- Corresponding Author:
- Dr. Arun Shirwaikar
Dean, Gulf Pharmacy College, Gulf Medical University, Ajman, UAE
E-mail: arunshirwaikar@yahoo.co.in
| Date of Submission | 2 September 2006 |
| Date of Revision | 16 August 2007 |
| Date of Acceptance | 12 November 2007 |
| Indian J Pharm Sci, 2007, 69 (6): 753-758 |
Abstract
Dispersible tablets of Metformin Hydrochloride were prepared using Ocimum gratissimum mucilage powder and Ocimum gratissimum seed powder as disintegrants. Specifications for herbal raw materials and finished products were set according to the Committee for Proprietary Medicinal Products. Several formulations were prepared and evaluated for physical parameters such as thickness, hardness, friability, weight variation, drug content, and disintegration time and drug dissolution. The formulations were prepared with different proportions of Ocimum gratissimum mucilage powder and Ocimum gratissimum seed powder. The formulated tablets had good appearance and better drug release properties as compared to the marketed conventional tablets. The study revealed that Ocimum gratissimum mucilage powder and Ocimum gratissimum seed powder were effective as disintegrants in low concentrations (5%). The study further revealed a poor relation between the swelling index and disintegrating efficiency. Mucilage extracted from Ocimum gratissimum seeds was subjected to toxicity studies for its safety and preformulation studies for its suitability as a disintegrating agent. The mucilage extracted is devoid of toxicity. Studies indicated that the extracted mucilage is a good pharmaceutical adjuvant, specifically a disintegrating agent.
Keywords
Metformin hydrochloride, Ocimum gratissimum, mucilage, seed powder, disintegrating agent
Mouth dissolving tablets rapidly disperse, when kept on the tongue, and instantaneously release the drug. It may be absorbed from the mouth, pharynx and oesophagus as the saliva passes into the stomach and may produce rapid onset of action [1-6]. The advantages of mouth dissolving dosage forms are increasingly being recognized in both, industry and academia. Their growing importance was underlined recently when the European Pharmacopoeia [7] adopted the term “orodispersible tablet” as a tablet that is to be placed in the mouth where it disperse rapidly before swallowing. The main criterion for mouth dissolving tablets is its rapid disintegration or dissolution in the oral cavity with saliva in 15 to 60 s, without any water [8] and it should have a pleasant mouth feel [9].
Metformin HCl, chemically, N,N-dimethylimidodidicarbonimidicdiamine hydrochloride, has been widely used for the management of non-insulin dependent diabetes mellitus (NIDDM). Unlike other biguanide drugs, metformin HCl does not induce lactic acidosis. It is freely soluble in water and has a limited window of absorption i.e. less than 60% which further decreases with an increase in dose. Almost 80-100% of the drug is excreted unchanged [10,11]. Oral absorption is confined to upper part of the intestine.
Mucilage and gums have been known since ancient times for their medicinal uses. In the modern era also they are widely used in the pharmaceutical industries as thickeners, water retention agents, emulsion stabilizers, suspending agents, binders and film formers [12,13]. Apart from its use in finished medicines, newer uses have been found in the preparation of cosmetics, textiles, and paint paper. Hence the demand for these substances is increasing and new sources are getting tapped [14,15]. Though, India, due to geographical and environmental positioning has traditionally been a good source for such products among the Asian countries, a large quantity of this is still being imported from the European countries to meet up the ever-increasing demand [16].
Ocimum gratissimum Linn., Var. cinerifolium, Fam: Labiatae, is a semi woody tender perennial 3 to 8 feet in height, which is grown widely in India, because of its social and religious importance. The plant oil is an established mosquito repellant, antibacterial and local anasthetic17. The present work is an attempt to extract and investigate the pharmaceutical properties of the gum and to assess its suitability as a disintegrant agent in the pharmaceutical formulations.
Materials and Methods
Metformin HCl, talc, magnesium stearate and aspartame were all obtained from Zydus Research Centre, Ahmedabad, India as gift samples. Ocimum gratissimum seeds were procured from the local market. All the other solvents and chemicals used were of either pharamcopoeial or analytical grade. Vernier calipers, Monsanto hardness tester, Roche friabilator and disintegration apparatus were supplied by Campbell Electronics, Mumbai. USP XXIII dissolution apparatus-2 was from Tab-Machines, Mumbai and 1601 PC UV Spectrophotometer is from Shimadzu, Tokyo, Japan.
Extraction of mucilage
The mucilage of Ocimum gratissimum was extracted and precipitated separately by previously reported methods [18,19]. The precipitated gum was filtered, treated with acetone to remove the traces of water and dried in an oven at 40°.
Determination of swelling index
The swelling index is the volume in ml occupied by 1 g of drug; including any adhering mucilage after it has been swollen in an aqueous liquid for 4 h. The swelling index of Ocimum gratissimum mucilage powder, seed powder were determined according to the BP method [20]. One gram of each disintegrant was taken in a 25 ml ground glass stoppered cylinder graduated over a height of 120 to 130 mm in 0.5 divisions. To this 25 ml of water was added and this was shaken vigorously every 10 m for 1 h and then allowed to stand for 24 h. The volume occupied by the disintegrating agent including adhering mucilage was measured. The swelling index was calculated from the mean of three determinations.
Evaluation of toxicity
Toxicity studies were carried out according to the method of Knudsen and Curtis [21]. Preformulation studies were carried out according to official monographs and drug&-excipient compatibility studies were done by using FTIR.
Standard calibration curve of metformin HCl
Solutions ranging from 2 to 4 µg/ml were prepared in phosphate buffer (pH 6.8 fluid). Absorbance was measured for each solution at λmax of 233 nm, using 1601 PC Shimadzu UV Spectrophotometer. Correlation coefficient was found to be 0.9998 in phosphate buffer.
Formulation of mouth dissolving tablets
Mouth dissolve tablets of metformin HCl were prepared by the conventional direct compression technique using Ocimum gratissimum mucilage powder and seed powder at concentrations of 5, 10, 15, 20%. The composition of each formulation is given in Table 1.
| Ingredients | A | B | C | D |
|---|---|---|---|---|
| Metformin HCl | 500 | 500 | 500 | 500 |
| OGM*/OGS* | 26.6 | 53.24 | 79.9 | 106.5 |
| Aspartame | 12 | 12 | 12 | 12 |
| Magnesium stearate | 9 | 9 | 9 | 9 |
| Talc | 9 | 9 | 9 | 9 |
| Flavour (orange) | 2.4 | 2.4 | 2.4 | 2.4 |
OGM* Ocimum gratissimum mucilage and OGS* Ocimum gratissimum seed powder
Table 1: Formulation of dispersible tablets of metformin hcl by using ogm*/ogs*
Preparation and evaluation of mixed blend of drug and excipients
All ingredients were passed through mesh no. 60. Required quantity of each was taken for particular formulation and the blend was mixed by tumbling in a polythene bag. Prior to compression into tablets, the blend was evaluated for the following properties.
Angle of repose was determined by using funnel method [22]. Powder was poured from a funnel that can be raised vertically until a maximum cone height, ‘h’ was obtained. Diameter of heap, ‘D’ was measured. The angle of repose ‘Ө’ was calculated by formula tan Ө= h/r, Ө= tan-1(h/r), where, Ө is the angle of repose, ‘h’ is the height in cm and ‘r’ is the radius.
Apparent bulk density was determined by pouring pre- sieved drug excipient blend into a graduated cylinder and measuring the volume and weight “as it is”. It is expressed in g/ml and is given by Db= M/V0, where, M is the mass of powder and V0 is the Bulk volume of the powder. Tapped density was determined by placing a graduated cylinder, containing a known mass of drug-excipient blend, on mechanical tapping apparatus. The tapped volume was measured by tapping the powder to constant volume. It is expressed in g/ml and is given by Dt= M/Vt, where M is the mass of powder and Vt is the tapped volume of the powder.
The flow properties [23] were determined by Carr’s Index and Hausner ratio. Carr’s index is expressed in percentage by I= Dt-Db/Dt, where, Dt is the tapped density of the powder and Db is the bulk density of the powder. Hausner ratio is expressed in percentage by H= Dt/Db, where, Dt is the tapped density of the powder and Db is the bulk density of the powder. Mixed blend of drug and excipients were compressed on a single punch Cadmach machine with 500 mg die cavity under hardness of 3-4 kg/cm2.
Evaluation of tablets
Twenty tablets were selected at random and average weights were determined. Then individual tablets weighed and the individual weight was compared with the average. Hardness [24] of the tablet was determined by using a Monsanto hardness tester, which is expressed in kg/cm2. Friability of the tablet was determined using Roche Friabilator, which is expressed in percentage (%). Twenty tablets were initially weighed (Winitial) and transferred into the friabilator. The friabilator was operated at 25 rpm per min for 4 mins (100 revolutions). The tablets were weighed again (Wfinal) and the % friability was then calculated by using the following formula, F= Winitial- Wfinal/Winitial×100.
Content uniformity for 20 tablets was determined according to the monograph specification for metformin HCl [25], by UV Spectrophotometer. Thickness of the tablets was measured by Vernier Calipers, and is expressed in mm. In vitro disintegration time was determined using disintegration test apparatus. For this, a tablet was placed in each of the six tubes of the apparatus and one disc was added to each tube. The time in seconds taken for complete disintegration of the tablet with no palpable mass remaining in the apparatus was measured. Disintegration time in the oral cavity of human volunteers was measured by placing the tablet on the tongue until no lumps remain, and is expressed in seconds. Wetting time was determined by keeping a piece of tissue paper folded twice, which was placed in a small Petri plate (internal diameter= 6.5 cm) containing 6 ml of water. A tablet was placed on the paper, and the time for complete wetting of the tablet was measured in seconds. The method was slightly modified by maintaining water at 37°. In vitro dissolution study [26,27] was carried out in USP dissolution test apparatus Type II.
Results and Discussion
The average yield of dried mucilage obtained from Ocimum gratissimum seeds was 15%. To determine the safety level of extracted mucilage, acute toxicity studies were carried out. In this study it showed no manifestations of toxic syndromes. During acute toxicity studies were performed to assess the suitability of extracted mucilage for oral delivery. For that we have assessed hematological parameters for 15 days were observed. This study has inferred that there was no change in hematolological parameters, which was comparable with the control.
Swelling indices of Ocimum gratissimum mucilage powder and seed powder were found to be 20 and 25, respectively. Tablets prepared by using the Ocimum gratissimum seed powder imparted its color to the tablet. The hardness of the Ocimum gratissimum mucilage powder was high compared to seed powder, which shows that the mucilage has strong intermolecular binding efficiency to hold the powder firmly during compression. This study revealed that the mucilage not only act as a disintegrant but also a strong binder for the tablets.
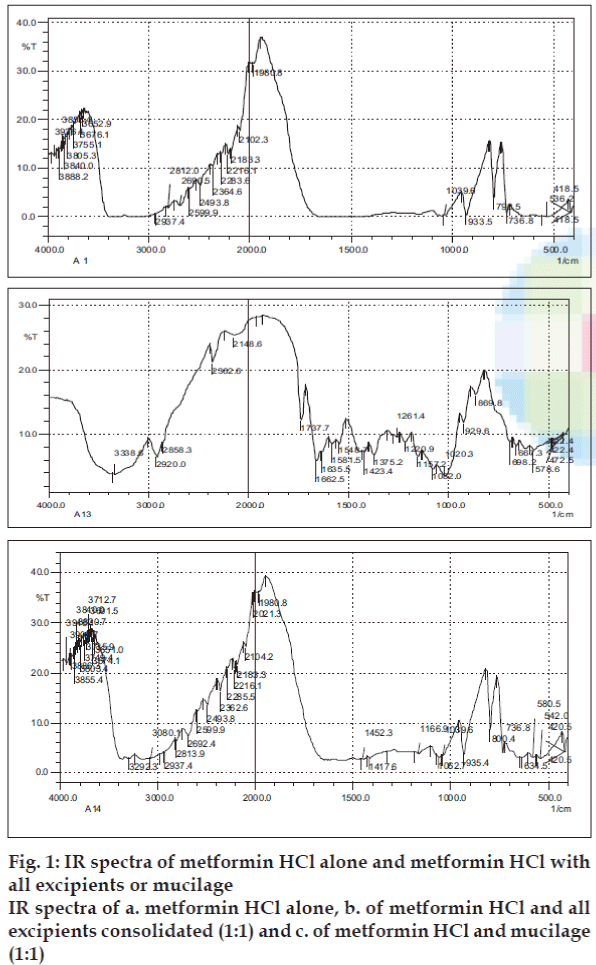
The IR spectra revealed that there was no compatibility related problems between the drug and excipients used in the formulation as shown in figs. 1a to 1c. Pre compression parameters for the formulations of Ocimum gratissimum mucilage powder and seed powders are shown in Table 2.
| Parameters | A | B | C | D |
|---|---|---|---|---|
| OGM* | ||||
| Bulk density (g/cc) | 0.56 | 0.57 | 0.54 | 0.57 |
| Tapped density (g/cc) | 0.67 | 0.73 | 0.71 | 0.73 |
| Bulkiness (cc/g) | 1.78 | 1.75 | 1.90 | 1.85 |
| Carr’s indeed (%) | 16.4 | 21.9 | 22.8 | 23 |
| Hausner ratio | 0.97 | 1.22 | 1.28 | 1.40 |
| Angle of repose (θ) | 28 | 30.6 | 32 | 31.28 |
| OGS* | ||||
| Bulk density (g/cc) | 0.57 | 0.58 | 0.52 | 0.56 |
| Tapped density (g/cc) | 0.74 | 0.75 | 0.72 | 0.74 |
| Bulkiness (cc/g) | 1.77 | 1.73 | 1.92 | 1.77 |
| Carr’s indeed (%) | 22.9 | 22.6 | 27.7 | 24.1 |
| Hausner ratio | 1.29 | 1.293 | 1.38 | 1.34 |
| Angle of repose (θ) | 28.1 | 27.6 | 32 | 30.4 |
OGM* Ocimum gratissimum mucilage and OGS* Ocimum gratissimum seed powder
Table 2: Precompression parameters of metformin hcl by using ogm*/ogs*
Bulk density was found to be between 0.52 to 0.58 g/cc and tapped density between 0.67 to 0.75 g/ cc, bulkiness between 1.73 to 1.9, Carr’s index between 16 to 27%, Hausner ratio between 0.97 to 1.4 and angle of repose was found to be between 28 to 32°, indicating fair to good flow properties. The formulations containing Ocimum gratissimum mucilage powder and seed powder at concentrations of 15 and 20% (Formulation C and D) showed the angle of repose values > 30o indicating only a fair flow properties of the powder mixture. Hardness of the all the formulations were measured in kg/cm2. The hardness of all formulations were found to be 3-4 kg/cm2.
Post compression parameters of the formulations of Ocimum gratissimum mucilage powder and seed powders are shown in Table 3. Hardness of all the formulations was kept constant within the abovementioned range to compare the disintegration time between the formulations prepared using different disintegrant combinations. Friability values of all the formulations were with in the limit i.e. is less than 1.0% except for formulation C and D of both which clearly inferred that the mucilage and seed powder from Ocimum gratissimum has strong binding capacity at 5 and 10% beyond this concentration the binding efficiency decreased. Thickness of all tablets was between 5.60-5.62 mm. As the powder blend had good flow properties, tablets obtained were of uniform weight. Drug content of all the formulations were found to be in the range of 96-99%, which is within acceptable limits.
| Parameters | A | B | C | D |
|---|---|---|---|---|
| OGM* | ||||
| Hardness | 2.9 | 2.8 | 4.3 | 3.8 |
| Friability (%) | 0.96 | 0.88 | 1.03 | 1.1 |
| Thickness (mm) | 5.61 | 5.60 | 5.61 | 5.62 |
| Weight variation | 4.5 | 4.0 | 4.5 | 4.0 |
| Content Uniformity (%) | 98.9 | 98.0 | 97.0 | 98.6 |
| OGS* | ||||
| Hardness | 2.8 | 3.0 | 3.2 | 3.0 |
| Friability (%) | 0.82 | 0.92 | 1.21 | 1.32 |
| Thickness (mm) | 5.60 | 5.61 | 5.60 | 5.61 |
| Weight variation | 4.5 | 4.0 | 4.5 | 4.0 |
| Content Uniformity (%) | 98.2 | 96.0 | 97.3 | 98.5 |
OGM* Ocimum gratissimum mucilage and OGS* Ocimum gratissimum seed powder
Table 3: Post compression parameters of metformin hcl by using ogm*/ogs*
In vitro and in vivo dispersion time was 40-68 s for all the formulations. The disintegration times of all the formulations were within official requirements that is less than 180 s. Comparison between disintegration time in oral cavity, wetting time, and disintegration time (in vitro) for Ocimum gratissimum mucilage and seed powder formulations are shown in Table 4, (fig. 2), respectively. Wetting time was used as a parameter to correlate with disintegration time in oral cavity. This is an important criterion for understanding the capacity of disintegrants to swell in presence of little amount of water. Disintegration time in oral cavity was found between 43-68 s and 45 -65 s for Ocimum gratissimum mucilage powder, and seed powder respectively. Wetting time was found between 40-88 s and 38-75 s for Ocimum gratissimum mucilage powder and seed powder respectively. This showed good correlation between disintegration time in oral cavity and wetting time for all formulations. All designed formulations using Ocimum gratissimum mucilage powder and seed powder showed rapid dissolution and percent cumulative drug release (%CDR) at the end of 5 min was 75-97% for all formulations, shown in Table 5, while conventional marketed tablet of Metformin HCl (Glyciphage®) required around 35 minutes for same amount of drug to be released.
| Parameters | A | B | C | D |
|---|---|---|---|---|
| OGM* | ||||
| Disintegration time in oral cavity (sec) | 43 | 55 | 62 | 68 |
| Wetting time (sec) | 39 | 58 | 72 | 88 |
| Disintegration time in vitro (sec) | 40 | 51 | 58 | 62 |
| OGS* | ||||
| Disintegration time in oral cavity (sec) | 45 | 52 | 65 | 65 |
| Wetting time (sec) | 38 | 62 | 74 | 75 |
| Disintegration time in vitro (sec) | 43 | 56 | 60 | 65 |
OGM* Ocimum gratissimum mucilage and OGS* Ocimum gratissimum seed powder
Table 4: Comparison between disintegration time and wetting time for metformin hcl formulations by using ogm*/ogs*
| Formulation of OGM | % CDR | Formulation of OGS | % CDR |
|---|---|---|---|
| A | 77.4 | A | 75.0 |
| B | 84.15 | B | 81.0 |
| C | 93.15 | C | 91.0 |
| D | 97.65 | D | 93.0 |
| Marketed product | 12.825 | Marketed product | 12.825 |
OGM Ocimum gratissimum mucilage and OGS Ocimum gratissimum seed powder
Table 5: Comparison of in vitro release of various formulations
Acknowledgements
The authors thank Manipal Academy of Higher Education for providing facilities to carry out this work and to Zydus Research Center, Ahemedabad, India for providing gift samples.
References
- Seager H. Drug-delivery products and the Zydis fast-dissolving dosage form. J Pharm Pharmacol 1998;50:375-82.
- Chang RK, Guo X, Burnside BA, Couch RA. Fast-dissolving tablets. Pharm Tech 2000;24:52-8.
- Dobetti L. Fast-melting tablets: Developments and technologies. Pharm Tech 2001;(Suppl.), 44.
- Kuchekar BS, Bhise SB, Arumugam V. Design of fast dissolving tablets. Indian J Pharm Edu 2001;35:150.
- Parfitt K. editor. In; Martindale: The complete drug reference, 32nd ed. UK: Pharmaceutical Press; 1999. p. 758.
- Dollery C. In; AHFS drug information. American Society of Health-System Pharamacists: Bethesda, MD, USA; 1992. p. 663.
- European directorate for the quality of medicines and healthcare. In: Pharmeuropa, Electronic Issue 19.3, Strasbourg, (France): TheEuropean Pharmacopoeia Forum; 1998. p. 547.
- Sastry SV, Nyshadham JR, Fix JA. Recent technological advances in oral drug delivery: A review. Pharm Sci Tech 2000;3:138-45.
- Indurwade NH, Rajyaguru TH, Nakhat PD. Novel approach: Fast dissolving tablets. Indian Drugs 2002;39:405-9.
- Stepensky D, Friedman M, Srour W, Hoffmann A. Preclinical evaluation of pharmacokinetic-pharmacodynamic rationale for oral CR metformin formulation. J Control Release 2001;71:107-15.
- Vidon N, Chaussade S, Noel M, Franchisseur C, Huchet B, Berneir JJ. Metformin in the digestive tract. Diabetes Res ClinPract 1988;4:223-9.
- Monif T, Mahlhotra AK, Kapoor VP. Cassia fustula seed galactomannan: Potential binding agent for pharmaceutical formulation. Indian J Pharm Sci 1992;54:234-40.
- Kapoor VP, Banerji R, Prakash D. Leguminous seeds: Potential industrial sources for gum, fat and protein. J SciInd Res 1992;51:1-22.
- Kakrani HK, Jain NK. A study on binding properties of guggul gum. Indian J Hosp Pharm 1981;100-2.
- Bhunvara NS, Khorana ML. Studies on suspending property of mucilages of hyprophilaspinosa. Indian Drugs 1985;22:500-2.
- Whistler RL. In; Industrial gums, 2nd ed. New York: Academic Press; 1973.
- The Wealth of India, Vol. VIII, New Delhi: CSIR Publication; 2001. p. 80.
- Khanna M, Nandhi RC, Singh S, Jain GK, Sarin JP. Standardization of pure isapgol mucilage for pharmaceutical use. Indian J Pharm Sci 1988;50:238-40.
- Baveja SK, RangaRao KV, Arora J. Examination of natural gums and mucilages as sustaining materials in tablet dosage forms-Part II. Indian J Pharm Sci 1989;51:115-8.
- British Pharmacopoeia Vol. II, London: Her Majesty’s Stationery Office; 1988. p. 140.
- Knudsen LF, Curtiss JM. The use of the angular formulation in biological assays. J Am Stat Soc 1947;42:282-96.
- Marshall K. In: Lachman, Leon, Liberman, Kanig HA, editors. The Theory and practice of Industrial Pharmacy, 3rd ed. Mumbai: Varghese Publishing House; 1987. p. 67.
- Fiese EF, Hagen TA. In: Lachman, Leon, Liberman HA, Kanig JL, editors. The Theory and practice of Industrial Pharmacy, 3rd ed. Mumbai: Varghese Publishing House; 1987. p. 183.
- Banker GS, Anderson GR. In: Lachman L, Liberman HA, Kanig JL, editors. The Theory and practice of Industrial Pharmacy, 3rd ed. Mumbai: Varghese Publishing House; 1987. p. 293.
- Indian Pharmacopoeia, 4th ed. Vol. II, New Delhi: Controller of Publications, Govt. of India; 1996. p.735.
- Klancke J. Dissolution testing of orally disintegrating tablets. Diss Tech May 2003;6-8.
- Siewert M, Dressman J, Brown CK, Shah VP. FIP/AAPS guidelines to dissolution/in vitro release testing of novel/special dosage forms. AAPS Pharm Sci Tech 2003;4:article 7.
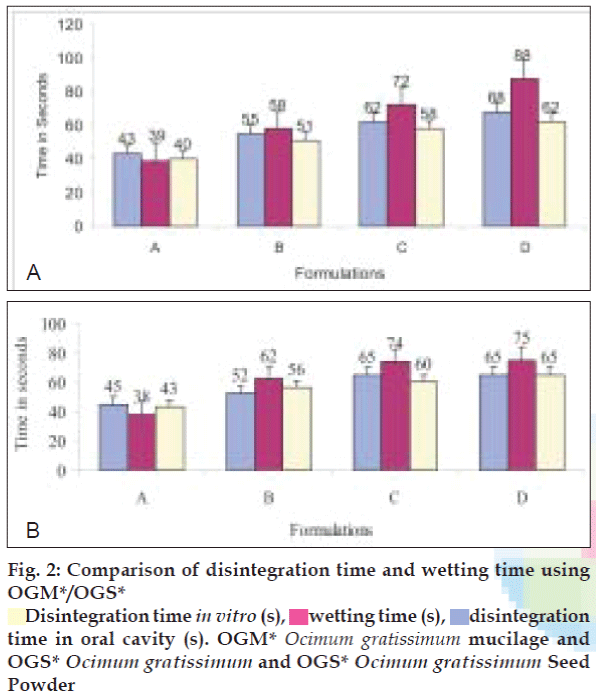
 Disintegration time in vitro (s),
Disintegration time in vitro (s),  wetting time (s),
wetting time (s),  disintegration
time in oral cavity (s). OGM* Ocimum gratissimum mucilage and
OGS* Ocimum gratissimum and OGS* Ocimum gratissimum Seed
Powder
disintegration
time in oral cavity (s). OGM* Ocimum gratissimum mucilage and
OGS* Ocimum gratissimum and OGS* Ocimum gratissimum Seed
Powder



