Sheeja Velayudhan Kutty*, C. Niranjana Muraleedharan, H. Yellina, Swapna Surendran, K. Khairunnisa1 and Sincy Mathew2
Department of Pharmaceutical Analysis, 1Department of Pharmacology, 2Department of Pharmaceutical Chemistry, Grace College of Pharmacy, Palakkad 678004, Kerala, India
- *Corresponding Author:
- Sheeja Velayudhan Kutty
Department of Pharmaceutical Analysis, Grace College of Pharmacy, Palakkad 678004, Kerala, India
E-mail: shakilahmad56@gmail.com
| Date of Received | 08 September 2023 |
| Date of Revision | 08 May 2024 |
| Date of Accepted | 16 October 2024 |
| Indian J Pharm Sci 2024;86(5):1894-1899 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Most relevant analytical technique has been established for the simultaneous quantification of rosuvastatin and teneligliptin in its specific and formulation utilizing reverse phase high performance liquid chromatography. The developed method was found to be linear, accurate, precise, robust and rugged. The mobile phase used was acetonitrile:water (65:35) with flow rate of 0.8 ml/min. The pH was set to 2.5 using orthophosphoric acid. The elution time obtained were 5.48 min for rosuvastatin along with 2.35 min for teneligliptin. The smallest amount of analyte which can be detected and quantified considered being 7.58 µg/ml and 22.98 µg/ml for rosuvastatin and 6.96 µg/ml and 21.10 µg/ ml for teneligliptin subsequently. Every single estimated parameters complied with the relevant criteria, therefore specified the usefulness of reverse phase liquid chromatographic method for quantification of tablet dosage form. Forced degradation studies have been performed using the developed method. Degradation of rosuvastatin was performed under photoalkaline condition and degradation of teneligliptin under photoacidic condition. Structural characterization tools such as fourier transform infrared, nuclear magnetic resonance and electron spray ionization mass spectrometer were used for depiction of degradation products. The fragmentation pathways of both the drugs were depicted. Additionally, in silico toxicity of the degradants was predicted using ProTox-II software. This investigation indicates an extensive new method for degradation studies which can be useful in the course of drug development phase.
Keywords
Reverse phase high performance liquid chromatography, rosuvastatin, teneligliptin, validation, forced degradation study, in silico toxicity
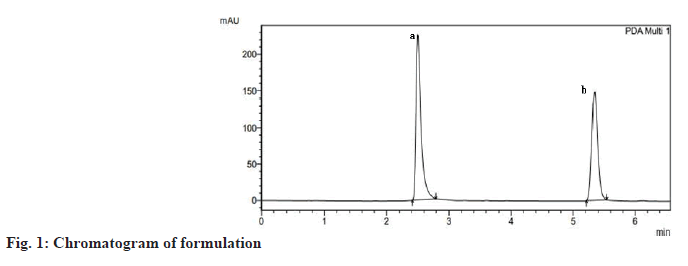
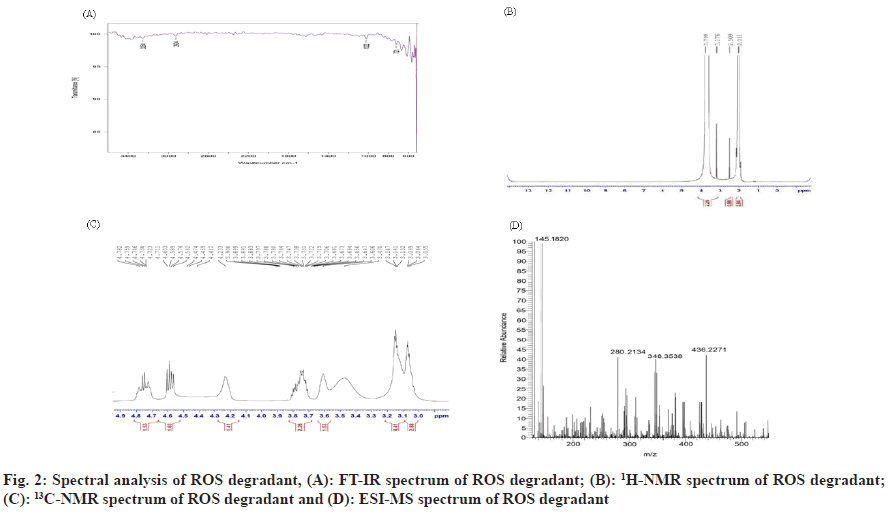
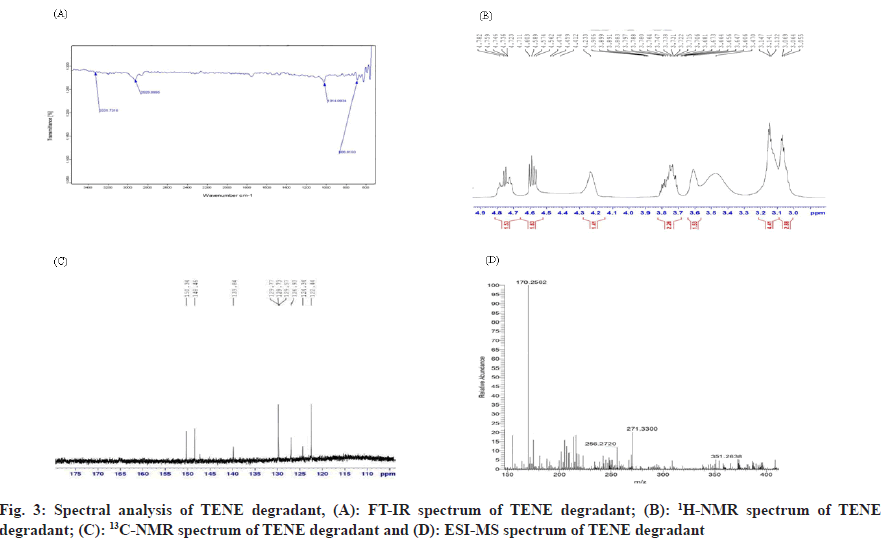
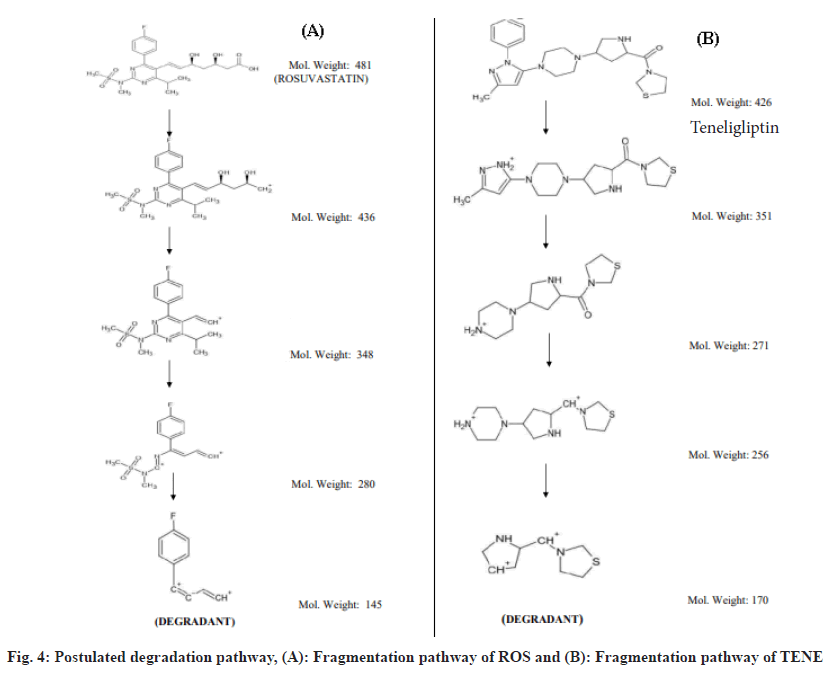
Combination of Rosuvastatin (ROS) and Teneligliptin (TENE) is utilized for the treatment of type 2 diabetes mellitus and dyslipidemias. ROS belong to a group of drugs called statins, which decreases Low-Density Lipoprotein (LDL) and Triglycerides (TG) and shoot up High-Density Lipoprotein (HDL) in the plasma. It competitively inhibit the enzyme Hydroxymethylglutaryl-Coenzyme A (HMG-CoA) reductase[1], which catalyzes the conversion of HMGCoA to mevalonic acid and is the 3rd step in a sequence of metabolic reactions involved in the production of several compound including cholesterol, LDL, and Very LDL (VLDL). TENE is used to lower blood sugar level. It acts by enhancing the liberation of insulin from the pancreas and reducing the hormones that shoot up blood sugar levels. This decreases the fasting and post-meal sugar levels. The instruments employed in the studies are Ultra Violet-Visible Spectroscopy (UV-VIS), Reverse Phase High Performance Liquid Chromatography (RP-HPLC), Fourier Transform Infrared (FT-IR), Nuclear Magnetic Resonance (NMR) and Electron Spray Ionization Mass Spectrometer (ESI-MS). Diabetes, and increasing resistance to insulin, even in persons considered to have normal insulin sensitivity, has been associated with higher concentrations of cholesterol, LDL and TG and lower concentrations of HDL cholesterol[2,3] which results in Atherogenic Dyslipidemia (AD) and worsens the prognosis of diabetic individuals by synergistically accelerating the atherosclerosis and development of Cardiovascular Disease (CVD). It has been hypothesized that most of the oral antidiabetic drugs have significant lipid lowering effect besides achieving effective glycaemic control and thus can modify dyslipidemia and help in decreasing the risk of atherosclerosis, coronary heart disease, stroke, nephropathy and retinopathy[4]. In individuals with diabetes, adherence to statin therapy was described to be indigent, with a reduction by 87 % in starting 3 mo to less than 50 % half yearly. Through combination it has been proved to improve medication when compared to its free drug component regimens. Dipeptidyl Peptidase (DPP) IV inhibitor TENE is an anti-hyperglycaemic agent that has a well recognized clinical efficacy in the treatment of diabetes. ROS is a well known inhibitor of HMG-CoA reductase and is used to treat dyslipidaemia, and combination ensured a remarkable reduction in Hemoglobin A1C (HbA1C), reduction in LDL level at 24 w and reduces the threat of heart disease in diabetic individuals[5]. The literature survey reveals one HPLC and High- Performance Thin-Layer Chromatography (HPTLC) in synthetic mixture of ROS and TENE has been performed but no reverse phase LC studies were established for the simultaneous assessment of ROS and TENE in its medicated form. The present study envisions a validated technique for the estimation of ROS and TENE in tablet dosage form using RPHPLC. Though there are some literature who has conducted forced degradation and characterization of degradants present in ROS and TENE in its pure form. The present research work put special emphasis on degradation of ROS under photoalkaline condition and degradation of TENE under photoacidic condition along with its in silico toxicity assessment. From the literature it was clear that Reactive Oxygen Species (ROS) was stable to basic pH even under thermal stress similarly in case of TENE it was stable to acidic pH under thermal stress, so present work shows that photoalkai condition for ROS and photoacidic condition for TENE has produced degradation impurities. Hence current study desinged to develop clear, fast, exact, reproducible technique for the evaluation of ROS and TENE in specific and medicated form. As method validation is a vital measure in analytical method development, the present method been validated following the guidelines of International Council for Harmonisation (ICH)[6,7]. Though there are some literature explaining forced degradation and characterization of degradants present in ROS and TENE in its pure form. The present research work put special emphasis on degradation of ROS under photoalkaline condition and degradation of TENE under photoacidic condition along with its in silico toxicity assessment which has not been reported yet. Structural elucidation of degradation products in active pharmaceutical ingredients is an essential part of pharmaceutical dosage development. Degradants present in the active pharmaceutical have to be quality reviewed to ensure no mutagenic or toxic substances will be dispensed to individuals. A pure drug sample of ROS was provided by Rubicon Pharma Ltd., Mumbai, and TENE was provided by Glenmark Pharmaceuticals Ltd., Nashik. Acetonitrile and water HPLC grade (Thermo Fisher Scientific India Pvt. Ltd.), O-phosphoric acid (HiMedia Laboratories Pvt. Ltd.), methanol HPLC grade (S D Fine Chem Ltd.)[8], Hydrochloric acid (HCL) and sodium hydroxide (NaOH) (Spectrum Chemicals Pvt. Ltd.) were used for present study. The marketed formulation (Cedaglip-R 10) manufactured by Chemo Bilogical Ltd. New Delhi with ROS 10 mg and TENE 20 mg purchased from nearby pharmacy. The RP-HPLC system (Shimadzu LC-20AT model) consists of a Prominence LC-20AT dual pump, a Phenomenex Luna 5 μm C18(2) 100 Å column (250 × 4.6 mm, Inner Diameter (ID) 5 μm), a Rheodyne 7725i injector with a 20 μl loop capacity, and an Photodiode Array Detector (SPD-M20A) Prominence diode array detector. LCsolution software was used for integration, and a UV chamber by Chemi S.p.A. was employed for degradation studies. Characterization of degradants was performed using an NMR spectrometer (700 MHz Bruker Avance III High Definition (HD), a MS (Waters Xevo G2-XS QTof with a Probe Electrospray Ionization (PESI) source), and an FTIR spectrophotometer (Bruker with Online Publication network of the University of Stuttgart (OPUS) software). The mobile phase consisted of acetonitrile:water (65:35) with a pH of 2.5 using orthophosphoric acid. 20 mg of ROS and 40 mg of TENE pure drug was precisely weighed were transferred into separate 50 ml standard flask. Volume was then made up to 50 ml with HPLC methanol to obtain 400 μg/ml of ROS and 800 μg/ml TENE. Further dilution was done to obtain 120 μg/ ml along with 240 μg/ml of ROS in addition to TENE subsequently, thereby injected into the chromatographic systems. Average weight was calculated by taking weight of 20 tablets and was finely ground to a fine powder. Exactly weighed quantity corresponding to 20 mg of ROS, in addition to 40 mg of TENE was transferred, HPLC methanol was added for dissolving and it was ultra sonicated for 10 min, finally volume was produced to 50 ml with HPLC methanol. The solution was sieved using a membrane filter and the filter out was collected. Appropriate aliquots containing 120 μg/ml ROS and 240 μg/ml TENE were estimated by the suggested method and the chromatogram was recorded (fig. 1). The analytical operation was redone thrice. The percentages of specific drugs were considered to be within the limit that is not less than 90 % and not more than 110 % in formulations. Relative Standard Deviation (RSD) is obtained as 1.05 and 0.57 for ROS and TENE respectively. The low % RSD indicates that the technique can be employed for the regular product quality analysis. The method was validated using ICH guidelines by determining the following parameters such as linearity, accuracy, precision, robustness, ruggedness, precision, detection limit and quantification limit [9]. 5 different concentrations of standard ROS (80, 120, 160, 200, and 240) μg/ml and TENE (160, 240, 320, 400, and 480) μg/ml were prepared, and linearity was evaluated using linear regression analysis[8]. A good linear relationship was found with regression coefficient 0.999 for ROS (y=11263x+2704) and 0.999 for TENE (y=6450x-4164). The recovery analysis of the established technique was evaluated using standard addition method and a certain amount of blended pure drug was added up to the analyte solution at 50 %, 75 %, and 100 % levels[9]. The accuracy studies were performed 3 times and the percentage amount recovered appeared to be within the acceptable range (90 %-93 % for ROS and 100 %-106 % for TENE). With aim of determining the reproducibility of the present method, formulation at a specific concentration level 120 μg/ml of ROS and 240 μg/ml of TENE were prepared and analysed in 3 replicates during the same day (intra-day) and on 3 consecutive days (inter-day). Robustness of the developed technique has been evaluated by making minor changes in the pH of mobile phase and flow rate, and the results were studied. Ruggedness was calculated by carrying out assessment of the formulation following the suggested process by 3 individual evaluators. As the low % RSD values were obtained indicating the method was accurate, precise, robust and rugged. The Limits of Detection (LOD) and the Limits of Quantification (LOQ) were evaluated in regard to the intercept, standard deviation and slope of the curve[8]. The LOD values for ROS in addition to TENE were 7.58 μg/ml and 6.96 μg/ml subsequently and LOQ values were 22.98 μg/ml and 21.10 μg/ml subsequently, which denotes the sensitivity of the established analytical technique. Degradation behaviour of TENE was studied by photoacid hydrolysis and ROS was studied by photoalkali hydrolysis. 20 mg of TENE and ROS were accurately weighed into separate 25 ml standard flask and dissolved in HPLC methanol. 10 ml 5 M HCl was added to TENE and 10 ml 5 M NaOH was added to ROS and kept in UV chamber at 254 nm for 7 d. The degraded sample is neutralized using suitable base for TENE and suitable acid for ROS to avoid further decomposition and then sample was infused into the chromatographic mode in addition to it the degradants were recovered for characterization by developed chromatographic condition. Structural elucidation of the recovered degradants were accomplished using advanced instruments, viz., ESIMS (positive mode), FT-IR and NMR spectrometer 700 MHz Bruker Avance III HD. FTIR interpretation of ROS revealed that the proposed degradant contains peaks at 3100-3000 cm-1 (aromatic C-H stretching), 1100-1000 cm-1 (C-F stretching), 3300-2900 cm-1 (C-H stretching), and 900-675 cm-1 (out-of-plane aromatic C-H bending). For TENE, peaks were observed at 3350-3310 cm-1 (secondary amine), 3300-2900 cm-1 (C-H stretching), 1250-1020 cm-¹ (unconjugated C-N stretching), and 700-600 cm-1 (C-S stretching). Hydrogen-1 (1H) NMR spectrum of pure ROS revealed that number of hydrogen atoms was found to be 6 and for TENE it was found to be 16. Carbon-13 (13C) NMR spectrum of ROS degradant revealed that the number of carbon atoms was found to be 10 and for TENE it was 8. ESI-MS spectrum of ROS degradant showed 100 % intensity at 145.1. Hence, molecular weight of ROS degradant was found to be 145.1. ESI-MS spectrum of TENE degradant was obtained at 170.2. Hence, molecular weight of TENE degradant was found to be 170.2. The results of spectral analysis were shown in fig. 2 and fig. 3. The postulated fragmentation pathway for the degradants was depicted in fig. 4. In silico toxicity of degradation product was predicted using ProTox- II software and outcome revealed the anticipated median Lethal Dose (LD50) in mg/kg weight, predicted toxicity, and its accuracy. The toxicity assessment of the ROS degradant predicted an LD50 of 300 mg/kg. The toxicity class was identified as class 3. The compound was found to be both carcinogenic and mutagenic in nature. The toxicity assessment of TENE degradant predicted were LD50 of 600 mg/kg. The toxicity class was found to be 4. The compound was found to be practically non-toxic and non-irritant. The established chromatographic technique deemed to be fast as it has a shorter retention time in addition to the developed technique appeared to be intelligible, cost effective when compared to already developed method. The developed method uses volatile buffer system which has an advantage of column life stability and can be used when a mass spectrometer is used as LC detector. So in contrast to the previous established techniques, the current method owns superiority in the entire manner. For this reason the recent established analytical technique conceivably employed for the regular quality assurance of ROS and TENE in mixed dosage form. Forced degradation studies of the pure forms of ROS and TENE were performed using the same developed RP-HPLC method. Degradation of ROS was performed under photoalkaline condition and degradation of TENE under photoacidic condition. Characterization of the degradants generated was performed using FT-IR, 1H, 13C NMR and ESI-MS, with structure elucidation of the proposed degradants. Along with that, in silico toxicity of the degradants were assessed by using ProTox-II software and the toxicity endpoints were determined. It was found that degradant of ROS belong to toxicity class 3 and the compound was found to be carcinogenic and mutagenic in nature. Similarly, the degradant of teneligiptin belong to class 4 and found to be practically non-toxic and not an irritant.
Conflict of interest:
The authors declared no conflict of interests.
References
- Tripathi KD. Essentials of medical pharmacology. 7th ed. Jaypee brothers; New Delhi; 2013. p. 636-7.
- Patil M, Jani H, Khoja S, Pirani N, Khoja S. A review on chemistry and pharmacological activity of metformin hydrochloride and teneligliptin hydrobromide hydrate in combined dosage form. Pharma Tutor. 2017;5(3):24-30.
- Krauss RM, Siri PW. Dyslipidemia in type 2 diabetes. Med Clin North Am 2004;88(4):897-909.
[Crossref] [Google Scholar] [PubMed]
- Motgi S, BNV RR, Sattar MA. Study of lipid lowering effects of oral antidiabetic drugs in type 2 diabetes mellitus patients. Int J Basic Clin Pharmacol 2018;7(1):126-32.
- Bae JC, Min KW, Kim YH, Kim KA, Hong EG, Park CY, et al. Efficacy and safety of fixed?dose combination therapy with gemigliptin (50 mg) and rosuvastatin compared with monotherapy in patients with type 2 diabetes and dyslipidaemia (BALANCE): A multicentre, randomized, double?blind, controlled, phase 3 trial. Diabetes Obes Metab 2019;21(1):103-11.
[Crossref] [Google Scholar] [PubMed]
- ICH. Q2A harmonization tripartite guidelines, test on validation of analytical procedures, IFPMA. In: Proceedings of the International Conference on Harmonization. Geneva 1994. p. 1-5.
- Mokal R, Jadhav A. Development and validation of a novel high performance thin layer chromatography method for simultaneous estimation of mangiferin and berberine in an ayurvedic medicine. Indian J Pharm Sci 2022;84(5):1338-42.
[Crossref] [Google Scholar] [
- Srivastava B, Sheeja VK, Haribabu Y, Ebin CJ. Method development and validation of simultaneous estimation of emtricitabine and tenofovir alafenamide in bulk and tablet dosage form using LC-MS/MS. Res J Pharm Technol 2021;14(5):2493-6.
- Haribabu Y, Nihila K, Sheeja VK, Akhil MB. Method development and validation for simultaneous estimation of lamivudine, dolutegravir and tenofovir disoproxil fumarate in bulk and pharmaceutical dosage form using RP-HPLC and its application to in vitro dissolution study. J Med Pharm Allied Sci 2021;10.