- Corresponding Author:
- K. Dixit
Department of Pharmaceutical Sciences, Dr. H. S. Gour University, Sagar-470 003, India.
E-mail: vkdixit111@rediffmail.com
| Date of Submission | 16 May 2005 |
| Date of Revision | 10 October 2005 |
| Date of Acceptance | 16 July 2006 |
| Indian J Pharm Sci, 2006, 68 (4): 448-455 |
Abstract
Transformed dedifferentiated cultures of Artemisia annua L. were established using four wild type strains of Agrobacterium tumefaciens . From the Ti-transformed in vitro gall calli so obtained cell suspension cultures were developed. Growth kinetics of Ti-transformed cell suspension cultures were studied and compared with untransformed (control) cultures. Ti transformed cell suspension cultures were found to exhibit faster growth rate than the control. Gall callus synthesized 0.2011 g/100 g DW of artemisinin while only 0.0178 g/100 g DW was detected in untransformed callus cultures Likewise. 38.6 µg/ml was found in spent medium of cell suspension culture at day 21, which shows a five fold increase in artemisinin over control (7.5 µg/ml).Cell suspension cultures are found to be practically more feasible than static cultures, hence an empirical approach is taken to scale up the Ti-transformed cell suspension culture in laboratory scale bioreactor (Batch process). The bioreactor cultivation yielded 45.56 µg/ml of artemisinin at day 21.The result suggests that the Erlenmeyer flasks results could be successfully scaled-up into the bioreactor and condition can be further optimized to have best artemisinin yield.
About 8000 herbal remedies are codified in Ayurveda and are still in use in many areas today. Large numbers of bioactive compounds, obtained from herbs are life saving drugs [1]. In recent years market of plant products expand rapidly and this trend will continue in the 21st century because more and more people prefer natural products. Many of these products are difficult to synthesize chemically or difficult to produce in large amounts. In this perspective plant tissue culture technology holds promise specially plant cell cultures has been looked at as a potential alternative for efficient production of natural Tibioactive compounds [2,3].
The plant Artemisia annua Linn. has been used in China as a medicinal herb having antimalarial properties for nearly 2000 y [4]. Artemisinin (AN) obtained from A. annua is rapidly acting schizontocide that is effective against chloroquine-sensitive and resistant strains of Plasmodium as well as in cerebral malaria, which is the most lethal stage of disease [5,6]. The compound occurs in low yield in the plant 0.01-0.5% w/w of tissue dry weight [7-9] and also chemical synthesis is economically unattractive.
AN is well established antimalarial molecule and has long been explored through tissue culture technology. In vitro shoot cultures have shown to yield 0.16% w/w AN [10], while 0.42% w/w {Dry weight (DW)} has been reported in hairy root cultures [11]. Though the plant cells cultured in suspensions have all the properties that can be explored for large scale production, there is only one report indicating 8μg/ml AN in cell suspension cultures [12]. Recently Agrobacterium tumefaciens mediated transformation has been found effective for the study of shoot derived secondary product formation in vitro [9]. Hence the studies have been carried out on Ti transformed culture of A. annua for improving the yield of AN.
Materials and Methods
Sources of explants
The seeds of A. annua were procured from medicinal garden, Department of Pharmaceutical Sciences, Sagar (M.P.). The seeds were sterilized by treating first with 90% alcohol for 3 s, followed by 0.1% (w/v) mercuric chloride for 5 m. The seeds thus sterilized were then rinsed thoroughly 3 times with sterile water and allowed to germinate on MS media supplemented with 5% sucrose along with padding of filter paper and absorbent cotton moistened with sterile water. The seeds were then incubated at 25±2° with 16-8 h photoperiod. Germination started within 2-3d, since around 2-3 days were required for germination to commence. Explants utilized for the studies included entire seedling, epicotyl and hypocotyl segments of 14 d old aseptically germinated seedlings and lamina segments of 3 mo old field grown plants.
Callus and cell suspension cultures of A. annua
All the explants were appropriately sized and aseptically transferred to culture tubes containing MS medium with Naphthalene acetic acid (NAA) 0.5 ppm + Kinetin (Kn) 0.5 ppm and incubated at 25±2° in white cool fluorescent light (1600 lux) with 16/8 h photoperiod at 50-60% relative humidity. Suspension cultures were initiated by transferring the most friable segments of epicotyl callus to the Erlenmeyer flasks (250 ml) each containing 50 ml of MS medium with NAA (0.1 ppm)+Kn (0.1 ppm). The contents were agitated on Remi rotary flask shaker at 100110 rpm at temperature 25±2° in low intensity light.
Bacterial strains
Four different strains of Agrobacterium were used for genetic transformation. This includes, A. tumefaciens MTCC 431 (ATCC 15955), A. tumefaciens MTCC 2250 (DSM 30199), A. tumefaciens MTCC 609 (ATCC33970) and A. tumefaciens MTCC 2251 (DSM 30200). All the bacterial strains were obtained from the Institute of Microbial Technology (IMTECH) Chandigarh.
Triparental mating
Triparental mating was performed for introduction of GUS, a reporter gene for selection of transformed tissue, into the cells of Agrobacterium strains. E. coli strain pBI 121, grown on LB medium with kanamycin (50 μg/ml) at 37° overnight was utilized. pBI 121 was grown along with the strains of Agrobacterium, which were separately maintained on LB medium with the following antibiotics at 28°: A. tumefaciens MTCC 431 with ampicillin (25 μg/ml), A. tumefaciens MTCC 2250 with ampicillin (25 μg/ml), A. tumefaciens MTCC 609 with Streptomycin (25 μg/ml) and A. tumefaciens MTCC 2251 with ampicillin (25 μg/ml).
Each of the four strains of Agrobacterium thus obtained was streaked individually on minimal medium with kanamycin and the antibiotic to which the particular strain is resistant followed by incubation for 3-4 d at 28°. On every 3rd or 4th d the colonies were streaked on the fresh medium.
Infection with A. tumefaciens and culture of tumorous tissue
All the explants (seedlings and lamina) were surface sterilized by treating first with 90% alcohol for 3 s, followed by 0.1% (w/v) mercuric chloride for 2-3 m. Explants were then thoroughly rinsed 3 times with sterile water, pricked with a needle and immersed in bacterial suspensions of each strain having GUS gene. In vitro explants were then placed on sterile Petri dishes containing sterile filter paper soaked with liquid MS medium without growth hormones, for 24 h. Co-cultivation was carried out under controlled environmental conditions viz. 16/8 h photoperiod at 3000 lux intensity light and 24±2° temperature.
After co-cultivation, the infected explants were washed with sterile water. The explants were then transferred to solid MS medium lacking in growth hormones but containing cefotaxime (500 mg/l). The explants were allowed to grow under 16/8 h photoperiod at 24°. Approximately 3 weeks later, the tumors were transferred to fresh MS medium devoid of growth hormones but containing cefotaxime 250 mg/l. Tumors were sub cultured every 2 w. Cefotaxime concentration in medium was gradually reduced and totally omitted after 12 w. Control callus and untransformed cell suspension cultures were also cultivated simultaneously under identical conditions.
Confirmation of transformation
A. tumefaciens transformed cells usually synthesize novel amino acids called opines. Detection of opines is a firm indication of transformation in tissues and these can be detected by electrophoresis. The tissues were homogenized with 0.1 N hydrochloric acid and centrifuged. The supernatant was collected and subjected to electrophoresis. The supernatant was applied on Whatman Paper No. 1, wetted with the electrophoretic buffer [formic acid:acetic acid:water (5:15:80)] and dried in air. These strips were then subjected to electrophoresis for 1 h at 300 V.
Electrophoretograms were dipped in silver nitrate solution (0.25 g of silver nitrate in 0.25 ml of water and diluted to 50 ml with acetone) and air dried for 20 min followed by development with methanolic sodium hydroxide (20% w/v NaOH mixed with 90 ml of methanol) and air drying for 30 m. These electrophoretograms were fixed by immersing in 5% w/v sodium thiosulfate solution. Appearance of black spots on electrophoretogram shows presence of opines in transformed tissue.
Histochemical GUS assay was performed on developing callus to verify the transformation. Expression of GUS reporter gene leads to formation of ß-glucuronidase enzyme which can be detected by using GUS stain [13].
Growth kinetics and product formation
Growth kinetic studies were carried out on three samples withdrawn at weekly intervals and various parameters for callus as well as cell suspension cultures were studied. Fresh weights (FW) for callus cultures were determined at 2 w intervals up to 8 w and growth indices were determined. In case of cell suspension cultures, growth parameters such as FW, DW, PCV and Cell count/ mm3 were studied for 28 d. Depletion of nutrient in the media as a result of utilization for growth was also studied to determine the growth kinetics. Samples of the media were withdrawn every week and the sucrose content was estimated using anthrone reagent [14]. Calibration curve was prepared using fresh solution of sucrose (1 mg/ml).
Analysis of artemisinin
Quantitative analysis of AN was performed according to method of Zhao and Zeng [15]. Samples of different callus and cell suspension cultures were dried under vacuum and extracted with diethylether for 48 h. The filtrate so obtained was again dried under vacuum and the residue was dissolved in 1 ml methanol followed by derivatization by treating with 4 ml NaOH (0.2%) and incubating at 50° for 30 m. After cooling to room temperature the solution was neutralized with 5 ml of Glacial acetic acid (0.2 M in 20% MeOH). AN was then analyzed using HPLC using Methanol: 0.1 mmol phosphate buffer pH 7.9 (40:60) as mobile phase on LunaC-18-ODS, 250 × 4.6 mm column having photo diode array detector (Shimadzu).
Culture of ti-transformed cells in bioreactor
Plant cell cultures have been looked at as a potential alternative for production of natural bioactive compounds [2,3]. As suspended cells can be accommodated in large-scale bioreactor, their biosynthetic potential can be utilized via biomass production. From the results of cell suspensions transformed with 4 different strains of A. tumefaciens, the one having best growth as well as maximum AN content was selected and an attempt was made to scale up the cell suspension culture in 1.5l capacity bioreactor (batch process).
Inoculum
The transformed cultures of A. annua (Agrobacterium tumefaciens MTCC 2250) were subcultured every 2 w. 10.0 ml of the cultures of A. annua were inoculated in 50 ml of fresh MS medium lacking in growth hormones in a 125 ml Erlenmeyer flask and incubated at 25±2° at 100-110 rpm on rotary shakers. A ratio of 1:2 for inoculum and new media is generally recommended in bioreactor. 200 ml of inoculum was thus prepared for 1 l of suspension culture. Preparation of inoculum was undertaken 2 w before seeding in bioreactor in sufficient amount so that extra quantity of inoculum is available.
Preparation of bioreactor
The bioreactor was cleaned properly and then it was filled with the MS medium (800 ml). The bioreactor along with the medium was autoclaved at 121° and 15 lb pressure for 45 m and cooled.
Inoculation and sampling
After 14 days the inoculum (200 ml) was ready. The inoculum was then transferred aseptically to the bioreactor under laminar air-flow and then the bioreactor was closed properly and assembled. The reactor was allowed to run at 25±2° and 150 rpm with aeration. 50 ml samples of cell suspension were withdrawn weekly and growth parameters were determined.
Results and Discussion
Seeds were germinated on three different media and almost 100% germination observed without any contamination. Seed germination started from 3-4th d and within 8-10 d 100% germination was observed in seeds placed on filter paper-absorbent cotton wool padding and also in seeds placed on seedling growth medium solidified with 0.6% agar. In seeds placed on solidified MS medium supplemented with 5% sucrose, germination was observed after 4-6 d with simultaneous callus initiation.
The callus was initiated on optimized MS medium containing 0.5 ppm NAA+0.5 ppm BAP (6-benzyl aminopurine). Calluses were initiated on all explant types within 12-14 d. Callus induction capacity was found to be maximum with aseptically germinated entire seedlings (80%) and cultivated plant Lamina segments (80%). In epicotyl explants callus induction (75%) was comparable with callus induction in entire seedling and lamina segments, while only 40% of hypocotyl explants exhibited callus initiation.
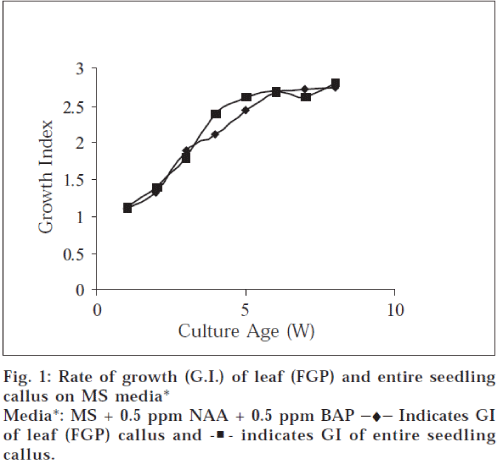
Best callus growth was observed when entire seedling (aseptically germinated) and leaf segments of field grown plant were used as explant, which was indicated by their growth indices (field grown plant leaf 2.75 and entire seedling callus 2.81 at 8 w, fig. 1).
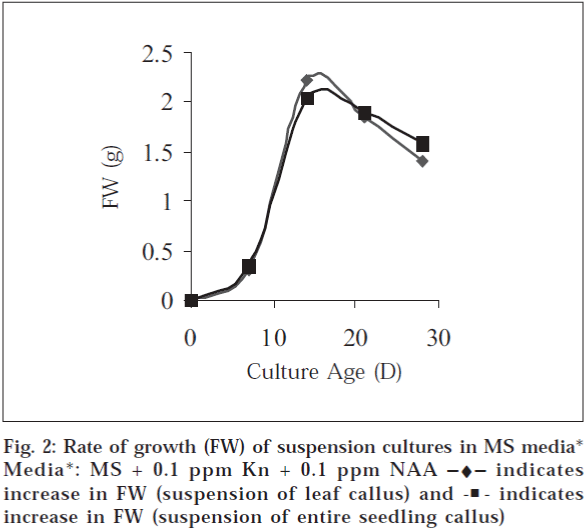
In case of suspension cultures initiated from leaf (FGP) callus 1.30±0.40 g (FW) and from entire seedling callus 1.34±0.04 g (FW) was obtained at 7th d of culture. Maximum biomass (FW g) obtained at day 15, 3.210±0.17 and 3.23±0.07 g for suspensions initiated form leaf callus and entire seedling callus respectively and then it declines at day 21 and 28 (fig. 2). This profile suggests lag phase (slow growth) up to 7 d, which is followed by rapid growth of cells up to 15 d resulting in maximum biomass. After this, the cells enter in stationary phase and a decline in growth rate due to cell death is noted.
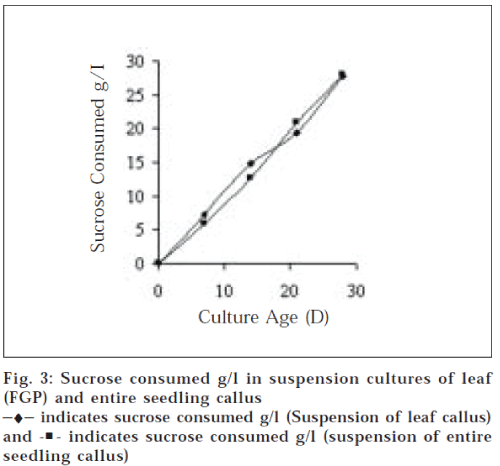
Nutrient depletion from media is indicated by sucrose content. Sucrose consumption was 19.4±0.33 and 27.8±50.18 g/l at day 21 and 28 respectively in case of suspension of leaf (FGP) callus and 20.9±0.14 and 27.91±0.22 g/l at day 21 and 28, respectively in suspension of entire seedling callus. The data shows that nutrient concentration gradually decreases and most of the nutrients are depleted from 3rd to 4th w (fig. 3). This suggests that from the medium nutrients are first taken up by the cells and when the cell reaches stationary phase, these are utilized for secondary metabolite production.
A. tumefaciens mediated genetic transformation of A. annua resulted in crown gall formation and efficiency of these in vitro culture systems for secondary metabolites production was studied using following four Agrobacterium strains having GUS reporter gene. A. tumefaciens MTCC 431, MTCC 2250, MTCC 609 and MTCC 2251. The callus initiation started within 14-18 d of infection in case of MTCC 431 and MTCC 2250, while initiation was late (21-30 d) in case of strain MTCC 2251. No callus formation observed in MTCC 609 strain.
The frequency of gall formation (transformation efficiency) varied with different types of explant and also with different types of strains. Cultivated plant lamina shows maximum transformation efficiency (72%) with MTCC 431 strain. Also good frequency of gall formation (64%) was observed with MTCC 2250 strain. MTCC 2251 strain was found to be the least effective in producing transformants. The transformation efficiency was found to be 30.2% with cultivated plant lamina and 26.7%, with entire seedlings, also a poor Aseptically germinated seedlings, MTCC 2250 showed maximum tumor formation frequency (76.6%), while it was 58.5% with MTCC 431. In MTCC 2250 infected entire seedling highly proliferating gall callus was observed. Thus both the field grown as well as in vitro grown plants of A. annua were found to be prone to Agrobacterium infection with MTCC 2250, MTCC 431 and MTCC 2251, while MTCC 609 strain failed to induce tumorigenesis in both the explants.
Tumorigenesis depended on two key factors: (a) choice of explants; (b) strain type. This differential response to Agrobacterium infectivity has been shown to depend on interaction between bacterial strains, types of explants and physiological state of the explants for other species [16-19].
The callus initiation started within 14-18 days of infection in case of MTCC 431 and MTCC 2250, while initiation was late (21-30 d) in case of strain MTCC 2251. No callus formation observed in MTCC 609 strain. The transformation was confirmed by carrying out Opine assay and GUS histochemical staining. Opines synthesized by Agrobacterium transformed cells were detected by electrophoresis and the calli transformed with MTCC 431, MTCC 2250 and MTCC 2251 showed presence of opines which confirms transformation.
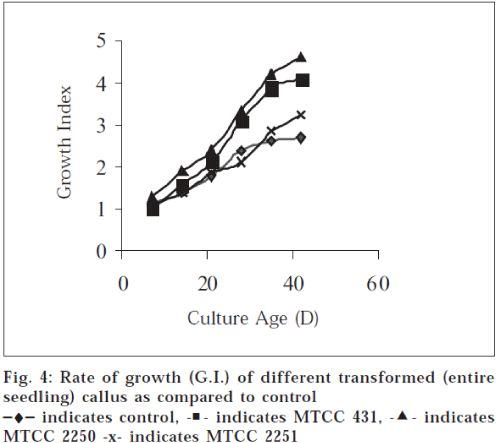
Growth index (GI) of transformed callus cultures from different explant sources and the different types of strains demonstrated rapid growth rate. Maximum growth rate was observed in entire seedling callus transformed with MTCC 2250 strain (GI, 4.72 at 6 w). Also appreciable growth rate was observed with lamina (FGP) (GI, 4.16 at 6 w) and entire seedlings (GI, 4.09 at 6 w) transformed with MTCC 431, while relatively slow growth rate was recorded in case of explants transformed with MTCC 2251. Transformed cultures show higher growth rate and greater biomass content than control (GI, 2.75 at 6 w) cultures as apparent from growth curve (fig. 4)
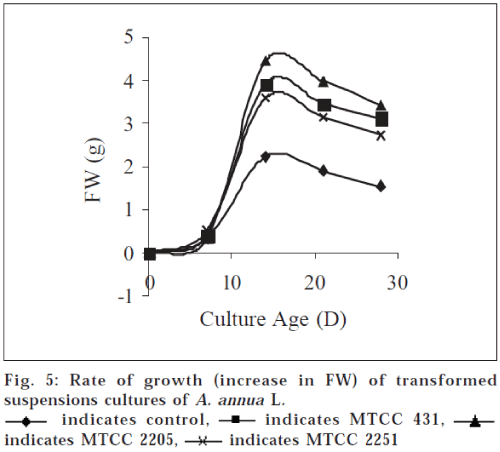
Among the transformed suspension cultures of all the three strains maximum biomass was obtained in MTCC 2250 transformed cultures (d 15). Transformed suspension yields 8.840±0.95 g FW (biomass at d 15) which is significantly higher (P<0.05) than untransformed cultures 3.23±0.07 g at d 15 (fig. 5). Transformed suspension also follow the same pattern as untransformed cultures suggesting that a lag phase exists up to 7 d of culture followed by rapid cell growth and maximum biomass (8.8400.95 g FW) was obtained at d 15 of the culture, after which there is decline in growth rate. When the biomass yields are compared with control cultures, 2-4 fold increase was observed in transformed cell cultures. This increase in biomass is attributed to tumorous nature of cell growth and cytokinin synthesis by the transformed cells.
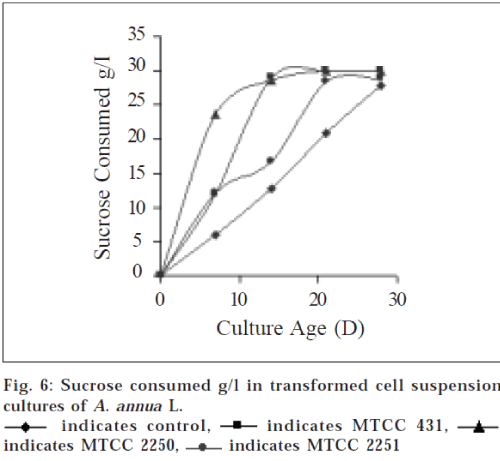
Nutrient depletion from the media in terms of amount of sucrose, was monitored regularly. Sucrose concentrations decreased rapidly from d 7 in case of transformed cell suspensions. Amount of sucrose consumed in case of transformed cell suspensions with MTCC 431 and MTCC 2250, were recorded at d 14 it was 29.00.33 and 29.3±0.16 g/ l respectively and up to d 21 it was completely depleted (fig. 6). It did not implicate that the growth stops at this stage (2 w), apparently it took time before absorbed nutrients were used for growth and the secondary metabolites are often expressed towards the end of a growth cycle, at the time primary nutrients become limited [10].
Suspension cultures of MTCC 2251 strain also followed the same pattern of utilization of sucrose at d 21 (28.34±0.13 g/l) and d 28 (28.80±0.09 g/l), however, the growth rate was found to be slow. This kinetic parameter suggests that growth rate of transformed cell cultures of MTCC 2250 is maximum and significantly higher (P<0.05) as compared to control cultures.
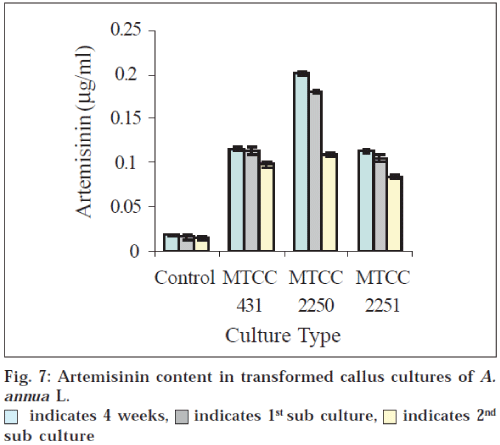
AN content was estimated in callus, suspension cultures and also in cultivated plants. In FGP the yield was 0.015 ±0.001g/100 DW (control) while in callus culture, 0.01781±0.0005 g/100g DW of AN was found (fig. 7). Transformed callus MTCC 431 showed 0.1151±.0001 g/100 g DW of AN, while MTCC 2250 showed 0.2011±0.0003 g/ 100 g DW, which was the maximum among all the transformed calli. The transformed callus with MTCC 2251 exhibited the least concentration of AN (0.114±0.0002 g/ 100 g DW) (fig. 7).
When compared with untransformed callus (0.0178±0.0005 g/100 g DW) 9-11 fold increase was observed in case of MTCC 2250 transformed cultures. This increase in AN content becomes more significant (P<0.05) when it is compared to field grown plants.
Subculturing further decreases the amount of AN in all types of calli (fig. 7). This could be attributed to structural rearrangements of the genomes of cultured cells caused by endoreduplication and/or nuclear fragmentation. This process might result in significant alterations in the genotypes of a portion of the cell population, thereby causing altered secondary metabolism in these cells. Since the population of cells containing altered genotypes might increase during prolonged culture periods, especially in the case where these genotypes produce fast-growing cell types, gradual decline in secondary metabolite productivity may be outcome.
In case of suspension cultures, it is known that secondary products are generally not produced in significant quantities until a culture reaches stationary phase [20]. Our results are in accordance with the findings. As apparent from the growth curve (fig. 2) cells reached the stationary phase at d 15 and the maximum concentration of AN 7.5 μg/ml is obtained at d 21 and 5.3 μg/ml at d 28 in control cultures (fig. 8).
In all the transformed cell suspension cultures significant quantities of AN were found in spent medium. The AN content was maximum at d 21 (after the culture reaches stationary phase), in all the strain types. Cultures with MTCC 2250 showed highest yield of AN i.e., 38.6 μg/ml. After d 21 the content of AN decreases (fig. 8). The results are in agreement with the findings that secondary products are generally not produced in significant quantities until a culture reaches stationary phase20. During the lag phase, cells generally produce, primary compounds that are required for building membranes, walls and other components needed for replication. When nutrients are depleted as the culture ages cell metabolism shifts into secondary metabolism [21,22]. The yield of AN in transformed suspension cultures is five times greater than that observed in control (untransformed suspension cultures).
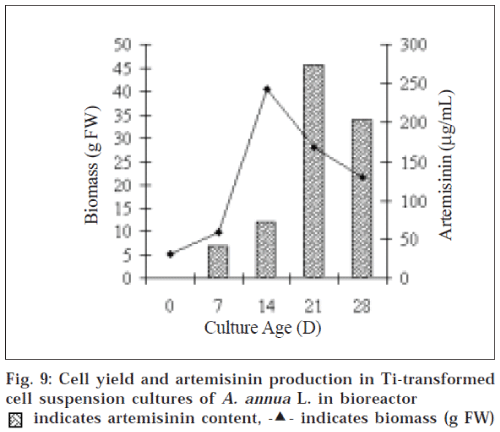
Growth kinetics of suspension culture of Ti-transformed cells of A. annua in bioreactor was consistent with the flask cultures. The maximum biomass (243.32±1.14 g FW) was obtained at d 15 of growth cycle and it yielded 45.56 μg/ml of AN at d 21. This increase in AN content is 6-7 fold greater than untransformed cultures in Erlenmeyer flasks (fig. 9).
Our studies represent the first ever approach towards suspension cultures of Ti-mediated (Agrobacterium tumefaciens) transformed cells of Artemisia annua L. As the results obtained from flask cultures are encouraging, the bioreactor process may be contemplated with a prototype small scale reactor system. Hence an empirical approach made to scale-up the Ti-transformed cell suspension culture in bioreactor. The bioreactor cultivations yielded 45.56 μg/ml AN, which is significant when we look upon low yield of field grown plant.
Suspension cultures offer a unique combination of physical and biological properties that must be accommodated in large scale bioreactor process aimed at exploring their biomass and synthesis of bioactive compounds. The studies suggests that Ti mediated transformation of Artemisia annua led to increase in artemisinin content in cultured cells and with judicious use of bioreactor process and optimized media conditions artemisinin can be obtained in significant amounts.
References
- Haung, P.L., Huang, H.I. and Lee-Huang, S. Chemistry andIndustry.,1992, 290.
- Choi, S.M., Son, S.H., Yun, S.R., Kwon, D.W., Seon, J.H. and Pack,K.Y., Plant Cell Tissue Org. Cult., 2000, 62, 187.
- Yu, K.W., Hahn, E.J. and Pack, K.Y., Enzyme Microb. Technol.,2000, 21, 59.
- Klayman, D.L., Science, 1985, 228, 1049.
- Roth, R.J. and Acton, N., J. Nat. Prod., 1989, 52, 1183.
- Pras, N., Visser, J.F. and Batterman S., Phytochem. Anal., 1991,2, 80.
- Akhila, A., Thakur, R.S. and Popoli, S.P., Phytochemistry.,1987, 26,1927.
- Nair, M.S.R., Basile, D.V., Indian J. Chem., 1992, 31 B, 880.
- Jha, S., Proc. Indian Natl. Sci. Acad., 1995, 61, 65.
- Woerdanbag, H.J., Luers, J.F.J., Van Uden, W., Pras, N., Malingre, T.M. and Alfermann, A.W., Plant Cell Tiss. Organ Cult.,1993,32, 247.
- Weathers, P.J., Cheetham, R.D., Follansbee, E. and Teoh, K.,Biotechnol. Lett.,1994, 16, 1281.
- Nair, M.S.R., Acton, N. and Klayman D.L., J. Nat. Prod., 1986, 49,504.
- McCabe, D.E., Martinell, B.J. and Christou, P., Biol. Technology,1988, 87, 923.
- Pollard, J.W. and Walker, J.M., Eds., In; Method in Molecular Biology (VI) Plant Cell and Tissue Culture, Humana Press, Clifton, NewJersey, 1990, 13.
- Zhao, S.S. and Zeng, M.Y., Planta Med., 1985, 51, 223.
- Godwin, I.D., Ford-Lloyd, B.V. and Newburg, H.J., Aus. J. Bot.,1992, 40, 751.
- Pawlicki, S., Sangwon, R.S. and Sangwon-Norrel, B.S., Plant CellTiss. Org. Cult., 1992, 31, 129.
- Berthomieu, P., Belin, F., Charlot, C. and Dore Jouonin, L., Plant Sci.,1994, 96, 223.
- Mukherjee, S., Ghosh, H. and Jha, S., Plant Cell Rep., 1996, 15, 691.
- Lindsey, K., Planta, 1985, 65, 126.
- Lindsey, K., Yeoman, M.M. and Black, G.M., FEBS Lett., 1983, 155, 143.
- Mavituna, F. and Park, U.M., Biotechnol. Lett. , 1985, 7, 637.
 indicates 4 weeks,
indicates 4 weeks,  indicates 1st sub culture,
indicates 1st sub culture, indicates 2nd sub culture
indicates 2nd sub culture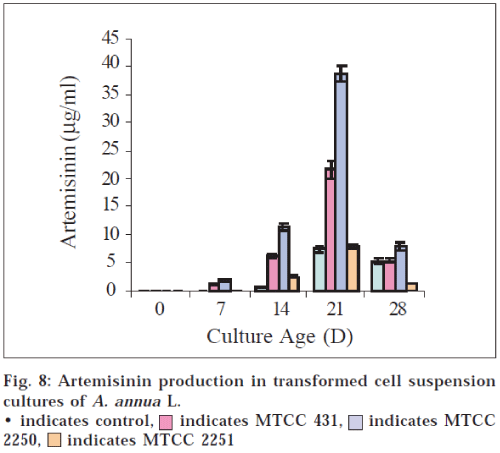
 indicates MTCC 431,
indicates MTCC 431, indicates MTCC 2250,
indicates MTCC 2250,  indicates MTCC 2251
indicates MTCC 2251