- *Corresponding Author:
- A. N. Nagappa
Pharmacy practice department, 4th floor, Shirdi sai baba cancer hospital, Manipal-576 104; 1Therdose pharma (P) ltd., Plot no: 30-32, Survey no. 400, Prasanth nagar, IE, Hyderabad-500 072, India
E-mail: anantha1232000@gmail.com
| Date of Submission | 20 February 2007 |
| Date of Revision | 17 February 2008 |
| Date of Acceptance | 23 February 2008 |
| Indian J Pharm Sci, 2008, 70 (2): 145-153 |
Abstract
Treating central nervous system diseases is very challenging because of the presence of a variety of formidable obstacles that impede drug delivery. Physiological barriers like the blood-brain barrier and blood-cerebrospinal fluid barrier as well as various efflux transporter proteins make the entry of drugs into the central nervous system very difficult. The present review provides a brief account of the blood brain barrier, the P-glycoprotein efflux and various strategies for enhancing drug delivery to the central nervous system.
Keywords
CNS drug delivery, P-glycoprotein, Nano particles, Blood brain barrier
The brain is a delicate organ. The brain-microvascular endothelial cells of the blood-brain barrier protect this organ from exogenous substances. Certain efflux transporters such as P- glycoprotein also perform the same function. However, these protective barriers restrict the entry to the brain from the periphery of compounds that might be of therapeutic value in the treatment of fatal central nervous system (CNS) diseases, such as brain tumors, HIV encephalopathy, epilepsy, cerebrovascular disease and neurodegenerative disorders, and of other pathologies. Delivery of drugs to the brain is a challenging task because of the presence of efficient protective mechanisms. The existing conventional drug delivery systems have been ineffi cient in selectively targeting drugs to the CNS. In response to this, aggressive research efforts have focused on the development of new strategies to more effectively deliver drug molecules to the brain [1].
The blood brain barrier (bbb) and P-glycoprotein (p-gp)
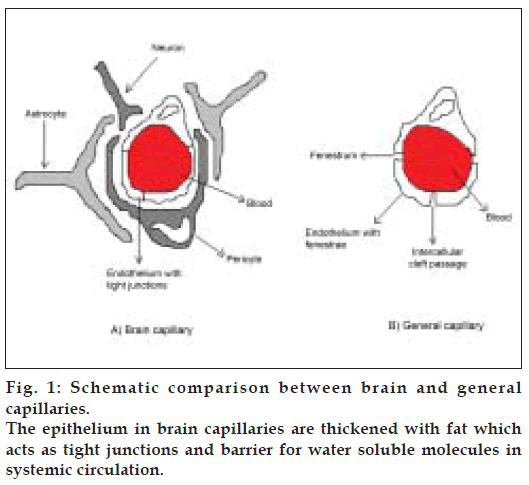
The BBB is one of the most challenging barriers in the body. It is created by the way the blood vessels in the brain are organized. Brain capillaries are different from capillaries of other parts of the body in that normal brain endothelia have fewer pinocytic vesicles, more mitochondria, no fenestrations and adjacent cells are maintained in close apposition by tight junctions [2-4]. The shielding effect of the BBB is further strengthened by the presence of certain efflux transporters such as P-gp in the luminal membrane of the cerebral capillary endothelium. (Schematic depiction of comparison between brain capillaries and capillaries in general is given in fig. 1). P-gp is a brain microvascular endothelial cell protein, which possesses several essential pharmacological functions of drug portage and expulsion [5]. It is present in high concentration on the apical surface of these endothelial cells. P-gp is an active drug efflux transporter protein. While P-gp is involved in protecting the brain exposure to a variety of pharmacologically active hydrophobic agents, it is an impediment to the treatment of various CNS diseases such as primary brain tumors and cerebral human immuno deficiency virus (HIV) infection [5]. P-gp has affinity for a broad range of structurally unrelated large hydrophobic compounds including Vinca alkaloids, epipodophyllotoxins, anthracyclines, cyclosporine A, digoxin, and various HIV protease inhibitors [6-9]. Various approaches are being tried to bypass P-gp efflux. Reversal agents such as R-verapamil, PSC 833 (cyclosporine analog), and biricodar, inhibit P-gp mediated drug transport and increase the influx of therapeutic agents they are coadministered with[10]. Certain non-ionic surfactants like Tween-80 and Cremophor EL have also been found to have the reversal activity [11,12]. However, most of these agents have been found to be pharmacologically active and elicit signifi cant toxicity at doses required for P-gp inhibition.
Strategies for enhanced cns drug delivery
Various strategies have been studied to circumvent the multitude of barriers inhibiting brain penetration by therapeutic agents. These strategies generally fall into one or more of the following three broad categories viz., manipulating drugs, disrupting the BBB, and exploiting alternative routes for drug delivery
Enhancing bbb permeability by drug manipulation
Lipophilic analogs
Drug penetration through the BBB is favored by lipophilicity. Because a drug’s lipophilicity correlates so strongly with cerebrovascular permeability, hydrophobic analogs of small hydrophilic drugs ought to more readily penetrate the BBB. However, the drug molecule should have an optimum octanolwater partition coefficient with Log P value of approximately 1.5 to 2.5 to be efficacious when delivered via the circulatory system [13].
Prodrugs
A prodrug consists of a drug covalently attached to an unrelated chemical moiety that improves the drug’s pharmacokinetic properties. The prodrug itself is inactive but becomes active when the attached moiety is cleaved in vivo by enzymatic or hydrolytic processes. To enhance a drug’s penetration through the BBB, prodrugs are often designed by attaching chemical moieties that increase the drug’s lipophilicity. The best example of this approach is the series of analogs of morphine. Morphine does not readily cross the BBB. Latentiation via acetylation of both hydroxyl groups yields the hallucinogenic heroin, which readily traverses the BBB, and subsequent hydrolytic cleavage of the acetyl groups yields high concentrations of morphine trapped in the brain due to its hydrophilicity [2].
Chemical drug delivery systems (CDS)
The chemical delivery systems cross the BBB by smuggling compounds across as their lipophilic precursors. The CDS approach is a part of retro metabolic drug design approach. These are inactive chemical derivatives of a drug obtained by one or more chemical modifications that provide sitespecifi c or site enhanced delivery through multi-step enzymatic and/or chemical transformations. They include two types of bio-removable moieties: a targeter (T) responsible for targeting, site-specifi city, and lock-in; and modifi er functions (F1-Fn) that serve as lipophilisers, protect certain functions, or fine tune the necessary molecular properties to prevent premature, unwanted, metabolic conversions. Targeting is achieved by design: CDSs will undergo sequential metabolic conversions, disengaging the modifier functions and finally the targeter, after they fulfill their site- or organ-targeting role.
There are a wide variety of CDSs possible both theoretically and in practice. For convenience, the major CDSs can be divided into three classes. They are a) enzymatic physico-chemical CDSs that exploit site-specifi c traffi c properties by sequential metabolic conversions resulting in altered transport properties. b) Site-specifi c enzyme activated CDSs that exploit specific enzymes found primarily, exclusively, or at higher activity at the site of action and c) Receptor-based CDSs that enhance selectivity and activity through transient, reversible binding to target receptors.
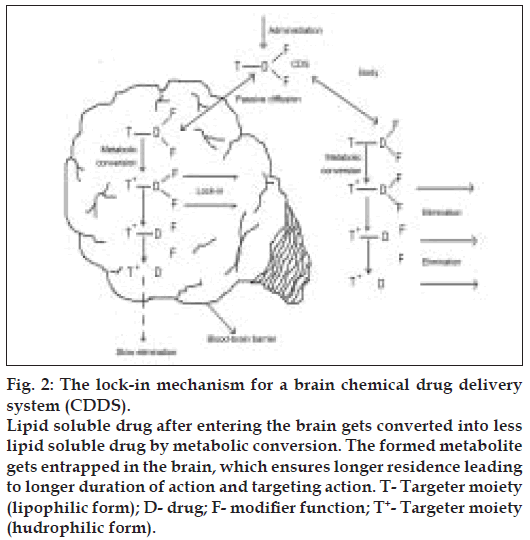
Brain targeting CDSs based on altered transport properties are the most studied ones, that are based on the idea that if a lipophilic compound that enters the brain is converted there into a lipid insoluble molecule, it will become ‘locked in’. Bodor et al, developed a creative approach to exploit the permeability characteristics of the BBB to achieve site-specifi c and/or sustained release of drugs to the brain [14-16]. Targeting is assisted because the same conversion-taking place in the rest of the body accelerates elimination.
In principle, many targeter moieties are possible, but the one based on the 1,4-dihydrotrigonelline- Trigonelline system, where the lipophilic 1,4-dihydro form (T) is converted in-vivo to the hydrophilic quaternary form (T+), proved the most useful. This conversion takes place easily everywhere in the body because it is closely related to the ubiquitous NADH-NAD+ coenzyme system associated with cell respiration. Oxidation takes place with direct hydride transfer, without generating highly active or reactive radical intermediates, providing a non-toxic targeter system. The resulting T+ is locked in the brain (fig. 2), but is easily eliminated from the body due to acquired positive charge, which enhances water solubility. After a relatively short time, the delivered drug D (as the inactive, locked-in T+--D) will be present essentially only in the brain, providing sustained, brain-specifi c release of the active drug. These systems have been tried for a wide range of drug classes such as steroid hormones, antiinfective agents, anticancer agents, and anti retroviral agents among these, oestradiol-CDS is the most advanced and is currently undergoing Phase 1 and 2 clinical trials.
Figure 2: The lock-in mechanism for a brain chemical drug delivery system (CDDS). Lipid soluble drug after entering the brain gets converted into less lipid soluble drug by metabolic conversion. The formed metabolite gets entrapped in the brain, which ensures longer residence leading to longer duration of action and targeting action. T- Targeter moiety (lipophilic form); D- drug; F- modiÞ er function; T+- Targeter moiety (hudrophilic form).
Molecular packaging
Delivering the peptides like enkephalin, TRH (thyrotropin-releasing hormone), and Kyotorphin analogs through the BBB is an even more complex problem because they can be rapidly inactivated by ubiquitous peptidases [17-20]. Three issues are to be solved simultaneously to enhance penetration through BBB. They are, to enhance passive transport by increasing the lipophilicity, assure enzymatic stability to prevent premature degradation, and exploit the lock-in mechanism to provide targeting. This complex approach is known as molecular packaging strategy, where the peptide unit is part of a bulky molecule, dominated by groups that direct BBB penetration and prevent recognition by peptidases. In general, a brain targeter packaged peptide delivery system contains a red-ox targeter (T), a spacer function (S), consisting of strategically used amino acids to ensure timely removal of the charged targeter from the peptide, the peptide itself (P) and a bulky lipophilic moiety (L) attached through an ester bond or sometimes through a C-terminal adjuster (A) at the carboxy terminal to enhance lipid solubility and to disguise the peptide nature of the molecule.
The first successful delivery with a package was for Tyr-D-Ala-Gly-Phe-D-Leu (DADLE), an analogue of leucine enkephalin, a naturally occurring linear pentapeptide (Tyr-Gly-Gly-Phe-Leu) that binds to opioid receptors. A similar strategy was used to deliver a thyrotropin-releasing hormone (TRH) analogue to the CNS [17-18]. These analogues are potential agents for treating neurodegenerative disorders such as Alzheimer’s disease.
Carrier-mediated drug delivery
This strategy takes advantage of the facilitative transport systems present in the brain endothelium. Active transport systems have been found for various substrates such as monosaccharides, monocarboxylic acids, amines, vitamins, hormones, purines, acidic and basic amino acids.
The cerebro vascular membranes are rich in facilitative carrier systems for both glucose and large neutral amino acids (LNAAs). The carrier system for glucose is highly specific for monosaccharides, rendering it useless for drug delivery. However, the LNAA carrier system is capable of transporting numerous endogenous as well as exogenous LNAAs with great structural variety [2,21]. This characteristic has made exploitation of the LNAA carrier system an attractive strategy for CNS drug delivery.
The LNAA carrier system has been exploited to deliver L-3,4-dihydroxyphenalanine (levodopa), an endogenous precursor to dopamine, to the brain. Unlike dopamine, levodopa has a high affi nity for the LNAA carrier system. After traversing the anti-luminal membrane of the cerebral endothelium, levodopa is decarboxylated to yield dopamine. The LNAA carrier system has also been exploited to deliver the antineoplastic agent melphalan into the brain.
Receptor/vector-mediated drug delivery
This strategy exploits several specific transcytosis systems that are actually meant for the extravasation of important nutrients and signaling molecules that cannot diffuse through the cerebromicrovasculature. These include systems for the transport of insulin, transferrin, and insulin like growth factor. Chimeric peptide delivery is one of such strategies, the principle of which lies in the coupling of a non-transportable peptide pharmaceutical to a transportable peptide or protein, which undergoes receptor mediated or absorptive mediated transcytosis through the BBB [22-23]. For example, the non-transportable protein β-endorphin was linked to the transportable protein-cationized albumin via a disulfi de linkage. This chimeric peptide was successfully transcytosed into the brain and enzymatically cleaved in the parenchyma by thiol reductase.
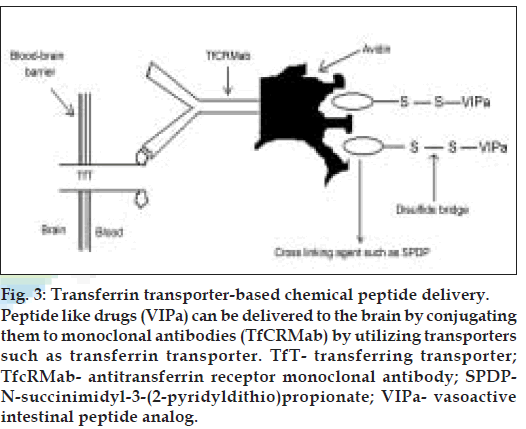
Binding of the vector to its receptor on the luminal surface of brain capillary endothelial cells initiates endocytosis. Following exocytosis at the abluminal plasma membrane and release into brain interstitial space, the pharmacologically active moiety of the chimeric peptide may be released by enzymatic cleavage if a cleavable linkage between the vector and the drug is employed. The free peptide drug would then be able to interact with its specifi c target receptor on brain cells. A covalent conjugate of cationised albumin and the opioid peptide D-Ala-β-endorphin (DABE) was the first example of a chimeric opioid peptide to be investigated in vitro [24] and in vivo [25] with regard to its transport at the BBB. This chimeric peptide was linked by the disulfi de based cross-linking agent, N-succinimidyl-3-(2-pyridyl-dithio) propionate (SPDP). The same technology has been applied to the vasoactive intestinal peptide analog (VIPa) (nontransportable pharmaceutical) [26-28]. This chimeric peptide consists of VIPa and a covalent conjugate of an antitransferrin receptor monoclonal antibody (mouse Mab OX26) and avidin vector (fig. 3).
Figure 3: Transferrin transporter-based chemical peptide delivery. Peptide like drugs (VIPa) can be delivered to the brain by conjugating them to monoclonal antibodies (TfCRMab) by utilizing transporters such as transferrin transporter. TfT- transferring transporter; TfcRMab- antitransferrin receptor monoclonal antibody; SPDPN- succinimidyl-3-(2-pyridyldithio)propionate; VIPa- vasoactive intestinal peptide analog.
The chimeric peptide technology has been applied to PNAs to achieve intracranial delivery of Polyamide (peptide) nucleic acids (PNAs) [29]. This PNA can successfully be delivered to the murine brain parenchyma when covalently coupled to the OX-26 monoclonal antibody. This technique has been expanded to successfully deliver drug-loaded immunoliposomes to the brain [30]. Immunoliposomes consist of liposomes coated with the inert polymer PEG conjugated to the OX-26 antitransferrin-receptor monoclonal antibody. Liposomes and proteins can be used as a cargo of membrane-permeable proteins for the improvement of their intracellular permeability [31].
Nanoparticles have been used as cargo for peptides. PLGA nanoparticles are the most studied ones in this regard. These nanoparticles consist of a colloidal polymer particle of poly-butyl cyanoacrylate (PBCA) with the desired peptide adsorbed onto the surface and then coated with Polysorbate 80. Nanoparticles have been used as a vector for delivery of hexapeptide dalargin (an enkephalin analog) [32-33]. Drugs that have successfully been transported across the BBB with the nanoparticles include loperamide, tubocurarine and doxorubicin [34,35]. The mechanism of nanoparticle transport has not yet been fully elucidated. The most probable transport pathway seems to be endocytosis by the blood capillary endothelial cells following adsorption of blood plasma components, most likely apolipoprotein E (apoE), after intravenous injection. Alternatively, transport may occur by transcytosis of the nanoparticles with drug across the endothelial cells. Percoating of nanoparticles with polysorbate led to adsorption of apoE and possibly other plasma components, which seem to be able to interact with the LDL receptors on the brain endothelial cells that could lead to their endocytosis. In addition to these processes, polysorbates seem to be able to inhibit efflux pump. This inhibition could contribute to the brain transport properties of the nanoparticles [36]. Luca et al, found an increased permeability of PLGA nanoparticles when conjugated with five short peptides [37]. Permeability of zidovudine and lamivudine across the BBB has been found to increase by 8-20 and 10-18 folds, respectively when administered as PBCA (polybutylcyanoacrylate) nanoparticles [38].
Disrupting the bbb
This invasive technique for enhanced CNS drug delivery involves the systemic administration of drugs in conjunction with transient BBB Disruption (BBBD). A variety of techniques that transiently disrupt the BBB have been investigated. However, many of these have been proved to be toxic and are not clinically useful. These include the infusion of solvents such as dimethyl sulfoxide or ethanol and metals such as aluminum, X-irradiation, and the induction of pathological conditions including hypertension, hypercapnia, hypoxia, or ischemia3, [39-41].
Osmotic BBBD
The most frequently applied clinical technique for achieving BBBD is the intracranial infusion of a hyperosmolar solution of mannitol [42,43]. By infusing the solution directly into an artery that feeds the target area of the brain; it is somewhat possible to achieve localized BBBD and/or drug delivery [44]. As the hyperosmolar solution flows through the cerebral capillaries, acute dehydration of endothelial cells results in cell shrinkage, which in turn widens the tight junctions connecting adjacent membranes. Subsequent rehydration in the presence of normal plasma leads to complete restoration of the BBB about 4 h. following treatment. An unfavourable toxic/ therapeutic ratio often is observed with hyper osmotic BBBD. One reason for this is that this methodology results in a 25% increase in the permeability of the tumor microvasculature, in contrast to a 10- fold increase in the permeability of normal brain endothelium [45]. Therefore the normal brain is left vulnerable to potentially neurotoxic chemotherapeutic agents.
Biochemical BBBD
These safer biochemical techniques selectively disrupt the intratumoral BBB while minimally altering the normal BBB [45]. Selective opening of brain tumor capillaries, by the intracarotid infusion of leukotriene C4 was achieved without concomitant alteration of the adjacent BBB [46]. It has been demonstrated in experimental animals that bradykinin, histamine and the synthetic bradykinin analog RMP-7 (receptormediated permeabilizer) infusion also selectively open the blood-tumor barrier .The biochemical mechanism has yet to be elucidated, but it has been established that the effect of the RMP-7 is mediated specifi cally through bradykinin B2 receptors.
Ultrasound-induced disruption
The possibility of enhanced drug delivery to the CNS by inducing hyperthermia has been investigated recently [47]. Ultrasound induced mild hyperthermia, which can be controlled and localized to a small volume within the tissue, may offer promise [48]. This technology is still in its infancy.
Alternative routes for cns drug delivery
Changes at molecular levels and the mode of drug delivery may not always help in an increased penetration of drugs into the brain parenchyma. Certain methodologies have been proposed to enhance drug penetration into the brain, which are based on drug administration by alternative routes bypassing the cardiovascular system.
Olfactory and Trigeminal pathways to the CNS
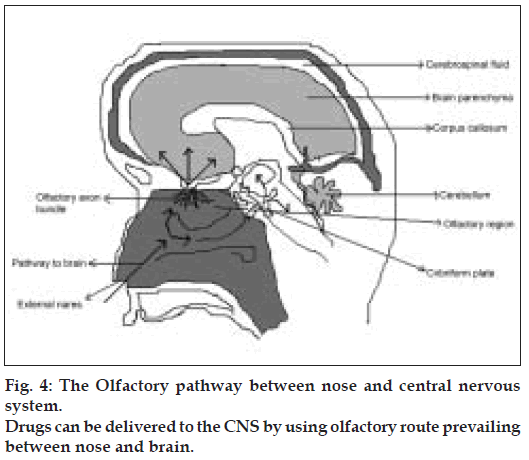
The neural pathway between the nasal mucosa and the brain provide a unique pathway for noninvasive delivery of therapeutic agents to the CNS [49-51] (fig. 4). The olfactory neural pathway provides both intraneuronal and extra neuronal pathways in to the brain. The intraneuronal pathway involves axonal transport and requires hours to days for drugs to reach different brain regions [52-56]. The extra neuronal pathway probably relies on bulk transport through perineural channels, which deliver drug directly to the brain parenchymal tissue, to the cerebro spinal fluid (CSF), or to both. This extra neuronal pathway allows therapeutic agents to reach the CNS with in minutes. Intranasal delivery of agents to the CSF is not surprising as CSF normally drains along the olfactory axon bundles as they traverse the cribriform plate of the skull and approach the olfactory submucosa in the roof of the nasal cavity where the CSF is then diverted in to the nasal lymphatics [57,58]
The Trigeminal neural pathway also may be involved in rapidly delivering protein therapeutic agents, such as insulin like growth factor-1 to the brain following intranasal administration. The trigeminal nerves innervating areas of the nasal cavity are responsible for most chemo-perception other than olfaction and sense diverse stimuli, including hot spices, formaldehyde, and other chemicals [59]. The properties of small molecules, including size and lipophilicity, have been reported to affect delivery to the CNS following intranasal administration [60,61]. The lower molecular weight and higher lipophilicity are favourable characteristics for rapid intranasal uptake of small molecules into the CNS.
There are limitations on the use of intranasal delivery as a means to bypass the BBB, including limitation on the concentrations achievable in different regions of the brain and spinal cord, which will vary with each agent. Delivery is expected to decrease with increasing molecular weight of the drug. Additionally, some therapeutic agents may be susceptible to partial degradation in the nasal mucosa or may cause irritation to the mucosa. Finally, nasal congestion from colds or allergies may interfere with this method of delivery.
Intraventricular/intrathecal delivery
Drugs can be infused intraventricularly using an Ommaya reservoir, a plastic reservoir implanted subcutaneously in the scalp and connected to the ventricles within the brain via an outlet catheter. Drug solutions can be subcutaneously injected into the implanted reservoir and delivered to the ventricles by manual compression of the reservoir through the scalp. This strategy has several advantages compared to vascular drug delivery. This route bypasses the BBB and results in high CSF drug concentrations. A smaller dose is enough and systemic toxicity can be greatly reduced [21]. Drugs in the CSF face minimized protein binding and decreased enzymatic activity relative to drugs in plasma, leading to longer drug half-life in the CSF [62]. However, this strategy has certain disadvantages, including a slow rate of drug distribution within the CSF and increase in intracranial pressure associated with fluid injection or infusion into small ventricular volumes, resulting in to high clinical incidence of haemorrhage, CSF leaks, neurotoxicity and CNS infections [22]. CSF-brain barrier also limits the success of this approach.
Interstitial delivery
This route of administration bypasses BBB. High CNS drug concentrations can be obtained with minimal systemic exposure and toxicity. Intracranial drug concentrations can be sustained, which is crucial in the treatment of many neurodegenerative disorders and for the antitumor effi cacy of many chemotherapeutic agents. Ommaya reservoir, infusaid pump, MiniMed PIMS system and Medtronic SynchroMed system are some of the systems, which have been developed for delivering drugs directly to the brain interstitium. Until recently the most widely used method has been the interstitial injection or infusion of drugs using an ommaya reservoir or implantable pump. The adaptation of the ommaya reservoir to achieve interstitial drug delivery simply involves placing the outlet catheter directly in the intracranial target area. This technique has often been applied to neurooncological patients in whom the outlet catheter is placed in the resection cavity following surgical de-bulking of a brain tumor. Chemotherapeutic agents can be periodically injected into the subcutaneous reservoir and then delivered directly to the tumor bed. This technique, however, does not achieve truly continuous drug delivery.
The ommaya reservoir or infusion pumps have thus far been used in various clinical trials with brain tumor patients to interstitially deliver the chemotherapeutic agents BCNU or its analogs, methotrexate, adriamycin, bleomycin, fluodeoxyuridine, cisplatin, and interleukin 2(IL-2). In most of these studies the intratumoral drug concentrations were often high, and the side effects of the therapy were mild. The success of these techniques is limited by catheter clogging or blocking by tissue debris, inadequate distribution throughout the tumor, and a high degree of burden to the patient.
Biotechnological approaches
Achieving interstitial drug delivery using biological tissues is another promising technique. It involves implanting into the brain, a tissue that naturally secretes a desired therapeutic agent. Transplantation of embryonic dopamine-releasing neurons is an attractive therapeutic strategy because these cells demonstrate good post-transplantation survival and growth characteristics in animal models [63]. However, lack of neovascular innervation limits this strategy. With out rapid neovascularization, implanted solid grafts undergo irreparable ischemic injury, leading to cell death.
Gene therapy has also been attempted to deliver drugs to the CNS. Prior to implantation, cells will be genetically modified to synthesize and release specifi c therapeutic agents. The therapeutic potential of this technique in the treatment of brain tumor was demonstrated. The utility of non-neuronal cells for therapeutic protein delivery to the CNS has been reviewed recently [64]. The survival of foreign tissue grafts may be improved by advancements in techniques for culturing distinct cell types. Cografted cells engineered to release neurotropic factors with cells engineered to release therapeutic proteins may enhance the survival and development of foreign tissue [65]. Direct application of proteinbased therapeutics to the brain could soon include variations of diphtheria toxin to combat refractory gliobastomas and engineered anti-apoptotic factor (FNK) with powerful cytoprotective activity, to protect against ischemia [66,67]. As for neurodegeneration, one seemingly attractive new therapy has been the use of growth factors, such as glial-derived neurotrophic factor (GDNF) as a potential means of reducing the depletion of certain key population of cells lost in Alzheimer’s or Parkinson’s diseases [68,69].
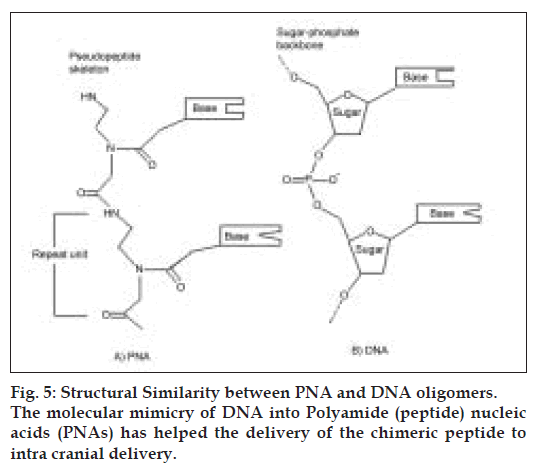
Antisense drug delivery is another recent technology in CNS drug delivery. Peptide nucleic acids (PNAs) (fig. 5) are antisense oligonucleotides containing a polypeptide backbone. Receptor mediated transcytosis has been exploited to promote PNA delivery to the CNS. For example, the attachment of PNAs to the anti-transferrin (OX26) receptor antibodies has been shown to increase the brain uptake of the PNAs, with out loss of the ability of the PNAs to hybridize to target mRN [70].
References
- Martin B, Robert L, Henry B, Mathiowitz E. Encyclopedia of controlled drug delivery, Vol. l, New York: John Wiley and Sons Inc; l999. p. l84-2lO.
- Pardridge WM. Recent advances in blood-brain barrier transport. Annu Rev Pharmacol Toxicol l988;28:25-39.
- Tamargo RJ, Brem H. Transporting therapeutics across the blood-brain barrier. Neurosurg l992;2:259-79.
- Abbott NJ, Romero IA. Transporting therapeutics across the blood-brain barrier. Mol Med Today l996;3:lO6-l3.
- Gelperina SE, Khalansky AS, Skidan IN, Smirnova ZS, Bobruskin AI, Severin SE, et al. Toxicological studies of doxorubicin bound to polysorbate 8O-coated poly (butyl cyanoacrylate) nanoparticles in healthy rats and rats with intracranial glioblastoma. Toxicol Lett 2OO2;l26:l3l-4l.
- Pankajavalli R. The Role of P-glycoprotein in the Blood-Brain Barrier. Einstein Quart J Biol Med 2OO3;l9:l6O-5.
- Dwibhashyam VS, Vijayaratna J. The Permeability Glycoprotein (P-gp): A Threat to Effective Drug Therapy? Indian Drugs 2OO6;43:6O9-l8.
- Tsuji A, Tamai I. Blood-brain barrier function of P-glycoprotein. Adv Drug Deliv Rev l997;25:287-98.
- Masereeuw R, Jaehde UM, Langemeijer W, DeBoer AG, Breimer DD. In vitro and in vivo transport of zidovudine (AZT) across the blood brain barrier and the effect of transport inhibitors. Pharm Res l994;ll:324-3O.
- Varma, MV, Yasvanth A, Chinmoy SD, Ramesh P. P-glycoprotein inhibitors and their screening: A perspective from bioavailability enhancement. Pharmacol Res 2OO3;48:347-59.
- Bhagvant D, Joseph PYK, James PE. Effects of nonionic surfactants on membrane transporters in Caco-2 cell monolayers. Eur J Pharm Sci 2OO2;l6:237-8.
- Yu-li Lo. Relationships between the hydrophilic-lipophilic balance values of pharmaceutical excipients and their multidrug resistance modulating effect in Caco-2 cells and rat intestines. J Control Release 2OO3;9O:37-48.
- Madrid Y, Langer LF, Brem H, Langer R. New directions in the delivery of drugs and other substances to the central nervous system. Adv Pharmacol l99l;22:299-324.
- Bodor N, Buchwald P. Drug targeting via retrometabolic approaches. Pharmacol Therp l997;76:l-27.
- Bodor N, Farag HH, Brewster ME. Site-specific, sustained release of drugs to the brain. Science l98l;2l4:l37O-2.
- Omar FA, Farag HH, Bodor N. Synthesis and evaluation of a redox chemical delivery system for brain-enhanced dopamine-containing an activated carbamate-type ester. J Drug Target l994;2:3O9-l6.
- Bodor N. A strategy for delivering peptides into the central nervous system by sequential metabolism. Science l992;257:l698-7OO.
- Prokai L. Chemical delivery system to transport a pyroglutamyl peptide amide to the central nervous system. J Am Chem Soc l994;ll6:2643-44.
- Prokai K, Tatrai PL, Bodor N. Brain-targeted delivery of a leucine- enkephalin analogue by retrometabolic design. J Med chem l996;39:4775.
- Brownlees J, Williams CH. Peptidases peptides and the mammalian blood-brain barrier. J Neurochem l993;6O:793.
- Greig NH. Optimizing drug delivery to brain tumors. Cancer Treat Rev l987;l4:l-28.
- Scheld WM. Drug delivery to the central nervous system: General principles and relevance to therapy for infections of the central nervous system. Rev Infect Dis l989;2:l669-9O.
- Bickel U, Yoshikawa T, Pardridge WM. Pharmacokinetics and saturable blood-brain barrier transport of biotin bound to a conjugate of avidin and a monoclonal antibody to the transferrin receptor. Adv Drug Del Rev l993;lO:2O5-45.
- Kumagai AK, Eisenberg J, Pardridge WM. Absorptive-mediated endocytosis of cationized albumin and a beta-endorphin-cationized albumin chimeric peptide by isolated brain capillaries: Model system of blood-brain barrier transport. J Biol chem l987;262:l52l4-9.
- Pardridge WM, Triguero D, Buciak JL. Beta-endorphin chimeric peptides: Transport through the blood-brain barrier in vivo and cleavage of disulfide linkage by brain. J Endocrinol l99O;l26:977- 84.
- Jefferies WA, Brandon MR, Hunt SV, Williams AF, Gatter KC, Mason DY. Transferrin receptor on endothelium of brain capillaries. Nature l984;3:l62-3.
- Yoshikawa T, Pardridge WM. Biotin delivery to brain with a covalent conjugate of avidin and a monoclonal antibody to the transferrin receptor. J Pharmacol Exp Ther l992;263:897-9O3.
- Friden PM, Walus LR, Watson P, Doctrow SR, Kozarich JW, Backman C, et al. Blood-brain barrier penetration and in vivo activity of an NGF conjugate. Science l993;259:373-7.
- Pardridge WM, Boado RJ, Kang YS. Vector-mediated delivery of a polyamide (?peptide?) nucleic acid analogue through the blood-brain barrier in vivo. Proc Natl Acad Sci USA l995;92:5592-6.
- Huwyler J, Wu D, Pardridge WM. Brain drug delivery of small molecules using immunoliposomes. Proc Natl Acad Sci USA l996;93:l4l64-9.
- Lindgren M, Hallbrink M, Prochiantz A, Langel U. Cell penetrating peptides. Trends Pharmacol Sci 2OOO;2l:99-lO3.
- Misra A, Jain NK. Progress in controlled and novel drug delivery systems, lst ed. New Delhi: CBS Publishers and Distributors; 2OO4. p. 23-47.
- Kreuter J, Alyautdin RN, Kharkevich DA, Ivanor AA. Passage of peptides through the blood- brain barrier with colloidal polymer particles nanoparticles. Brain Res l995;674:l7l-4.
- Kreuter J. Nanoparticlate systems for brain delivery of drugs. Adv Drug Deliv Rev 2OOl;47:65-8l.
- Kreuter J. Transport of drugs across the blood-brain barrier by nanoparticles. Curr Med Chem 2OO2;2:24l-9.
- Zordan-Nudo T, Ling, Liv Z, Georges E. Effect of nonionic detergents on P-glycoprotein drug binding and reversal of multidrug resistance. Cancer Res l993;53:5994-6OOO.
- Luca C, Francesca G, Giovanni T, Francesco R, Vandelli MA, Flavio F. Peptide-derivatized biodegradable nanoparticles able to cross the blood-brain barrier. J Control Release 2OO5;lO8:84-96.
- Yung-Chih K, Hung-Hao C. Effect of nanoparticulate polybutylcyanoacrylate and methylmethacrylate-sulfopropylmethacrylate on the permeability of zidovudine and lamivudine across the in vitro blood-brain barrier. Pharm Nanotechnol 2OO6;327:l6O-9.
- Gulati A, Nath C, Shanker K, Srimal, RC, Dhawan KN, Bhargava KP. Effect of alcohols on the permeability of blood-brain barrier. Pharmacol Res Commun l985;l:85-93.
- Borisenko SA. Effects of drugs on blood-brain barrier permeability in rats chronically intoxicated by ethanol. Ann lst Super Sanita l99O;26:39-42.
- Mayordomo F, Renau-Piqueras J, Megias L, Guerri C, Iborra FJ, Azorin I, et al. Cytochemical and stereological analysis of rat cortical astrocytes during development in primary culture: Effect of prenatal exposure to ethanol. Int J Dev Biol l992;36:3ll-2l.
- Kroll RA, Neuwelt EA. Outwitting the blood-brain barrier for therapeutic purposes: Osmotic opening and other means. Neurosurgery l998;42:lO83-99.
- Kroll RA, Pagel MA, Muldoon LL, Roman-Goldstein S, Fiamengo SA, Neuwelt EA. Improving drug delivery to intra cerebral tumor and surrounding brain in a rodent model: A comparison of osmotic versus Bradykinin modification of the blood-brain and/or blood-tumor barriers. Neurosurgery l998;43:879-86.
- Bullard DE, Bigner SH, Bigner DD. Comparison of intravenous versus intracarotid therapy with l,3-bis(2-chloroethyl)-l-nitrosourea in a rat brain tumor model. Cancer Res l985;45:524O-5.
- Inamura T, Nomura T, Bartus RT, Black KL. Intracarotid infusion of RMP-7, a bradykinin analog: A method for drug delivery to brain tumors. J Neurosurg l994;8l:752-8.
- Chio CC, Baba T, Black KL. Selective blood-tumor barrier disruption by leukotrienes. J Neurosurg l992;77:4O7-lO.
- Shivers RR, Wijsman JA. Blood-brain barrier permeability during hyperthermia. Prog Brain Res l998;ll5:4l3-24.
- Cho C W, Lin Y, Cobb WN. Ultrasound-induced mild hyperthermia as a novel approach to increase drug uptake in brain micro vessel endothelial cells. Pharm Res 2OO2;ll5:4l3-24.
- Thorne RG, Frey WH. Delivery of neurotrophic factors into the central nervous system. Clin Pharmacokinet 2OOl;4O:9O7-46.
- Illum L. Transport of drugs from the nasal cavity to the central nervous system. Eur J Pharm Sci 2OOO;ll:l-l8.
- Mathison S, Nagilla R, Bhaskar KU. Nasal route for direct delivery of solutes to the central nervous system: Fact or fiction? J Drug Target l998;5:4l5- 4l.
- Thorne RG, Emory CR, Ala TA, Frey WH. Brain, Quantitative analysis of the olfactory pathway for drug delivery to the brain. Brain Res l995;692:278-82.
- Balin BJ, Broadwell RD, Salcman M, El-Kalliny M. Avenues for entry of peripherally administered protein to the central nervous system in mouse, rat, and squirrel monkey. Comp Neurol l986;25l:26O-8O.
- Baker H, Spencer RF. Transneuronal transport of peroxidase-conjugated wheat germ agglutinin (WGA-HRP) from the olfactory epithelium to the brain of the adult rat. Exp Brain Res l986;63:46l-73.
- Broadwell RD, Balin BJ. Endocytic and exocytic pathways of the neuronal secretory process and trans-synaptic transfer of wheat germ agglutinin-horseradish peroxidase in vivo. J Comp Neurol l985;242:632-5O.
- Shipley MT. Transport of molecules from nose to brain. Brain Res Bull l985;l5:l29-42.
- Kida S, Pantazis A, Weller RO. CSF drains directly from the sub arachnoid space into nasal lymphatics in the rat -Anatomy, histology and immunological significance. Neuropathol Appl Neurobiol l993;l9:48O-8.
- Foldi M. The brain and the lymphatic system. Lymphol l996;29:l-9.
- Feron VJ, Arts JH, Kuper CF, Slootweg PJ, Woutersen RA. Health risks associated with inhaled nasal toxicants. Crit Rev Toxicol 2OOl;3l:3l3- 47.
- Sakane T, Akizuki M, Yamashita S, Sezaki H, Nadai T. Direct drug transport from the rat nasal cavity to the cerebrospinal fluid: The relation to the dissociation of the drug. J Pharm Pharmacol l994;46:378-9.
- Sakane T, Akizuki M, Taki Y, Yamashita S, Sezaki H, Nadai T. Direct drug transport from the rat nasal cavity to the cerebrospinal fluid: The relation to the molecular weight of drugs. J Pharm Pharmacol l995;47:379-8l.
- Harbaugh RE, Saunders RL, Reedert RF. Use of implantable pumps for central nervous system drug infusions to treat neurological disease. Neurosurgery l988;23:693-8.
- Sladek J, Gash D. Nerve-cell grafting in Parkinson?s disease. J Neurosurg l988;68:337-5l.
- Snyder EY, Senut MC. The use of non neuronal cells for gene delivery. Nerobiol Dis l997;4:69-lO2.
- Yurek DM, Sladek JR. Dopamine cell replacement: Parkinson?s disease. Annu Rev Neurosci l99O;l3:4l5-4O.
- Cohen KA, Liu T, Bissonette R, Puri RK, Frankel AE. DAB389EGF fusion protein therapy of refractory Glioblastoma Multiforme. Curr Pharm Biotechnol 2OO3;4:39-49.
- Asoh S, Ohsawa I, Mori T, Katsura K, Hiraide T, Katayama Y, et al. Protection against ischemic brain injury by protein therapeutics. Proc Natl Acad Sci USA 2OO2;99:l7lO7-l2.
- Alexi T, Venero JL, Hefti F. Protective effects of neurotrophin-4/5 and transforming growth factor-alpha on striatal neuronal phenotypic degeneration after excitotoxic lesioning with quinolinic acid. Neuroscience l997;78:73-86.
- Susan A. Biotechnology, brain and future. Trends Biotechnol 2OO5;23:34-4l.
- Banks WA. Oligonucleotides targeting prion diseases. J Pharmacol Exp Ther 2OOl;3:lll3-2l.