- *Corresponding Author:
- Mona Pankhaniya
Department of Quality Assurance, S. J. Thakkar Pharmacy College, Rajkot‑360 005, India
E‑mail: monapankhania89@gmail.com
| Date of Submission | 13 July 2012 |
| Date of Revision | 05 March 2013 |
| Date of Acceptance | 15 March 2013 |
| Indian J Pharm Sci 2013;75(3):284-290 |
Abstract
A simple, specific, accurate, and stability-indicating reversed-phase high-performance liquid chromatographic method was developed for the simultaneous determination of montelukast and fexofenadine hydrochloride, using a Lichrospher ® 100, RP-18e column and a mobile phase composed of methanol:0.1% o-phosphoric acid (90:10 v/v), pH 6.8. The retention times of montelukast and fexofenadine hydrochloride were found to be 10.16 and 12.03 min, respectively. Linearity was established for montelukast and fexofenadine hydrochloride in the range of 2-10 μg/ml and 24-120 μg/ml, respectively. The percentage recoveries of montelukast and fexofenadine hydrochloride were found to be in the range of 99.09 and 99.81%, respectively. Both the drugs were subjected to acid and base hydrolysis, oxidation, photolytic, and thermal degradation conditions. The degradation products of montelukast and fexofenadine hydrochloride were well resolved from the pure drug with significant differences in their retention time values. This method can be successfully employed for simultaneous quantitative analysis of montelukast and fexofenadine hydrochloride in bulk drugs and formulations.
Keywords
Montelukast, fexofenadine hydrochloride, degradation products, stability-indicating method, HPLC
Montelukast (MTKT), 1‑ [ [ [(1R)‑1‑ [3‑ [(1E)‑2‑ (7‑chloro‑2‑quinolinyl)ethenyl]phenyl]‑3‑ [2‑(1‑ hydroxy‑1‑methylethyl)phenyl]propyl]thio] methyl] cyclopropaneacetic acid (fig. 1a) is a white crystalline powder, soluble in water, alcohol, dimethyl formamide, and dimethyl sulfoxide. MTKT is a selective leukotriene D4 receptor antagonist that is used as an antiasthmatic. It is official in Indian Pharmacopoeia, which recommends a high performance liquid chromatographic (HPLC) method for its analysis. Fexofenadine hydrochloride (FEX), α,α‑dimethyl‑4‑ [1‑hydroxy‑4‑ [4‑(hydroxydiphenylmethyl)‑1‑ piperidinyl] butyl]‑benzeneacetic acid (fig. 1b), is a white crystalline powder, slightly soluble in water and soluble in alcohol. FEX is histamine H1 receptor antagonist. It is official in Indian Pharmacopoeia, which recommends a HPLC method for its analysis [1].
MTKT and FEX combination tablet is a recently introduced tablet formulation in Indian market. Literature survey reveals that many analytical methods are reported for determination of MTKT and FEX individually. However, no method is reported for simultaneous estimation of these two drugs by reverse phase HPLC in combined dosage form.
Stability testing forms an important part of drug product development. The purpose of stability testing is to provide evidence on how the quality of a drug substance or drug product varies with time under the influence of a variety of environmental factors such as temperature, humidity, and light; enabling recommendation of storage conditions, retests periods, and shelf lives to be established. The two main aspects of drug product that play an important role in shelf‑life determination are assay of active drug, and degradants generated, during the stability study. The assay of drug product in stability test sample needs to be determined using stability‑indicating method, as recommended by the International Conference on Harmonization (ICH) guidelines [2] and United States Pharmacopoeia (USP 26) [3]. Although stability‑indicating methods have been reported for assay of various drugs in drug products, most of them describe assay procedures for drug products containing only one active drug substance. Only few stability‑indicating methods are reported for assay of combination drug products containing two or more active drug substances. The objective of this work was to develop an analytical LC procedure, which would serve as stability‑indicating assay method for combination drug product of MTKT and FEX.
Both the drugs MTKT and FEX are official in IP 2010. Detailed survey of literature for MTKT revealed several reported methods based on different techniques, viz. HPLC [4‑11], HPTLC [12,13], UV spectrophotometry [14‑22], voltammetric [23], and LC‑ESI‑MS/MS [24] for its determination from pharmaceuticals. Similarly, survey of literature for FEX revealed reported methods based on HPLC [25‑35], UV spectrophotometry [36‑39], UPLC [40], and LC‑MS [41] for its determination from pharmaceuticals. There is only one stability‑indicating method has been reported for determination of MTKT and FEX [42]. This manuscript describes the development and subsequent validation of a stability‑indicating isocratic reversedphase HPLC method for simultaneous determination of MTKT and FEX in the presence of their degradants. To establish the stability‑indicating nature of the method, forced degradation of drug substances and drug product was performed under different stress conditions (thermal, photolytic, UV exposure, acid and basic hydrolytic and oxidative), and stressed samples were analyzed by the proposed method. The proposed LC method was able to separate both drugs from degradants generated during forced degradation studies.
Materials and Methods
MTKT and FEX working standard were procured as gift sample from Torrent Research Centre, Gandhinagar and Sakar Healthcare Pvt. Ltd., Ahmedabad, respectively. Combination product of MTKT and FEX (Label claim: 10 mg and 120 mg, respectively), Montair‑Fx® tablet (Cipla) was purchased from the local pharmacy. Methanol and water used were of HPLC grade and purchased from Merck Specialities Pvt. Ltd., Mumbai. o‑phosphoric acid (OPA), sodium hydroxide, hydrochloric acid, and hydrogen peroxide used were of Analytical grade and purchased from Merck Specialities Pvt. Ltd., Mumbai.
The liquid chromatographic system (YL‑clarity 9100, Korea) consisted of the following components: YL9101 vacuum degasser, YL9110 quaternary pump, YL9160 PDA detector, a manual injection facility with 20 µl fixed loop. The chromatographic analysis was performed using Lichrospher® 100, RP‑18e column (250×4.6 mm, 5 µm particle sizes) as a stationary phase.
Preparation of mobile phase and stock solutions
A total of 0.1% OPA was prepared by diluting HPLC grade water. A total of 900 ml of methanol and 100 ml of 0.1% OPA were mixed and pH was adjusted to 6.8. This mixture was sonicated and filtered through 0.45 µm membrane filter and used as a mobile phase. Stock solution of MTKT was prepared by transferring 100 mg of MTKT into 100 ml volumetric flask. And stock solution of FEX was prepared by transferring 60 mg of FEX into 100 ml volumetric flask. Volumes were made upto the mark with mobile phase in both volumetric flasks to obtain a solutions containing 1000 μg/ml of MTKT and 600 μg/ml of FEX. The solution of MTKT was further diluted with the same solvent to obtain the final concentrations of 50 μg/ml of MTKT. The RP-HPLC was performed in with isocratic mode using a mobile phase of methanol: 0.1% OPA (90:10 v/v), pH 6.8 on Lichrospher® 100, RP‑18e column (250×4.6 mm, 5 µm particle size) with 1 ml/min flow rate at 226 nm using UV detector.
Calibration curves for MTKT and FEX
Tablet formulation contained MTKT and FEX in a ratio of 1:12. Appropriate aliquots of MTKT and FEX stock solutions were taken in different 10 ml volumetric flasks and diluted up to the mark with mobile phase to obtain final concentrations of 2-10 µg/ml and 24-120 µg/ml of MTKT and FEX, respectively.
Forced degradation studies
Forced degradation of each drug substances and the drug product was carried out under hydrolytic, oxidative, photolytic, and thermolytic conditions. Thermal and photo degradation of drug substances and drug product was carried out in solid state. After degradation these solutions were diluted with mobile phase to achieve a concentration of 5 μg/ml of MTKT and 60 μg/ml of FEX (on label claim basis for marketed formulation). Then 20 μl portions of degraded solutions were injected into the HPLC system and analyzed under the chromatographic analysis condition described earlier.
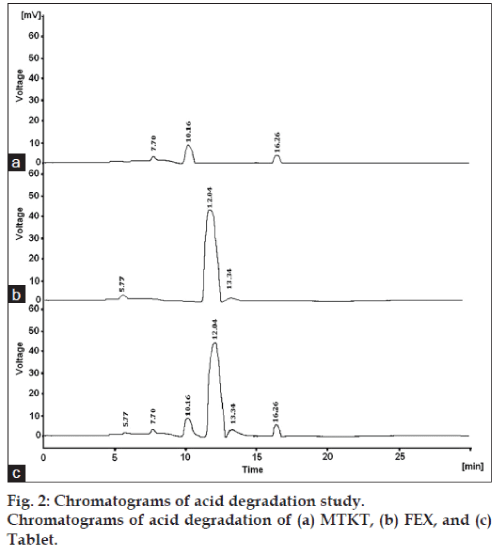
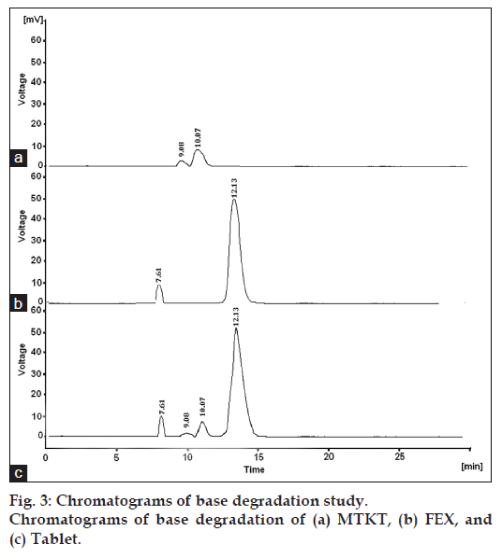
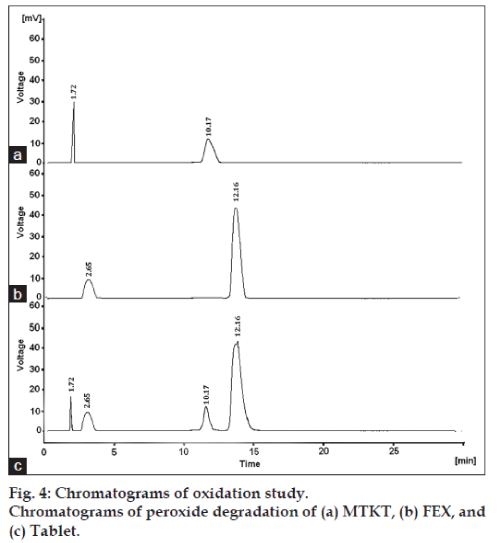
Acid hydrolysis of drug substance and drug product in solution state was performed in 0.1N hydrochloric acid for 2 h. Base hydrolysis of drug substance and drug product in solution state was performed in 1.0 N sodium hydroxide solution for 2 h. For oxidation, sample solutions of drug substance and drug product in 3% hydrogen peroxide were kept at room temperature for 4 h. For thermal, samples of drug substances and drug product were placed in a hot air oven at 80º for 24 h. For photolytic, samples of drug substances and drug product, both in solid state, were placed in UV light for 24 h.
Method validation
The method of analysis was validated as per the recommendations of ICH and USP for the parameters like accuracy, linearity, precision, detection limit, quantitation limit, and robustness. The accuracy of the method was determined by calculating percentage recovery of MTKT and FEX. For both the drugs, recovery studies were carried out by applying the method to drug sample to which the known amount of MTKT and FEX corresponding to 80, 100, and 120% of label claim had been added (standard addition method). At each level of the amount three determinations were performed and the results obtained were compared.
Intra‑ and inter‑day precision study of MTKT and FEX was carried out by estimating the corresponding responses three times on the same day and on three different days for the concentration of 5 μg/ml and 60 μg/ml for MTKT and FEX, respectively. The limit of detection (LOD) and limit of quantitation (LOQ) were calculated using following formula: LOD=3.3(SD)/S and LOQ=10(SD)/S, where SD is standard deviation of response (peak area) and S is average of the slope of the calibration curve.
System suitability tests are an integral part of any chromatographic analysis method which is used to verify reproducibility of the chromatographic system. To ascertain its effectiveness, certain system suitability test parameters were checked by repetitively injecting the drug solution at the concentration level of 5 and 60 μg/ml for MTKT and FEX, respectively to check the reproducibility of the system.
For robustness evaluation of HPLC method a few parameters like flow rate and pH of mobile phase were deliberately changed. Each factor selected was changed at three levels (−1, 0, +1) with respect to optimized parameters. Robustness of the method was done at the concentration level of 5 and 60 μg/ml for MTKT and FEX, respectively.
Analysis of marketed formulation
Twenty tablets were accurately weighed and finely powdered. The quantity of the powder equivalent to 10 mg MTKT or 120 mg FEX was accurately weighed and transferred into 100 ml volumetric flask. The volume made up to the mark with mobile phase. The solution was filtered through Whatman filter paper. The both solutions of MTKT and FEX were further diluted with the mobile phase to obtain final concentrations of 6l and 72 µg/ml for MTKT and FEX, respectively.
Results and Discussion
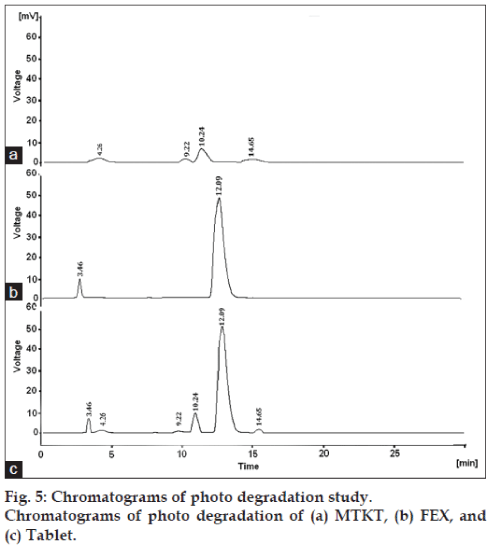
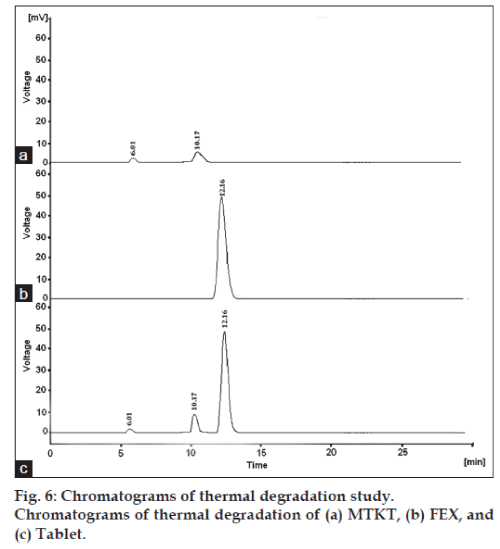
The degradation study indicated that MTKT was susceptible to acid, base, oxidation, photo and thermal degradation. FEX was found to be susceptible to acid, base, oxidation and photo while it was stable to thermal degradation conditions. Typical chromatograms of stressed samples are shown in figs. 2-6. In all degradations the drug degrades as observed by the decreased area in the peak of the drug when compared with peak area of the same concentration of the nondegraded drug, without giving any additional degradation peaks. Both the drugs showed no degradation at 0 h, in all the degradation conditions. Summary of the degradation studies of MTKT and FEX was shown in Table 1. In that percent degradation was calculated by comparing the areas of the degraded peaks in each degradation condition with the corresponding areas of the peaks of both the drugs under nondegradation condition. It also showed retention time of degraded products which were observed in different degradation conditions for both drugs.
| Degradation condition | % Assay | % Degradation | ||
|---|---|---|---|---|
| MTKT | FEX | MTKT | FEX | |
| Acidic/0.1 N HCl/2 h/solution | 89.2 | 96.33 | 10.6 | 3.65 |
| Alkaline/1.0 N NaOH/2 h/solution | 85.6 | 88.6 | 13.2 | 11.05 |
| Peroxide/3% H2O2/4 h/solution | 83.2 | 92.27 | 16 | 7.88 |
| Photo/under UV ligtht/24 h/solid | 92 | 99.51 | 8 | 0.47 |
| Thermal/80°/24 h/solid | 96.4 | 92.98 | 3.6 | 6.95 |
MTKT=Montelukast, FEX=Fexofenadine hydrochloride
Table 1: Summary of degradation of mtkt and fex in different condition
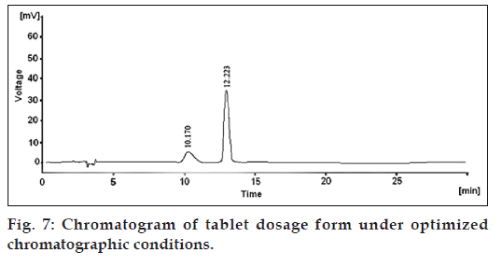
The mobile phase consisting of methanol: 0.1% OPA (90:10 v/v), pH 6.8, at 1 ml/min flow rate was optimized which gave two sharp, well resolved peaks with minimum tailing factor for MTKT and FEX (fig. 7). UV overlain spectra of both MTKT and FEX showed isoabsorptive point at 226 nm, so this wavelength was selected as the detection wavelength. The retention times for MTKT and FEX were 10.17 and 12.22 min, respectively. Resolution was found to be 2.195. The calibration curve for MTKT and FEX was found to be linear over the range of 2-10 and 24-120 µg/ml, respectively (Table 2). The proposed method was successfully applied to the determination of MTKT and FEX in their combined tablet dosage form. The developed method was also found to be specific, since there was no interference found of the degradation product, mobile phase and as well as excipients. The LOD for MTKT and FEX were found to be 0.036 and 0.283 µg/ml, respectively, while LOQ were 0.109 and 0.859 µg/ml, respectively (Table 2).
| Parameters | MTKT | FEX |
|---|---|---|
| Linearity range (μg/ml) | 2‑10 | 24‑120 |
| Slope | 54.21 | 28.046 |
| Intercept | 49.178 | −25.763 |
| Correlation coefficient | 0.9996 | 0.9992 |
| Regression equation | Y=54.21x+49.178 | Y=28.046x–25.763 |
| LOD (μg/ml) | 0.03622 | 0.28357 |
| LOQ (μg/ml) | 0.10975 | 0.859303 |
| Repeatability (% RSD, n=6) | 1.79% | 0.49% |
| Precision (%RSD) | ||
| Intraday (n=3) | 0.729‑1.266% | 0.266−0.675% |
| Interday (n=3) | 0.472−0.878% | 0.031−0.335% |
| System suitability(% RSD) | 0.487 | 0.059 |
| Theoretical plate | 3429 | 2211 |
| Accuracy % | 99.04‑99.72 | 99.60‑99.95 |
| Robustness (% RSD) | 0.401 | 0.298 |
| % Assay | 99.68 | 98.66 |
MTKT=Montelukast, FEX=Fexofenadine hydrochloride, LOD=Limit of detection, LOQ=Limit of quantitation, RSD=Relative standard deviation
Table 2: Summary of validation parameters
In this reported study, a stability‑indicating HPLC method was developed for the simultaneous determination of MTKT and FEX and validated as per ICH guidelines. The developed method was accurate, precise, and repeatable. It was also found to be simple, specific, and sensitive for analysis of MTKT and FEX in combination without any interference from the degraded products, diluents, and excipient. Assay results for the combined dosage form using the proposed method showed 99.68±1.1229% of MTKT and 99.66±0.2544% of FEX. It can be concluded that as the method could separate the drugs from their degradation products, it may be employed for analysis of stability samples of MTKT and FEX.
Acknowledgements
The authors thank Torrent Research Centre, Ahmedabad, for providing montelukast and Sakar Healthcare Pvt. Ltd., for providing fexofenadine hydrochloride as gift samples for this work. Authors also wish to thank the Principal and management for providing necessary facilities and encouragement.
References
- Indian Pharmacopoeia, Controller of Publication, Govt. of India, Ministry of Health and Family Welfare, New Delhi, Vol. 2; 2010. p. 1346, 1704.
- ICH harmonized tripartite guideline, stability testing of new drug substances and products, Q1A (R2) Feb, 2003. p. 1‑15.
- USP 26, The United States Pharmacopoeia, 26th Rev. Rockville, MD: United States Pharmacopoeial Convention Inc.; 2003. p. 1151.
- Singh RM, Saini PK, Mathur SC, Singh GN, Lal B. Development and validation of a RP‑HPLC method for estimation of montelukast sodium in bulk and in tablet dosage form. Indian J Pharm Sci 2010;72:235‑37.
- Alsarra IA. Development of a stability‑indicating HPLC method for the determination of montelukast in tablets and human plasma and its applications to pharmacokinetic and stability studies. Saudi Pharma J 2004;12:136‑43.
- Eldin AB, Shalaby AA, El‑Tohamy M. Development and validation of a HPLC method for the determination of montelukast and its degradation products in pharmaceutical formulation using an experimental design. Acta Pharm Sci 2011;53:45‑56.
- Raju KN, Swamy TG, Rao AL. Development and validation of RP‑HPLC method for the determination of montelukast sodium in bulk and in pharmaceutical formulation. Int J Pharm ChemBiolSci 2011;1:12‑6.
- Roman J, Breier AR, Steppe M. Stability indicating LC method to determination of sodium montelukast in pharmaceutical dosage form and its photodegradation kinetics. J ChromatogrSci 2011;49:540‑6.
- Patil S, Pore YV, Kuchekar BS, Khire VG. Determination of montelukast sodium and bambuterol hydrochloride in tablets using RP HPLC. Indian J Pharm Sci 2009;71:58‑61.
- Radhakrishna T, Narasaraju A, Ramakrishna M, Satyanarayana A. Simultaneous determination of montelukast and loratadine by HPLC and derivative spectrophotometric methods. J Pharm Biomed Anal 2003;31:359‑68.
- Eswarudu MM, Junapudi S, Chary TN. RP‑HPLC method development and validation for simultaneous estimation of montelukast sodium and levocetirizinedihydrochloride in tablet dosage form. Int J Pharm World Res 2011;2:145‑6.
- Rathore AS, Sathiyanarayanan L, Mahadik KR. Development of validated HPLC and HPTLC methods for simultaneous determination of levocetirizinedihydrochloride and montelukast sodium in bulk drug and pharmaceutical dosage form. Pharm Anal Acta 2010;1:1‑6.
- Rote A, Niphade V. Determination of montelukast sodium and levocetirizinedihydrochloride in combined tablet dosage form by HPTLC and first‑derivative spectrophotometry. J LiqChromatogrRelatTechnol 2011;34:155‑67.
- Garg LK, Kumar BR, Sait SS, Krishnamurthy T. Determination of montelukast sodium in oral granules dosage forms by a simple and accurate UV spectrophotometric methods. Int J Pharm Sci Rev Res 2011;7:69‑72.
- Pourghazi K, Khoshhesab ZM, Golpayeganizadeh A, Shapouri MR, Afrouzi H. Spectrophotometric determination of cetirizine and montelukast in prepared formulations. Int J Pharm PharmSci 2011;3:128‑30.
- Arayne MS, Sultana N, Hussain F. Spectrophotometric method for quantitative determination of montelukast in bulk, pharmaceutical formulations and human serum. J Anal Chem 2009;64:690‑5.
- Patel DJ, Patel SA, Patel SK. Simultaneous determination of montelukast sodium and bambuterol hydrochloride in tablet dosage form by ultraviolet spectrophotometry (Dual wavelength method). Int J Pharm Biol Res 2010;1:71‑5.
- Patel SA, Patel DJ, Patel NJ. Simultaneous spectrophotometric determination of montelukast sodium and bambuterol hydrochloride in tablets. Int Res J Pharm 2011;2:154‑8.
- Patel SA. Development and validation of spectrophotometric method for simultaneous determination of montelukast sodium and bambuterol hydrochloride in tablets. Int J Pharm Biomed Res 2011;2:112‑8.
- Choudhari V, Kale A, Abnawe S, Kuchekar B, Gawli V, Patil N. Simultaneous determination of montelukast sodium and levocetirizine dihydrochloride in pharmaceutical preparations by ratio derivative spectroscopy. Int J Pharm Tech Res 2010;2:4‑9.
- Patel NK, Pancholi SS. Spectrophotometric determination of montelukast sodium and levocetirizinedihydrochloride in tablet dosage form by AUC curve method. Der Pharm Chem 2011;3:135‑40.
- Patel PG, Vaghela VM, Rathi SG, Rajgor NB, Bhaskar VH. Derivative spectrophotometry method for simultaneous estimation of rupatadine and montelukast in their combined dosage form. J Young Pharm 2009;1:354‑8.
- Alsarra A, Al‑Omar M, Gadkariem EA, Belal F. Voltammetric determination of montelukast sodium in dosage forms and human plasma. Farmaco 2005;60:563‑7.
- Challa BR, Awen BZ, Chandu BR, Khagga M, Kotthapalli CB. Method development and validation of montelukast in human plasma by HPLC coupled with ESI‑MS/MS: Application to a bioequivalence study. Sci Pharm 2010;78:411‑22.
- Oliveira DC, Weigch A, Rolim CM. Simple and reliable HPLC analysis of fexofenadine hydrochloride in tablets and its application to dissolution studies. Pharmazie 2007;62:96‑100.
- Kozan I, Palabiyik IM, Karacan E, Onur F. Spectrophotometric and high performance liquid chromatographic determination of fexofenadine hydrochloride in pharmaceutical formulations. Turk J Pharm Sci 2008;5:175‑89.
- Sharaf El‑Din MK, Ibrahim F, Eid MI, Wahba ME. Validated stability indicating liquid chromatographic method for the determination of fexofenadine hydrochloride in presence of its degradation products. Application to tablets and content uniformity testing. J Pharm Res 2011;4:2377‑80.
- Arayne MS, Sultana N, Shehnaz H, Haider A. RP‑HPLC method for the quantitative determination of fexofenadine hydrochloride in coated tablets and human serum. Med Chem Res 2011;20:55‑61.
- Radhakrishna T, Reddy OG. Simultaneous determination of fexofenadine and its related compounds by HPLC. J Pharm Biomed Anal 2002;29:681‑90.
- Miura M, Uno T, Tateishi T, Suzuki T. Determination of fexofenadine enantiomers in human plasma with high‑performance liquid chromatography. J Pharm Biomed Anal 2007;43:741‑5.
- Uno T, Yasui‑Furukori N, Takahata T, Sugawara K, Tateishi T. Liquid chromatographic determination of fexofenadine in human plasma with fluorescence detection. J Pharm Biomed Anal 2004;35:937‑42.
- Zafar F, Shoaib MH, Yousuf RI. Development of RP‑HPLC method for fexofenadine determination in tablet formulations and development of dissolution method. Pak J Pharm 2011;28:43‑9.
- Maher HM, Sultan MA, Olah IV. Development of validated stability‑indicating chromatographic method for the determination of fexofenadine hydrochloride and its related impurities in pharmaceutical tablets. Chem Cent J 2011;5:76‑86.
- Karakus S, Kucukguzel I. Development and validation of a rapid RP‑HPLC method for the determination of cetirizine or fexofenadine with pseudoephedrine in binary pharmaceutical dosage forms. J Pharm Biomed Anal 2008;46:295‑302.
- Saeed AM, Najma S, Zeeshan MA, Ahmed SF. Simultaneous determination of gliquidone, fexofenadine, buclizine, and levocetirizine in dosage formulation and human serum by RP‑HPLC. J ChromatogrSci 2010;48:382‑5.
- Kumar KS, Ravichandran V, Mohan Maruga Raja MK, Thyagu R Dharamsi A. Spectrophotometric Determination of Fexofenadine hydrochloride. Indian J Pharm Sci 2006;68:841‑2.
- Polawar PV, Shivhare UD, Bhusari KP, Mathur VB. Development and validation of spectrophotometric method of analysis for fexofenadine HCl. Res J Pharm Tech 2008;1:539‑41.
- Srinivas LD, Kumar PR, Sastry BS. Ion association methods for the determination of fexofenadine in pharmaceutical preparations. E‑J Chem 2005;2:199‑202.
- Narayana B, Veena K. A new method for the spectrophotometric determination of fexofenadine hydrochloride. Indian J Chem Tech 2010;17:386‑90.
- Jain DK, Kumar AM, Jain D. Quantitative determination of fexofenadine in human plasma by UPLC. Indian J Pharm Educ Res 2010;44:295‑300.
- Isleyen EA, Ozden T, Ozilhan S, Toptan S. Quantitative determination of fexofenadine in human plasma by HPLC‑MS. Chromatographia 2007;66:109‑13.
- Singh RR, Rathnam MV. A stability indicating RPHPLC method for the estimation of Montelukast Sodium and Fexofenadine hydrochloride in pharmaceutical preparations.Int J Pharm PharmSci 2012;4:587‑93.