. The state of Assam is one of the 29th states of India blessed with rich flora and fauna. With the geographical location 89°50/ E to 96°10/ E and 24°30/ N to 28°10/ N Assam state is one among the richest biodiversity zones of North-East India[6]. Most of the people, especially ethnic tribal groups living in this state perform various traditional health-care practices and rely on traditional medicines as the primary source of healthcare needs. According to World Health Organisation (WHO), the use of traditional herbal medicine has spread not only in the developing countries but also in the industrialized ones, as a complementary way to treat and prevent illness[7]. Natural products could be a potential source of drugs for humans or livestock, and also the products and their analogs can act as intermediates for the synthesis of useful drugs[8]. Plant possesses many phytochemicals with various bioactivities including antioxidant, anti-inflammatory, anticancer, antiviral, antidiabetic, anthelmintic, etc[9-11]. Musa balbisiana colla belonging to the family Musaceae is an important monocotyledonous herb having several religious and medicinal values[12]. Among the different varieties of Musa species, M. balbisiana is native to India and has been utilized as folk medicine since ancient times[13]. Various parts of this plant are reported to be used for the treatment of diseases including diabetes, diarrhea, scabies, helminthiasis, stomach problem, and inflammation[14]. Several studies have reported the pharmacological properties of different parts of M. balbisiana. However, we did not find any literature regarding the corm extract of the plant. Therefore, the present study has been designed to study the phytochemical profile and antioxidant activities of Musa balbisiana corm extract. Sample plant was collected from Kokrajhar district of Assam and identified in the department of Botany, Bodoland University. The sample plant was identified as Musa balbisiana Colla and the identification number is BUBH2018067.
The corm parts of M. balbisiana were collected from Kokrajhar district of Assam with the help of local people. The plant part was brought to the laboratory and washed with distilled water and dried in hot air oven below 50° and processed for preparation of crude extract. Dried plant parts were ground into powdered form and soaked into 80% methanol. Solution was filtered after 72 hrs of soaking and fresh solvent was added. The process was repeated three times and the filtrate obtained was evaporated in a rotary evaporator. Dry, semi-solid M. balbisiana methanolic extract (MBME) obtained was kept at -20º for further use. The process was followed as per the method described in our earlier publication[6].
The protein content of the plant extract was estimated following the Lowry method[15]. The presence of total carbohydrate content in MBME was estimated following the Anthrone method[16]. The total phenolic content (TPC) was estimated using Folin‑Ciocalteu reagent[17]. The amount of TPC was calculated from a calibration curve of gallic acid and results were expressed as μg gallic acid equivalent (GAE)/mg plant extract. The total flavonoid content (TFC) was determined following the method of Ordonez et al.[18]. TFC was calculated from the standard curve of quercetin, and the values were expressed as μg quercetin equivalent (QE)/mg plant extract.
The total antioxidant capacity (TAC) of MBME was done by phosphomolybdate method using ammonium molybdate[19]. The reaction mixture was incubated at 95° for 30 min and absorbance measured at 695 nm against a blank solution. TAC was expressed as μg ascorbic acid equivalent (AAE)/mg plant extract.
Ferric reducing antioxidant power assay (FRAP) was performed following the method of Iloki-Assanga et al[20]. The FRAP activity of MBME was compared with the standard ascorbic acid and values were expressed as μg Fe2+ equivalent (FE)/mg plant extract. The 1,1‑diphenyl‑2‑picrylhydrazyl (DPPH) free radical scavenging activity of MBME was estimated using DPPH as described by Mamta et al.[21]. Lipid peroxidation inhibitory activity was studied following the modified thiobarbituric acid reactive species (TBARS) assay to measure the lipid peroxide formation using egg yolk homogenates as lipid‑rich media[22]. The colouration of the assay mixture was measured at 532 nm. The 2,2’‑Azinobis‑(3‑ethylbenzothiazoline‑6‑sulfonate) (ABTS) free radical scavenging activity of MBME was measured following the method of Re et al[23] using gallic acid as the standard reference. Seven elements such as lead (Pb), chromium (Cr), nickel (Ni), cadmium (Cd), copper (Cu), manganese (Mn), and zinc (Zn) were analyzed using Atomic Absorption Spectroscopy (AAS) following the method of Zheljazkov and Nielson with slight modification[24]. Briefly, 1 g of plant powder was digested with concentrated HNO3 at 90º for 45 min. The temperature is then slowly increased up to 100º and boiled for 6-7 h by the addition of HNO3 till complete digestion. The process was continued till the solution became colourless and the end volume was maintained at 10 ml. The solution was then diluted to 100 ml of distilled water and then filtered using Whatman filter no. 1. The solution was then analysed for trace elements at AAS, Shimadzu AA-7000. The chemical composition of MBME was determined using the GC-MS system (TQ- 8030 Shimadzu Corporation Kyoto, Japan) as described by Kalita et al.[12]. All the statistical calculations were carried out in Microsoft excel. IC50 (concentration of plant extract at 50% inhibition of activity) values were calculated using SPSS software. Correlation study and figures were drawn using OriginPro software. All the experiments were represented with mean ± standard deviation (SD). Statistical significance was calculated at P≤0.05 level.
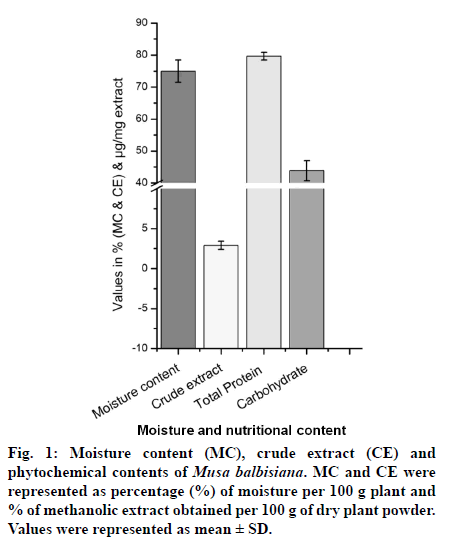
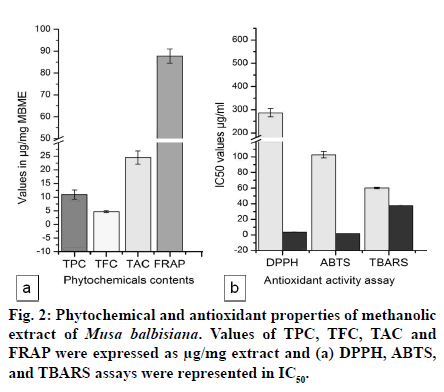
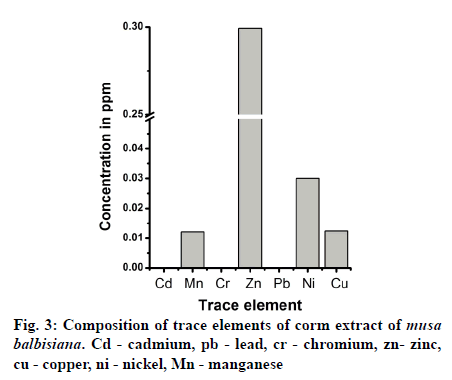
Medicinal plants are rich sources of phytochemicals and secondary metabolites. In the present study, phytochemical content of methanolic crude extract of M. balbisiana corm was analyzed. Fig. 1 showed the moisture content, crude extract obtained, phenolic, and flavonoid content of MBME. The plant part (corm) was found to contain high moisture content (75.30%) while the crude extract obtained was only 2.35% after three rounds of extraction process. The methanolic extract of corm was found to contain high protein content (79.65±1.22 μg/mg extract) while the carbohydrate content was found to be almost half of the protein (43.9±3.13 μg/mg extract). Statistical analysis showed significant difference (at P≤0.05 level) between the protein and carbohydrate content of M. balbisiana. MBME also showed considerable amount of phenolic and flavonoid content. M. balbisiana corm extract showed higher TPC content than TFC. The TPC and TFC were found to be 10.93±1.71 μgGAE/ mg and 4.72±0.33 μgQE/mg plant extract, respectively. Similarly, the TAC and FRAP activity was found to be 24.43±2.42 μgAAE/mg extract and 87.69±3.27 μg FeE/mg extract, respectively (fig. 2a). The methanolic crude extract of M. balbisiana was found to possess strong free radical scavenging activity. The IC50 values for DPPH, ABTS, and TBARS assays were found to be 287±17.45 μg/ml, 102.89±4.16 μg/ml, and 60.11±0.86 μg/ml, respectively. The standard reference chemical showed 3.64±0.365 μg/ml, 1.76±0.05 μg/ ml, and 37.65±0.91 μg/ml for DPPH, ABTS, and TBARS antioxidant assays, respectively (fig. 2b). Fig. 3 showed the metallic content of the methanolic crude extract of M. balbisiana. A total of seven trace elements were analysed out of which Zn was found to be in highest concentration (0.2993 ppm) followed by Ni (0.03 ppm), Cu (0.0124 ppm), and Mn (0.0121 ppm). On the contrary, three toxic elements, Cd, Pb, and Cr were not detected in the analysis. All the metallic contents were found to be in the permissible limit.
GC-MS analysis identified five compounds from the corm extract of M. balbisiana. Table 1 showed the GCMS parameters of all the five compounds identified from the plant. The five major compounds identified were difluoroisocyanatophosphine (1), 2’-methoxy- 2,3’,4,4’-tetrabromodiphenyl ether (2), isophthalic acid, ethyl 6-ethyloct-3-yl ester (3), phthalic acid, 2-(4-chlorophenoxy)ethyl hexylester (4), and pseudodiosgenin diacetate (5). The chromatograms of retention time and m/z intensities are presented in fig. 4a & b. The compound which shows highest peak was identified as Difluoroisocyanatophosphine and has the molecular formula CF2NOP and molecular weight of 110.987 with the retention time of 5.785 (Table 1).
| Name of the compound | Retention time | m/z | Area (%) | Height (%) | Mol. weight (g/mol) | Mol. Formula |
|---|---|---|---|---|---|---|
| 1. Difluoroisocyanatophosphine | 5.785 | 69.25 | 8.77 | 24.94 | 110.987 | CF2NOP |
| 2. 2'-Methoxy-2,3',4,4'-tetrabromodiphenyl ether | 9.793 | 569.00 | 10.42 | 20.49 | 515.8 | C13H8Br4O2 |
| 3. Isophthalic acid, ethyl 6-ethyloct-3-yl ester | 15.100 | 177.00 | 30.20 | 21.14 | 334.4 | C20H30O4 |
| 4. Phthalic acid, 2-(4-chlorophenoxy)ethyl hexyl ester | 20.460 | 193.00 | 29.98 | 16.67 | 404.9 | C22H25ClO5 |
| 5. Pseudodiosgenin diacetate | 23.588 | 81.00 | 20.63 | 16.76 | 498.7 | C31H46O5 |
Table 1: Gc-Ms Profiles of The Compounds Identified from Corm Extract of Musa Balbisiana
Phytochemical analysis revealed high protein, carbohydrate, phenolic, and flavonoid content in the methanolic corm extract of M. balbisiana. Similar to our study, Mahmood reported high content of TPC and less content of TFC in M. paradisiaca[25]. The presence of phenolic, flavonoid, and glycosides was also reported in M. acuminata[26]. Free radicals are generated continuously in our bodies as a result of several metabolic processes. Our body has an innate capacity to neutralise these free radicals. However, our innate antioxidant capacity to neutralize free radicals is limited to certain concentration and beyond that concentration our body fails to neutralize it. With rich phytochemicals and secondary metabolites, plants act as a source of antioxidant molecules which can increase the antioxidant property of our body[27,28]. Several studies have reported the antioxidant and free radical scavenging properties of several medicinal plants[29,30].
Metallic content plays a vital role in our day to day life. It can be harmful or toxic if it crosses the permissible level. In our analysis, we have found that the toxic heavy metals such as Pb and Cd were below the WHO permissible limits[31]. The study thus suggests that the corm extract do not contain metallic content at the range that is toxic to human consumption. Trace elements are important for several biological activities and human health. Trace elements such as Zn, Cu, Fe, and Mg functions as cofactors for many proteins and enzymes[32,33]. On the other hand, heavy metals like Pb, Cd, Ni, and Cr do not have any beneficial roles but are known to be toxic to the health[34]. GC-MS study of corm extract of M. balbisiana showed five major compounds from the plant. All the compounds were reported for the first time from M. balbisiana corm extract. We did not find any literature about the pharmacological properties of the identified compounds. Similarly, several ester compounds were reported from the fruit peel extract of Musa species[35]. Recent studies by Yingyuen et al.[36] reported the presence of rutin as the major compound in the leaves of M. balbisiana. Similarly, Kumari et al.[37] reported seven major phenolic content from the fruit pulp of M. balbisiana and also revealed that the extract contains cardioprotective activity.
Medicinal plants own the impression of significant success in the traditional system of disease treatment. This paper sheds a light on the phytochemical profile and antioxidant properties of the plant. Considering the different activities of plants, it therefore, justifies the traditional use of medicinal plants in the treatment of different health disorders. Musa balbisiana corm extract showed rich phytochemical and antioxidant properties as well as low toxic elements which are indicative enough for rich medicinal values of the plant. However, proper pharmacological studies and characterisation of bioactive compounds need to be carried out to understand the detailed mode of action.
Acknowledgements
Authors would like to acknowledge the instrumentation facilities supported by DST-SERB project under AS. Authors would also like to thank Department of Zoology, Bodoland University for providing necessary facilities. We also acknowledge Institute of Advanced Study in Science and Technology, Boragaon, Guwahati, for providing AAS and GC-MS facilities.
Conflict of Interest
Authors declares no conflict of Interest.
References
- Jamshidi-Kia F, Lorigooini Z, Amini-Khoei H. Medicinal plants: Past history and future perspective. J Herbmed Pharmacol 2018;7(1):1-7.
- Paudal KR, Panth N. Phytochemical profile and biological activity of Nelumbo nucifera. Evid Based Complement Alternate Med 2015:789124.
- Hossen MJ, Uddin MB, Ahmed SSU, Yu ZL, Cho YJ. Traditional medicine/plants for the treatment of reproductive disorders in Asia Nations. Pak Vet J 2015;36(2):127-33.
- Daimari M, Roy MK, Swargiary A, Baruah S, Basumatary S. An ethnobotanical survey of antidiabetic medicinal plants used by the Bodo tribe of Kokrajhar district, Assam. Indian J Tradit Know 2019;18(3):421-9.
- Swargiary A, Daimari M, Roy MK. Survey and documentation of anthelmintic plants used in traditional medicine system of tribal communities of Udalguri district Assam, India. J Appl Pharm Sci 2020;10(1):46-54.
- Swargiary A, Daimari A, Daimari M, Basumatary N, Narzary E. Phytochemicals, antioxidant, and anthelmintic activity of selected traditional wild edible plants of lower Assam. Indian J Pharmacol 2016;48:418-23.
- WHO. The world health report 2003-WHO https://www.who.int
- Makkar HPS, Norvsambuu T, Lkhavatsere S, Becker K. Plant secondary metabolites in some medicinal plants of Mongolia used for enhancing animal health and production. Tropicultura 2009;27(3):159-67.
- Twilley D, Langhansova L, Palaniswamy D, Lall N. Evaluation of traditionally used medicinal plants for anticancer, antioxidant, anti-inflammatory and anti-viral (HPV-1) activity. S Afr J Bot 2017;112:494-500.
- Sahaa MR, Dey P, Sarkar I, Sarker DD, Haldar B, Chaudhuri TK et al. Acacia nilotica leaf improves insulin resistance and hyperglycemia associated acute hepatic injury and nephrotoxicity by improving systemic antioxidant status in diabetic mice. J Ethnopharmacol 2018;210:275-86.
- Swargiary A, Verma AK, Singh S, Roy MK, Daimari M. Antioxidant and antiproliferative activity of selected medicinal plants of lower Assam, India: An in vitro and in silico study. Anticancer Agents Med Chem. 2020.
- Davey MW, Gudimella R, Harikrishna JA, Sin LW, Khalid N, Keulemans J. A draft Musa balbisiana genome sequence for molecular genetics in polyploid, inter- and intra-specific Musa hybrid. BMC Genomics 2013;14:683.
- Kalita H, Boruah DC, Deori M, Hazarika A, Sarma R, Kumari S et al. Antidiabetic and antilipidemic effect of Musa balbisiana root extract: A potent agent for glucose homeostasis in streptozotocin-induced diabetic rat. Front Pharmacol 2016;7:102
- Rai PK, Jaiswal D, Rai NK, Pandhija S, Rai AK, Watal G. Role of glycemic elements of Cynodon dactylon and Musa paradisiaca in diabetes management. Laser Med Sci 2009;24:761-8.
- Lowry OH, Rosebrough NJ, Farr AL Randal RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951;193(1):265-75.
- Sadasivam S, Manickam A. Biochemical methods. 3rd edition. New Age International: New Delhi; 2008, p. 8.
- Iloki S, Lewis L, Rivera G, Gil A, Acosta A, Meza C et al. Effect of maturity and harvest season on antioxidant activity, phenolic compounds and ascorbic acid of Morinda citrofolia L. (Noni) grown in Mexico. Afr J Biotechnol2013;12:4630-9.
- Ordonez AAL, Gomez JD, Vattuone MA, Isla MI. Antioxidant activities of Sechium edule (Jacq) Swartz extracts. Food Chem 2006;97:452-8.
- Huda-Faujan N, Norrakiah AS, Babji AS. Antioxidant activity of plants methanolic extracts containing phenolic compounds. Afr J Biotechnol 2009;8:484-9.
- Iloki-Assannga SB, Lewis-Lujan LM, Lara-Espinoza CL, Gil-Salido AA, Fernandez-Angulo D, Rubio-Pino JL et al. Solvent effects on phytochemical constituent profiles and antioxidant activities, using four different extraction formulations for analysis of Bucida buceras L. and Phoradendron californicum. BMC Res Notes 2015;8:396.
- Mamta, Mehrotra S, Amitabh, Kirar V, Vats P, Nandi SP et al. Phytochemical and antimicrobial activities of Himalayan Cordycepssinensis (Berk.) Sacc. Indian J Exp Biol 2015;53(1):36-43.
- Ohkawa H, Ohsini N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979;95(2):351-8.
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 1999;26(9-10):1231-7.
- Zheljazkov VD, Nielson, NS. Effect of heavy metals on peppermint and cornmint. Plant Soil 1996;178:59-66.
- Mahmood A, Ngah N, Omar MN. Phytochemicals constituent and antioxidant activities in Musa x Paradisiaca Flower. Eur J Sci Res 2011;66(2):311-8.
- Sumathy V, Lachumy SJ, Zakaria Z, Sasidharan S. In vitro bioactivity and phytochemical screening of Musa acuminata flower. Pharmacologyonline 2011;2:118-27.
- Padalia H, Poptani R, Chanda S. Evaluation of in vitro antioxidant properties of solvent extracts of selected medicinal plants and their synergistic efficacy. J Herbs Spices Med Plants 2018;24(1):15-27.
- Swargiary A, Brahma B. Phytochemical analysis and antioxidant activity of Hodgsonia heteroclita (Roxb). Indian J Pharm Sci 2017;79(2):212-9.
- Swargiary A, Nath P, Basumatary B, Brahma, B. Phytochemical, antioxidant, and trace element analysis of anthelmintic plants of north-east India. Int J Pharm Pharm Sci 2017;9(9):228-32.
- Swargiary A, Daimari M, Roy M, Haloi D, Ramchiary B. Evaluation of phytochemical properties and larvicidal activities of Cynodon dactylon, Clerodendrum viscosum, Spilanthes acmella and Terminalia chebula against Aedes aegypti. Asian Pac J Trop Med 2019;12(5):224-31.
- WHO. WHO permissible level of heavy metals in plants and soil. 1996. https://www.omicsonline.org/articles–images/2161-0525-5-334-t011.html
- Benabderrahim MA, Yahiaa Y, Bettaieb I, Elfalleh W, Nagaz K. Antioxidant activity and phenolic profile of a collection of medicinal plants from Tunisian arid and Saharan regions. Ind Crops Prod 2019;138:111427.
- Al-Fartusie FS, Mohssan SN. Essential trace elements and their vital roles in human body. Indian J Adv Chem Sci 2017;5(3):127-36.
- Anal JM, Chase P. Trace elements analysis in some medicinal plants using graphite furnace-atomic absorption spectroscopy. Environ Eng Res 2016;21(3):247-55.
- Villaverdea Lucia JJ, Oliveira L, Vilela C, Dominguesc RM, Freitas N Cordeiro N, Freire CSR et al. High valuable compounds from the unripe peel of several Musa species cultivated in Madeira Island (Portugal). Ind Crops Prod 2013;42:507-12.
- Yingyuen P, Sukrong S, Phisalaphong M. Isolation, separation and purification of rutin from Banana leaves (Musa balbisiana). Ind Crops Prod 2020;149.
- Kumari S, Katare P, Elancheran R, Nizami H, Paramesha B, Arava S et al. Musa balbisiana fruit rich in polyphenols attenuates isoproterenol-induced cardiac hypertrophy in rats via inhibition of inflammation and oxidative stress. Oxid Med Cell Longev 2020;27:7147498.