- *Corresponding Author:
- Ping Zang
Department of Geriatrics, Baiyun Hospital Affiliated to Guizhou Medical University, Guiyang, Guizhou Province 550058, China
E-mail: happyzhp1103@163.com
| This article was originally published in a special issue, “Emerging Therapeutic Interventions of Biopharmaceutical Sciences” |
| Indian J Pharm Sci 2024:86(3) Spl Issue “280-286” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The effects of anti-inflammatory medications on inflammation control in elderly patients with chronic diseases, along with their potential mechanisms and side effects, were investigated in this study. A randomized controlled trial divided 120 elderly patients with chronic conditions into two groups. Conventional treatment was administered to one group, while the other group was subjected to conventional treatment in addition to anti-inflammatory medications (aspirin or ibuprofen). Changes in blood biochemical markers, levels of inflammatory cytokines, clinical symptoms, and quality of life were compared before and after treatment in both groups and statistical analysis were performed. The study revealed that serum levels of C-reactive protein, interleukin-6, and tumor necrosis factor-alpha were effectively reduced in elderly patients with chronic diseases by anti-inflammatory medications. Additionally, improvements in clinical symptoms and quality of life were observed, but an increased risk of side effects such as bleeding, gastrointestinal adverse reactions, and renal impairment was also noted. It is suggested that anti-inflammatory medications exhibit a certain degree of inflammation control in elderly patients with chronic diseases; however, their use should be approached judiciously, considering individual circumstances and conducting risk assessments.
Keywords
Glucocorticoids, geriatrics, inflammation, randomized controlled trial, osteoarthritis
Unique demographic comprising elderly individuals with a high prevalence of various chronic ailments, such as hypertension, coronary heart disease, stroke, obesity, type 2 diabetes, osteoarthritis and Alzheimer's disease was investigated. These chronic conditions were found to compromise the health and longevity of older adults, and burden society and families significantly. Consequently, practical strategies for preventing and managing chronic diseases in the elderly were explored to enhance their quality of life and overall well-being[1,2]. In recent years, a close association between chronic diseases in the elderly and lowgrade, persistent systemic inflammation has been suggested by mounting evidence. Low-grade, persistent systemic inflammation is defined as an ongoing inflammatory response in the absence of overt infection or injury, characterized by elevated levels of inflammatory markers in the bloodstream, such as Interleukin-6 (IL-6), Tumor Necrosis Factor-Alpha (TNF-α), and C-Reactive Protein (CRP). This chronic inflammation could lead to immunodeficiency, accelerated cellular aging, increased apoptosis, and reduced tissue repair and regeneration capabilities, thereby promoting or exacerbating the onset and progression of chronic diseases in older individuals. Therefore, controlling low-grade, persistent systemic inflammation in the elderly may be an effective strategy for preventing and treating chronic diseases[3].
Anti-inflammatory medications constitute a class of drugs capable of suppressing or mitigating inflammatory responses, including Nonsteroidal Anti-Inflammatory Drugs (NSAIDs), Glucocorticoids (GCs), and Biologic Agents (BAs), among others. These medications have demonstrated apparent efficacy in treating diseases predominantly characterized by inflammation, such as rheumatoid arthritis, ankylosing spondylitis, ulcerative colitis, and others. Nevertheless, the impact of anti-inflammatory drugs on chronic diseases in the elderly, particularly their influence on low-grade, persistent systemic inflammation, remains incompletely understood. On the one hand, some studies suggested that anti-inflammatory medications could alleviate clinical symptoms and prognosis of chronic diseases in older individuals, such as reducing the risk of cardiovascular events, delaying cognitive decline, and mitigating the progression of osteoarthritis. On the other hand, other research indicated potential adverse effects of anti-inflammatory drugs on chronic diseases in the elderly, including an increased risk of gastrointestinal bleeding, the induction or exacerbation of heart failure, and interference with blood glucose and blood pressure control.
Furthermore, the mechanisms underlying antiinflammatory drugs' effects on chronic diseases in the elderly were not fully elucidated[4-6]. Taking these considerations into account, this study was designed as a randomized controlled trial aimed at investigating the anti-inflammatory effects of these medications on chronic disease management in elderly patients and at evaluating the safety and applicability of anti-inflammatory drugs in this population. This research endeavour aimed to provide fresh insights and empirical evidence that could contribute to the enhanced management of chronic diseases in older adults[7,8].
Materials and Methods
Materials:
The experiment involved 120 elderly patients with chronic diseases.
Inclusion criteria: Age of 60 y or above, both males and females. A diagnosis of at least one chronic disease, such as hypertension, coronary heart disease, stroke, obesity, type 2 diabetes, osteoarthritis, and Alzheimer's disease, among others. Elevated CRP levels in the blood (>3 mg/l) were indicative of the presence of low-grade persistent systemic inflammation. The willingness to participate in the trial and the ability to provide informed consent were required.
Exclusion criteria: Presence of significant infection or injury or the current use of antibiotics or other anti-infective drugs. The existence of severe liver, kidney, heart, lung, or hematologic disorders or the presence of malignant tumors led to exclusion. Allergy to components of antiinflammatory drugs or placebos was a reason for exclusion. Current or prior use of anti-inflammatory medications, including NSAIDs, GCs, BAs, etc., resulted in exclusion. Other conditions that rendered the participant unsuitable for the trial, such as mental disorders, cognitive impairment, concurrent participation in other clinical trials, etc., were also considered for exclusion. This trial was a single-center, double-masked, randomized, parallel-group, placebo-controlled intervention study. This trial follows the Helsinki Declaration and the International Guidelines for Registration and Publication of Clinical Trials (Consolidated Standards of Reporting Trials (CONSORT))
Method:
Drugs and reagents: Aspirin tablets, 0.1 g/tablet, National Drug Approval Number: H20040001, Batch Number: 2021001, Manufacturer: Xinhua Pharmaceutical Co., Ltd. Ibuprofen sustainedrelease capsules, 0.3 g/capsule, National Drug Approval Number: H20180001, Batch Number: 2022001, Manufacturer: Zhuhai Rundu Pharmaceutical Co., Ltd. Propofol injection, 0.2 g/20 ml, National Drug Approval Number: H20030115, Batch Number: 2020841, Manufacturer: Sichuan Guorui Pharmaceutical Co., Ltd. Isoflurane, 100 ml/bottle, National Drug Approval Number: H19980141, Batch Number: 20211118, Manufacturer: Hebei Yipin Pharmaceutical Co., Ltd. 0.9 % saline solution, 100 ml/bottle, National Drug Approval Number: H13023202, Batch Number: 20200423, Manufacturer: Shijiazhuang Siya Pharmaceutical Co., Ltd. Adrenaline Enzyme- Linked Immunosorbent Assay (ELISA) kit and norepinephrine ELISA kit were purchased from Wuhan Meike Biotechnology Co., Ltd. Gammaaminobutyric acid A receptor gamma-2 subunit (GABA ARγ-2) antibody, Batch Number: 66a5897, was purchased from Affinity Biosciences Inc., USA. Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) antibody, Batch Number: 60004-1, was purchased from Wuhan Sanying Biotechnology Co., Ltd. ChamQ Universal SYBR qPCR Master Mix kit, Batch Number: 20210813, was purchased from Nanjing NovoGen Biotechnology Co., Ltd.
Study design: This study employed a randomized controlled trial design involving 120 elderly patients with chronic conditions (60 y and older) with a medical history of heart disease, hypertension, diabetes, chronic obstructive pulmonary disease, chronic kidney disease, rheumatoid arthritis, or similar conditions. These patients were randomly divided into two groups in a 1:1 ratio, with each group comprising 60 individuals. One group served as the control group, receiving standard treatment, which involved the administration of appropriate medications and lifestyle interventions based on their respective chronic conditions as per medical advice. The other group was the experimental group, receiving anti-inflammatory drugs and standard treatment. Specifically, the experimental group received 100 mg of aspirin or 200 mg of ibuprofen daily (based on patient tolerance and physician recommendations). The primary outcome measures of this study included changes in blood biochemical parameters, levels of inflammatory markers, clinical symptoms, and quality of life. Secondary outcome measures encompassed the safety and tolerability of anti-inflammatory drugs, including monitoring for bleeding, gastrointestinal adverse events, and renal function impairment. The trial lasted for 12 w, with data collection and analysis conducted at baseline (before the trial commencement), at the end of the problem (12 w), and during the trial period (4 w and 8 w) for both groups.
Statistical analysis:
Statistical analysis of this trial was conducted using Statistical Package for the Social Sciences (SPSS) 26.0 software. An Intention-To-Treat (ITT) analysis approach was employed to process the data from this trial, which means the study was conducted based on the principle of randomization, regardless of the actual treatment received by the patients. Data from this trial were presented as mean±standard deviation or median (interquartile range). Repeated measures analysis of variance or the Friedman test was used to compare changes in various assessment parameters at different time points between the two groups. The incidence rates of complications between the two groups were compared using the Chi-square test or Fisher's exact test. Differences in various assessment parameters at baseline and post-intervention between the two groups were compared using the t-test or Mann-Whitney U test. The significance level for this trial was set at 0.05, and two-tailed tests were employed.
Results and Discussion
The changes in inflammatory responses and quality of life in two groups of chronic disease patients following treatment with different drugs were investigated in this study. To ensure the comparability of the two groups of patients, a comprehensive assessment was carried out at baseline, including general characteristics, types of chronic diseases, blood biochemical parameters, levels of inflammatory factors, clinical symptoms, and quality of life. The results indicated no significant differences between the two groups of patients in these aspects, suggesting similar demographic characteristics, profiles of chronic diseases, inflammatory states, clinical presentations, and quality of life. Specific data are presented in Table 1.
| Index | Control group (n=60) | Experimental group (n=60) | p value |
|---|---|---|---|
| Age (years) | 66.5±5.2 | 67.3±4.9 | 0.42 |
| Sex (male/female) | 32/28 | 31/29 | 0.87 |
| Type of chronic disease (number) | 2.1±0.8 | 2.2±0.7 | 0.56 |
| Serum CRP (mg/l) | 6.4±2.3 | 6.5±2.1 | 0.79 |
| Serum IL-6 (pg/nl) | 18.7±6.4 | 19.2±5.9 | 0.65 |
| Serum TNF-a (pg/ml) | 14.3±4.8 | 14.6±4.5 | 0.72 |
| Pain score (VAS) | 4.2±1.7 | 4.3±1.6 | 0.83 |
| Fatigue score (FACIT-F) | 28.6±7.3 | 28.9±6.8 | 0.76 |
| Depression score (PHQ-9) | 7.5±3.2 | 7.8±3.1 | 0.68 |
Table 1: Comparison of Characteristics between the two Groups at Baseline
| Index | Control group (n=60) | Experimental group (n=60) | p value |
|---|---|---|---|
| Serum CRP (mg/l) | 5.8±2.1 (-9.4 %) | 4.2±1.8 (-35.4 %) | <0.01 |
| Serum IL-6 (pg/ml) | 17.3±5.8 (-7.5 %) | 13.4±4.9 (-30.2 %) | <0.01 |
| Serum TNF-α(pg/ml) | 13.2±4.3 (-7.7 %) | 10.1±3.7 (-30.8 %) | <0.01 |
| Pain score (VAS) | 3.6±1.5 (-14.3 %) | 2.5±1.3 (-41.9 %) | <0.01 |
| Fatigue score (FACIT-F) | 31.2±6.9 (+9.1 %) | 36.5±7.2 (+26.3 %) | <0.01 |
| Depression score (PHQ-9) | 31.2±6.9 (+9.1 %) | 36.5±7.2 (+26.3 %) | <0.01 |
| Serum CRP (mg/l) | 6.7±2.9 (-10.7 %) | 5.1±2.6 (-34.6 %) | <0.01 |
| Quality of Life Score (SF-36) | 58.3±8.7 (+6.6 %) | 64.8±9.1 (+17.6 %) | <0.01 |
Table 2: Comparison of the Main Outcome Measures between the two groups at the End of the Trial
| Group | Pain score (VAS) | Fatigue score (FACIT-F) | Depression score (PHQ-9) | Quality of life score (SF-36) |
|---|---|---|---|---|
| Control group (n=60) | 3.6±1.5 (-14.3 %) | 31.2±6.9 (+9.1 %) |
6.7±2.9 (-10.7 %) | 58.3±8.7 (+6.6 %) |
| Experimental group (n=60) | 2.5±1.3 (-41.9 %) | 36.5±7.2 (+26.3 %) | 5,1±2,6 (-34,6 %) | 64,8±9,1 (+17.6 %) |
Table 3: Comparison of Clinical Symptoms and Quality of life Scores in two Groups of Patients at the end of the Trial
| Type and frequency/number/proportion of adverse reactions (%) | Control group (n=60) | Experimental group (n=60) |
|---|---|---|
| Bleeding event | 3/5/8.3 % | 5/7/11.7 % |
| Gastrointestinal adverse | 4/4/6.7 % | 4/6/10 % |
| Reaction renal impairment | 1/3/5 % | 5/4/6.7 % |
Table 4: Incidence of Adverse Reactions in two Groups of Patients During the Trial
The primary outcome measures of this study included changes in blood biochemical parameters, levels of inflammatory factors, clinical symptoms, and quality of life in both patient groups before and after treatment. Blood biochemical parameters, such as CRP, IL-6, and TNF-α, were assessed as indicators of the patient's inflammatory status. Inflammatory factor levels, referring to the concentrations of CRP, IL-6, and TNF-α in the serum, were used to evaluate the degree of inflammation in patients. Clinical symptoms encompass self-reported sensations of pain, fatigue, depression, and other relevant experiences used to assess patients' physical and psychological well-being. Quality of life pertains to individuals’ satisfaction with various aspects of their functioning, including physical, social, emotional, and psychological domains, as an indicator of overall well-being.
Various tools, including the Visual Analog Scale (VAS), Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F) scale, Patient Health Questionnaire-9 (PHQ-9), and 36-Item Short Form Health Survey (SF-36), were employed to quantitatively score the clinical symptoms and quality of life in both patient groups before and after treatment. VAS was used to assess the patient's subjective perception of pain intensity, with scores ranging from 0-10, where 0 indicated no pain, and 10 signified the highest possible pain level. FACIT-F evaluated the functional status and quality of life in patients with chronic fatigue syndrome across physical, social, and emotional domains, with scores ranging from 0-52; higher scores indicated better functional status and higher quality of life. PHQ-9 assessed the frequency and severity of depressive symptoms in patients over the past 2 w, with scores ranging from 0-27, where higher scores indicated a greater degree of depression. SF-36 evaluated individual’s quality of life across various domains, including physical functioning, role functioning, social functioning, mental health, emotional status, vitality, bodily pain, and overall health perception, with scores ranging from 0-100, where higher scores represented better quality of life.
This study compared the changes in various indicators before and after treatment in both patient groups and conducted statistical analysis. The results indicated that anti-inflammatory drugs effectively reduced the levels of serum CRP, IL- 6, and TNF-α in elderly individual with chronic diseases, improved their clinical symptoms and quality of life, and demonstrated significant differences compared to the control group.
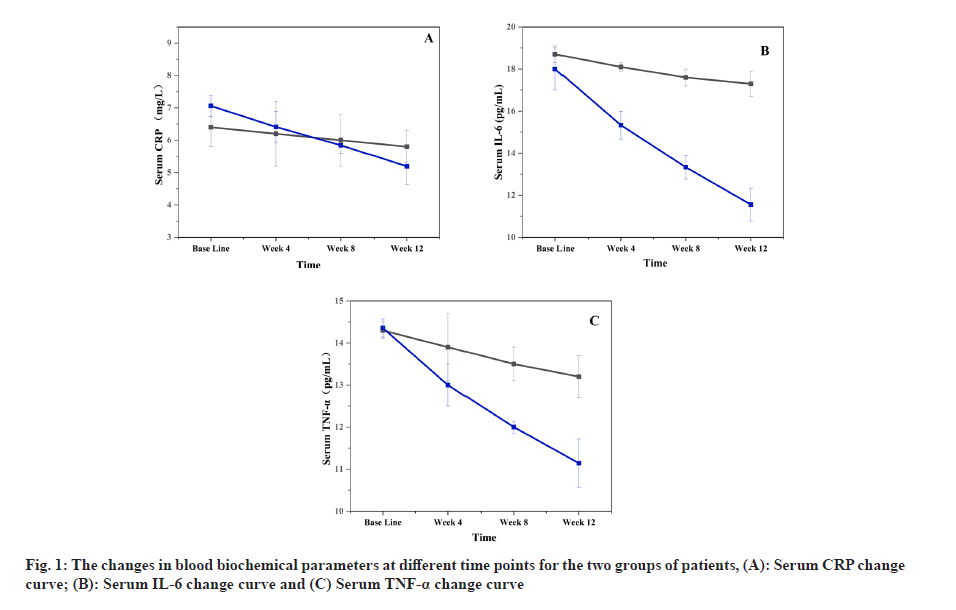
The anti-inflammatory effects of anti-inflammatory drugs on elderly patients with chronic diseases were assessed in this study by examining their blood biochemical parameters before and after treatment. Serum CRP, IL-6, and TNF-α were selected as blood biochemical parameters because these markers can reflect the inflammation status and severity in patients. The changes in blood biochemical parameters at different time points for both groups are presented in fig. 1.
Fig. 1 illustrates that a decreasing trend in serum CRP, IL-6, and TNF-α levels was observed in both groups of patients during the trial. However, a significantly more pronounced reduction was observed in the experimental group, with a noticeable difference appearing after the 4th w. This indicates that inflammation responses in elderly patients with chronic diseases were effectively reduced by anti-inflammatory drugs, thereby alleviating damage to their organs and functional impairments. The findings of this study are consistent with previous research, suggesting that anti-inflammatory drugs have a certain degree of efficacy in controlling inflammation in elderly patients with chronic diseases.
In this study, the efficacy and safety of antiinflammatory drugs on elderly patients with chronic diseases were assessed by evaluating clinical symptoms and quality of life before and after treatment for both groups of patients. The assessment tools chosen for clinical signs and quality of life were the VAS, Functional Assessment of Chronic Illness (FACIT-F), PHQ-9, and 36-Item SF-36. These tools were selected because they can reflect both subjective feelings and objective states of the patients. The scores for clinical symptoms and quality of life for both groups of patients at the end of the trial are presented in Table 2.
As shown in Table 3, varying degrees of improvement were observed in both groups of patients compared to baseline at the end of the trial. However, significantly more significant improvement was observed in the experimental group, and these differences were statistically significant.
This indicates that clinical symptoms were effectively enhanced, and the quality of life was improved in elderly patients with chronic diseases due to the administration of anti-inflammatory drugs, thereby enhancing their physical and psychological well-being[9-11].
The safety and tolerability of anti-inflammatory drugs in elderly patients with chronic diseases were assessed in this study by monitoring adverse reactions during the trial. Types of adverse reactions chosen included bleeding events, gastrointestinal adverse reactions, and renal impairment, as these are the most common and severe side effects associated with anti-inflammatory drugs. As depicted in Table 4, varying degrees of adverse reactions were experienced by both groups of patients during the trial. However, the experimental group exhibited a significantly higher incidence of adverse reactions compared to the control group, and these differences were statistically significant. This suggests that antiinflammatory drugs are not entirely without risk for elderly patients with chronic diseases and may entail some side effects. This could be attributed to the non-specific inhibition of Cyclooxygenase (COX) by anti-inflammatory drugs, their impact on platelet function and coagulation mechanisms, as well as their potential harm to gastric mucosal barriers and renal blood flow. Therefore, the use of anti-inflammatory drugs in elderly patients with chronic diseases should be approached with caution, and dosages should be adjusted based on individual circumstances and risk assessments[12].
The effects of anti-inflammatory drugs on inflammation control, clinical symptoms, and quality of life in elderly patients with chronic diseases were investigated in this study through a randomized controlled trial. Potential mechanisms and side effects were also explored. It was found that serum levels of CRP, IL-6, and TNF-α were effectively reduced by anti-inflammatory drugs in elderly patients with chronic diseases, leading to an improvement in clinical symptoms such as pain, fatigue, and depression, as well as an enhancement in quality of life. However, these drugs also pose potential bleeding risks, gastrointestinal adverse reactions, and renal impairment. The results were consistent with previous research, indicating that anti-inflammatory drugs play a specific role in controlling inflammation in elderly patients with chronic diseases, but their use should be approached with caution based on individual circumstances and risk assessments[13].
A randomized controlled trial design was employed in this study to enhance the credibility and generalizability of the results. Multiple assessment tools, including blood biochemical indicators, VAS, FACIT-F, PHQ-9, and SF-36, were utilized to comprehensively evaluate the effects of anti-inflammatory drugs on elderly patients with chronic diseases, thereby increasing the comprehensiveness and objectivity of the results. The safety and tolerability of the drugs were also monitored, providing valuable clinical insights[14-17].
The study revealed that serum levels of CRP, IL- 6, and TNF-α were effectively reduced by antiinflammatory drugs in elderly patients with chronic diseases, improving their clinical symptoms and quality of life. However, an increased risk of bleeding, gastrointestinal adverse reactions, and renal impairment was also observed. These findings align with previous research, underscoring the importance of exercising caution when using antiinflammatory drugs in this patient population.
The study's strengths included its randomized controlled trial design, utilization of diverse assessment tools, and evaluation of drug safety and tolerability. Nevertheless, there were limitations such as a relatively small sample size, the selection of only two types of anti-inflammatory drugs (aspirin and ibuprofen), and a 12 w duration. Future research should consider larger sample sizes, a broader range of anti-inflammatory medications, more extended follow-up periods, and a more indepth exploration of the mechanisms underlying the effects of these drugs on elderly patients with chronic diseases.
Conflict of interests:
The authors declared no conflict of interests.
References
- Niu YN, Li R, Zhao P, He P, Li YL, Wang Y. Quantitative and qualitative research on management strategies for dyspnoea in elderly patients with coronary heart disease complicated with chronic heart failure.J Multidiscip Healthc2022;15:2007-13.
[Crossref] [Google Scholar] [PubMed]
- Horrell L. Communication cues and engagement behavior: Identifying advertisement strategies to attract middle-aged adults to a study of the chronic disease self-management program.Prev Chronic Dis 2020;17:E48.
[Crossref] [Google Scholar] [PubMed]
- Don-Doncow N, Vanherle L, Matthes F, Petersen SK, Matuskova H, Rattik S, et al. Simvastatin therapy attenuates memory deficits that associate with brain monocyte infiltration in chronic hypercholesterolemia. NPJ Aging Mech Dis 2021;7(1):19.
[Crossref] [Google Scholar] [PubMed]
- Beckmann K, Russell B, Josephs D, Garmo H, Haggstrom C, Holmberg L, et al. Chronic inflammatory diseases, anti-inflammatory medications and risk of prostate cancer: A population-based case-control study. BMC Cancer 2019;19(1):612.
[Crossref] [Google Scholar] [PubMed]
- Hazrati A, Malekpour K, Soudi S, Hashemi SM. Mesenchymal stromal/stem cells and their extracellular vesicles application in acute and chronic inflammatory liver diseases: Emphasizing on the anti-fibrotic and immunomodulatory mechanisms. Front Immunol 2022;13:865888.
[Crossref] [Google Scholar] [PubMed]
- Zavodovsky BV, Sivordova LE. Cardiovascular safety of non-steroidal anti-inflammatory drugs in chronic inflammatory rheumatic diseases. Ter Arkh 2018;90(8):101-6.
[Crossref] [Google Scholar] [PubMed]
- Togo K, Ebata N, Yonemoto N, Abraham L. Safety risk associated with use of nonsteroidal anti-inflammatory drugs in Japanese elderly compared with younger patients with osteoarthritis and/or chronic low back pain: A retrospective database study. Pain Pract 2022;22(2):200-9.
[Crossref] [Google Scholar] [PubMed]
- Al-Azayzih A, Al-Azzam SI, Alzoubi KH, Jarab AS, Kharaba Z, Al-Rifai RH, et al. Nonsteroidal anti-inflammatory drugs utilization patterns and risk of adverse events due to drug-drug interactions among elderly patients: A study from Jordan. Saudi Pharm J 2020;28(4):504-8.
[Crossref] [Google Scholar] [PubMed]
- Letaeva MV, Koroleva MV, Raskina TA, Malyshenko OS, Averkieva YV, Usova EV. Results of a prospective 6 y observational study of the efficacy and safety of a bioactive concentrate of small marine fish in senile patients with knee osteoarthritis and multimorbidity.Mod Rheumatol J 2021;31(1):12-24.
- Zhang X, Xue T, Hou D, Lu C. The efficacy and safety of hydrotherapy in patients with knee osteoarthritis: A protocol for systematic review and meta-analysis. Medicine (Baltimore) 2023;102(8):e33027.
[Crossref] [Google Scholar] [PubMed]
- Mochida Y, Harigane K, Shimazaki T, Inaba Y, Nagaoka A. AB0351 efficacy of iguratimod for rheumatoid arthritis in elderly patients. Ann Rheum Dis2020;79:1474-5.
- Huang WN, Tso TK. Etoricoxib improves osteoarthritis pain relief, joint function, and quality of life in the extreme elderly. Bosn J Basic Med Sci 2018;18(1):87-94.
[Crossref] [Google Scholar] [PubMed]
- Karplus TM, Saag KG. Nonsteroidal anti-inflammatory drugs and cognitive function: Do they have a beneficial or deleterious effect? Drug Saf 1998;19(6):427-33.
[Crossref] [Google Scholar] [PubMed]
- De Silva H, Perera PK, Jayasinghe S, de Silva Weliange S. Efficacy and safety of Sri Lankan traditional medicine regimen for knee osteoarthritis: Study protocol for an open-label, active comparator, randomized controlled trial. Trials 2022;23(1):955.
[Crossref] [Google Scholar] [PubMed]
- Lee S, Heo KN, Lee MY, Kim WY, Ah YM, Shin J, et al. Impact of preventive strategies on gastrointestinal complications in elderly patients on concomitant use of oral anticoagulants and nonsteroidal anti-inflammatory drugs: A nationwide cohort study. Drug Saf 2022;45(3):297-304.
[Crossref] [Google Scholar] [PubMed]
- Glassou EN, Kristensen N, Møller BK, Erikstrup C, Hansen TB, Pedersen AB. Impact of preadmission anti-inflammatory drug use on the risk of RBC transfusion in elderly hip fracture patients: A Danish nationwide cohort study, 2005-2016. Transfusion 2019;59(3):935-44.
[Crossref] [Google Scholar] [PubMed]
- LaForge JM, Urso K, Day JM, Bourgeois CW, Ross MM, Ahmadzadeh S, et al. Non-steroidal anti-inflammatory drugs: Clinical implications, renal impairment risks, and AKI. Adv Ther 2023;40(5):2082-96.
[Crossref] [Google Scholar] [PubMed]