- *Corresponding Author:
- Nagashekhara Molugulu
Department of Pharmacy, Monash University Malaysia, Sunway, Selangor 16150, Malaysia
E-mail: nagashekhara.Molugulu@monash.edu
| Date of Received | 06 July 2024 |
| Date of Revision | 08 August 2024 |
| Date of Acceptance | 18 September 2024 |
| Indian J Pharm Sci 2024;86(4):1401-1408 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Baclofen is a food and drug administration approved medication to manage reversible spasticity, mainly targeting the relief of flexor spasms, clonus and concomitant pain, which are common issues following spinal cord lesions and multiple sclerosis. It can be administered orally or intrathecally. However, there is currently no standardised method for compounding the medication for oral administration, especially in paediatric patients or those with swallowing difficulties. It is often crushed and mixed with fruit juice or milk to aid in dosage adjustments and improve swallowability, potentially affecting the dosing accuracy. Therefore, this study aims to test the stability of oral powders of baclofen at concentrations of 1 mg/ ml and 10 mg/ml in X-Temp® oral suspension system. The suspension was stored in amber high density polyethylene bottles and stored either under refrigeration (5°±3°) or at room temperature (30°±2°) for duration of up to 90 d. The samples underwent thorough evaluation through visual inspection, pH measurement, high-performance liquid chromatography assay and microbiological stability assay. This study indicates that the baclofen suspension at concentrations of 1 mg/ml and 10 mg/ml remained consistent in physical, chemical, and microbiological characteristics for up to 90 d at both (5°±3°) and (30°±2°). Suggesting that the X-Temp® oral suspension system as a reliable medium for compounding of active pharmaceutical ingredients.
Keywords
Baclofen, high-performance liquid chromatography, high density polyethylene, gamma-aminobutyric acid B
Baclofen is an Food and Drug Administration (FDA) approved drug for managing reversible spasticity, particularly to relieve flexor spasms, clonus, concomitant pain, common sequelae of spinal cord lesions and multiple sclerosis[1]. The mechanism of action of baclofen is reported to bind to Gamma-Aminobutyric Acid B (GABAB) receptors in the brain and spinal cord, thus inhibiting the excitatory neurotransmitters that are implicated in causing spasticity and increased tone[2]. The chemical composition of baclofen is 4-amino-3- (4-chlorophenyl) butanoic acid (fig. 1). Its primary pharmacological function is as an antispasmodic agent, reducing muscular tension and it also demonstrates analgesic properties[3].
Baclofen can be administered orally or intrathecally[4]. Pharmacokinetic studies have indicated that in paediatric patients, dosages typically commence at 2.5 mg 3 times daily, which is half the adult dose and are gradually increased (from 2.5 mg 3 times daily to a maximum tolerated dose of 20 mg 4 times daily) until therapeutic efficacy is achieved[5]. Additionally, oral baclofen has been administered to neonates with Cerebral Palsy (CP) or neonatal hypertonia at an initial dose of 0.3-0.5 mg/kg/d[6,7]. A dosage recommendation advise initiating treatment with a conservative dose (approximately 0.3 mg/kg/d), administered in 2-4 divided doses, with cautious titration until optimal efficacy is attained for the child's needs. It is noteworthy that the administration of solid dosage forms such as tablets or capsules presents challenges for patients with swallowing difficulties[8]. When a medication is prescribed for an infant or child, and if there is no suitable commercially available paediatric formulation, an adult dosage form or the Active Pharmaceutical Ingredient (API) bulk powder will be modified to create the final product[9]. Compounding this medication involves multiple steps and calculations, which increases the risks related to drug stability, therapeutic efficacy, and product safety. The severe consequences of compounding errors in paediatric formulations have led to the development of safer compounding practices, as outlined by the National Association of Pharmacy Regulatory Authorities (NAPRA)[10]. Furthermore, system safeguards to minimize the risk of errors during compounding are detailed in the Medication Safety Self-Assessment (MSSA), which is focused on "never events" in community pharmacy.
Consequently, in paediatric compounding, baclofen tablets are often crushed and mixed with fruit juice or milk to facilitate dosage adjustments and enhance swallowability. Studies have reported that crushed tablets mixed with fruit juice or milk could alter a medication's bioavailability, introducing risk of variable or incomplete dosing[11,12]. Unlike the 'standardize 4 safety initiative' implemented by the American Society of Health-System Pharmacists[13], there is currently no standardized method for paediatric compounding of baclofen tablets. A recent study by Saito et al.[14] reported that approximately 19.2 % of facilities have implemented formulation modifications for oral baclofen tablets in paediatric patients. Understanding the compatibility of crushed baclofen tablets with prevalent diluents like juice and milk, commonly employed in Malaysian clinical settings, is crucial. Previous studies have reported the use of ready to use suspending vehicle X-Temp® oral suspension system (Pharm-D Health Science Sdn. Bhd.) is a suitable resource for compounding[8,15-17].
Therefore, in ensuring the efficacy and safety of baclofen, this study aims to conduct stability studies in accordance with the guidelines specified by the British Pharmacopeia[18]. The physicochemical and microbiological stability of baclofen tablets, compounded at 2 different concentrations from commercial tablets, utilizing the X-Temp® oral suspension system as the vehicle will be analysed. These compounded formulations will be stored under controlled conditions, including refrigerated (5°) and room temperature (30°) environments, throughout the study duration.
Materials and Methods
TThe baclofen tablets (Musafen, DuoPharma (M) Sdn. Bhd.) used in this study were prepared in 1 mg/ ml and 10 mg/ml suspension concentrations. Initially, the baclofen tablets were weighed to achieve the 1 mg/ml and 10 mg/ml concentrations, then transferred to a mortar and pestle and crushed into a fine powder. A small volume of X-Temp® oral suspension system (Pharm-D Health Science Sdn. Bhd.) was added to the powder to levigate it, forming a smooth paste. This paste was mixed with the mortar and pestle to achieve a thick consistency. Gradually, an additional X-Temp® oral suspension system was added to the paste with continuous mixing until a uniform liquid consistency was obtained. The mixture was then transferred to a graduated container, and the remaining residues in the mortar were rinsed with X-Temp® oral suspension system to ensure complete transfer.
Physical stability analysis:
Physical stability of the suspension was assessed through visual inspection, focusing on changes in colour, odour, and clarity. Stability is defined by the absence of any alterations in these characteristics. The suspension was divided into 2 groups of High Density Polyethylene (HDPE) bottles, each containing 10 bottles. One group was stored in a humidity chamber at 30° (±2°), while the other was refrigerated at 5° (±2°). One HDPE bottle was periodically sampled from each storage condition for physical at 1 d, 15 d, 30 d, 60 d, and 90 d. The specific gravity at different time points were determined to monitor the weights component of baclofen at 1 mg/ml and 10 mg/ml suspension. This test was conducted under 2 different temperature conditions include ambient room temperature (30°±2°) and refrigerated temperature (5°±2°) utilizing a specific gravity bottle. Specific gravity, defined as the relative weight of the sample in relation to the weight of water, was measured[19]. Acceptance criteria for the 2.5 mg/ml concentration were established within the range of 1.01- 1.15 g/ml in accordance with internal specifications.
Microbiological stability analysis:
Baclofen 1 mg/ml and 10 mg/ml samples were subjected to microbiological stability analysis to determine whether the microbiological attributes of non-sterile pharmaceuticals are met. Microbiological stability analysis was conducted with slight modification according to British Pharmacopoeia 2014. Microbiological stability analysis was conducted at 5° and 30° at the interval of 0 d, 15 d, 30 d, 60 d and 90 d. The Total Aerobic Microbial Count (TAMC) and the Total Combined Yeasts and Moulds Count (TYMC) were determined using the spread plate method. The sample was diluted with X-Temp® to achieve a 1:10 dilution, and 500 μl aliquots were transferred to each Petri dish containing mannitol salt agar, cetrimide agar, Xylose Lysine Deoxycholate (XLD) agar, soybean casein digest agar (Tryptone Soya Agar (TSB)), Sabouraud Dextrose Agar (SDA). The solutions were spread evenly and incubated at 30°-35° for 3-5 d. Microbial growth was then examined on the plates. A negative control was performed using a sterilized diluent.
A 10 ml sample was inoculated with 100 ml of soybean casein digest broth and incubated at 30°-35° for 18-24 h. After incubation, 1 ml was transferred to 100 ml of MacConkey broth, which was incubated at 42°-44° for 24-48 h. Then, a loopful was streaked onto a plate of MacConkey agar using a sterilized inoculating loop. The MacConkey agar plates were incubated at 30°-35° for 18-72 h in an inverted position. Growth of red, nonmucoid colonies on the MacConkey agar plates was then examined. The parameters for the TAMC and total combined yeasts/moulds count were set below 2×10 cfu/g, and the absence of Escherichia coli (E. coli) in 1 g. Negative controls are performed for each sample using the chosen diluent in place of the test preparation. There must be no growth of microorganisms.
Analytical analysis:
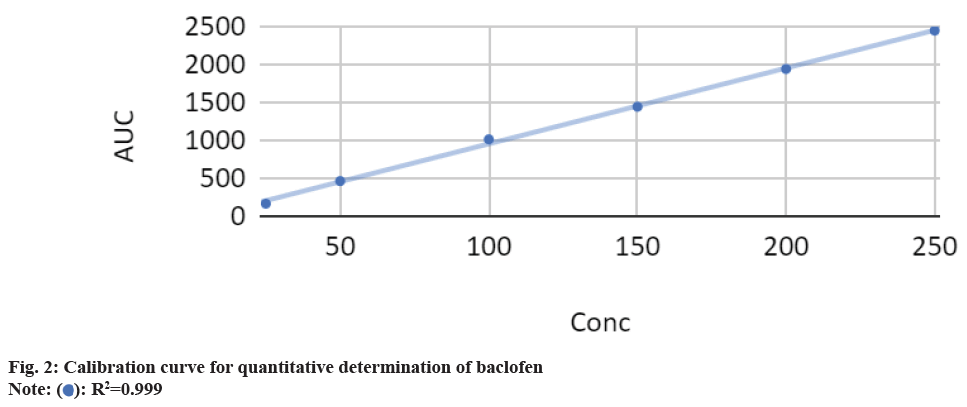
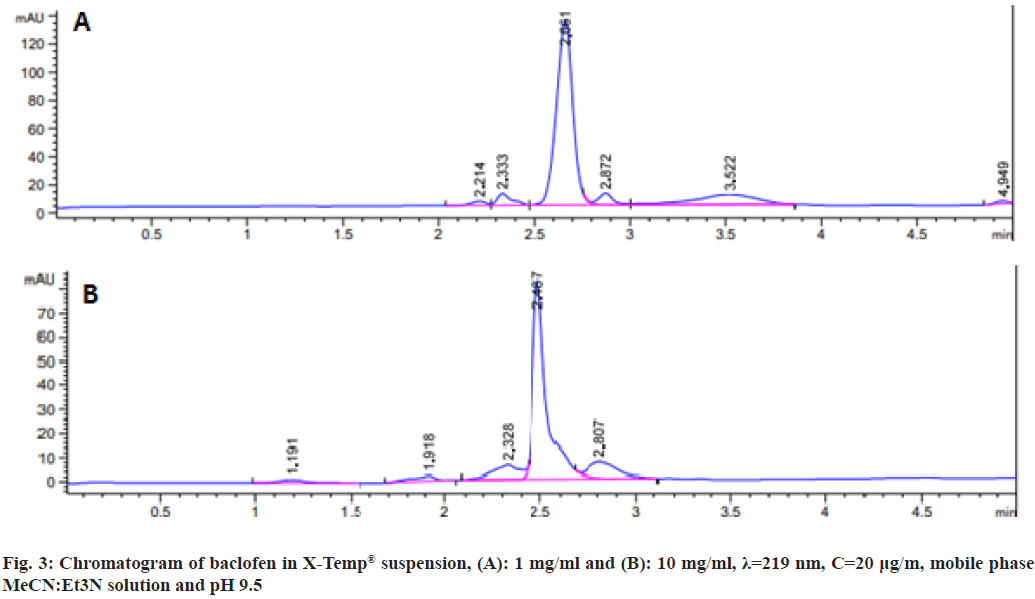
This analysis used the Agilent HPLC 1290 infinity system equipped with an auto injector. Diode-matrix detector with a reverse-phase column (C18, 250 mm) maintained at 30° with isocratic elution was used. The mobile phase was prepared from a mixture of 95 % acetonitrile and 5 % water. The flow rate for the analytical column was 1.0 ml/min. The column was thermostat at 30°. The sample volume was 20 l. Absorption was recorded at 219 nm because the baclofen absorption spectrum had maxima at these wavelengths. The accurate weight of the baclofen drug substance (10 mg) was dissolved in an Hydrogen Chloride (HCl) solution (10 ml, 0.1 m) to prepare a stock solution. Calibration curves were constructed by diluting the stock solution. The volume was then adjusted using the mobile phase, resulting in a standard stock solution of 250 μg/ml. Dilution with the mobile phase yielded standard solutions within the 10-0.625 μg/ml range. The linearity of the standard curve was assessed through linear regression analysis, with a correlation coefficient of 0.999 (fig. 2). Sample solutions for HPLC analysis were prepared by thoroughly shaking an extemporaneous baclofen suspension (approximately 2.5 mg/ml) for 1 min. This suspension was further diluted tenfold with the mobile phase. Triplicate sample solutions were filtered through a 0.45 μm membrane filter before analysis.
Results and Discussion
The stability of baclofen in X-Temp® suspension was successfully investigated to assess their stability at a low dose (1 mg/ml), and a high dose (10 mg/ml) was evaluated at room temperature (30°±1°) and under refrigerated conditions (5°±1°) (Table 1 and Table 2). The stability studies revealed that low and high doses of baclofen in X-Temp® suspension remained stable for 3 mo under these storage conditions. Table 1 and Table 2, shows the chemical, physical and microbiological stability of baclofen at 1 mg/ml and 10 mg/ml respectively. No changes in colour, odour or clarity were detected for both the 1 mg/ml and 10 mg/ml baclofen concentrations over a period of 90 d, whether stored at room temperature (30°±1°) or under refrigerated conditions (5°±1°). All suspensions were assayed, with baclofen content in both 1 mg/ml and 10 mg/ml samples exceeding 97 %. The pH of all suspensions stored under both storage conditions remained within the range of 4.30-5.51, showing minimal changes throughout the study period (Table 1). These results indicate that the buffering agents in X-Temp® were effective at maintaining the pH of the formulations, which is crucial for sustaining their chemical stability.
| Test | Specification | Temperature (C) | Days | ||||
|---|---|---|---|---|---|---|---|
| 1 | 15 | 30 | 60 | 90 | |||
| Visual appearance | Colour | 5 | Light yellow | Light yellow | Light yellow | Light yellow | Light yellow |
| 30 | Light yellow | Light yellow | Light yellow | Light yellow | Light yellow | ||
| Clarity | 5 | Opaque | Opaque | Opaque | Opaque | Opaque | |
| 30 | Opaque | Opaque | Opaque | Opaque | Opaque | ||
| Odour | 5 | Orange Flavour | Orange Flavour | Orange Flavour | Orange Flavour | Orange Flavour | |
| 30 | Orange Flavour | Orange Flavour | Orange Flavour | Orange Flavour | Orange Flavour | ||
| Physical properties | pH | 5 | 4.33 | 4.3 | 4.36 | 4.31 | 4.3 |
| 30 | 4.51 | 4.53 | 4.5 | 4.52 | 4.51 | ||
| Specific gravity | 5 | 0.9021 | 0.9019 | 0.9022 | 0.9021 | 0.902 | |
| 30 | 0.7145 | 0.715 | 0.7145 | 0.7122 | 0.7125 | ||
| Assay | 5 | 97.50 % | 97.10 % | 97.00 % | 97.00 % | 97.00 % | |
| 30 | 97.10 % | 97.10 % | 97.10 % | 97.10 % | 97.00 % | ||
| Microbial limit | Total aerobic bacteria (Not More Than (NMT) 2X10 cfu/g) | 5 | <2X10 cfu/g | <2X10 cfu/g | <2X10 cfu/g | <2X10 cfu/g | <2X10 cfu/g |
| 30 | <2X10 cfu/g | <2X10 cfu/g | <2X10 cfu/g | <2X10 cfu/g | <2X10 cfu/g | ||
| Total yeast and moulds (NMT 2X10 cfu/g) | 5 | <2X10 cfu/g | <2X10 cfu/g | <2X10 cfu/g | <2X10 cfu/g | <2X10 cfu/g | |
| 30 | <2X10 cfu/g | <2X10 cfu/g | <2X10 cfu/g | <2X10 cfu/g | <2X10 cfu/g | ||
| E.coli (Absence in 1 g) | 5 | Conforms | Conforms | Conforms | Conforms | Conforms | |
| 30 | Conforms | Conforms | Conforms | Conforms | Conforms | ||
Table 1: Stability Profile of 1 mg/ml Baclofen in X-Temp® Oral Suspension
| Test | Specification | Temperature (C) | Days | ||||
|---|---|---|---|---|---|---|---|
| 1 | 15 | 30 | 60 | 90 | |||
| Visual appearance | Colour | 5 | Light yellow | Light yellow | Light yellow | Light yellow | Light yellow |
| 30 | Light yellow | Light yellow | Light yellow | Light yellow | Light yellow | ||
| Clarity | 5 | Opaque | Opaque | Opaque | Opaque | Opaque | |
| 30 | Opaque | Opaque | Opaque | Opaque | Opaque | ||
| Odour | 5 | Orange Flavour | Orange Flavour | Orange Flavour | Orange Flavour | Orange Flavour | |
| 30 | Orange Flavour | Orange Flavour | Orange Flavour | Orange Flavour | Orange Flavour | ||
| Physical properties | pH | 5 | 4.33 | 4.3 | 4.36 | 4.31 | 4.3 |
| 30 | 4.51 | 4.53 | 4.5 | 4.52 | 4.51 | ||
| Specific gravity | 5 | 0.9021 | 0.9019 | 0.9022 | 0.9021 | 0.902 | |
| 30 | 0.7145 | 0.715 | 0.7145 | 0.7122 | 0.7125 | ||
| Assay | 5 | 97.50 % | 97.10 % | 97.00 % | 97.00 % | 97.00 % | |
| 30 | 97.10 % | 97.10 % | 97.10 % | 97.10 % | 97.00 % | ||
| Microbial limit | Total aerobic bacteria (NMT 2X10 cfu/g) | 5 | <2X10 cfu/g | <2X10 cfu/g | <2X10 cfu/g | <2X10 cfu/g | <2X10 cfu/g |
| 30 | <2X10 cfu/g | <2X10 cfu/g | <2X10 cfu/g | <2X10 cfu/g | <2X10 cfu/g | ||
| Total yeast and moulds (NMT 2X10 cfu/g) | 5 | <2X10 cfu/g | <2X10 cfu/g | <2X10 cfu/g | <2X10 cfu/g | <2X10 cfu/g | |
| 30 | <2X10 cfu/g | <2X10 cfu/g | <2X10 cfu/g | <2X10 cfu/g | <2X10 cfu/g | ||
| E.coli (Absence in 1 g) | 5 | Conforms | Conforms | Conforms | Conforms | Conforms | |
| 30 | Conforms | Conforms | Conforms | Conforms | Conforms | ||
Table 2: Stability Profile of 10 mg/ml BACLOFEN in X-Temp® Oral Suspension
The microbiological stability analysis demonstrated that the microbial quality of baclofen in X-Temp® suspension remained within the specified parameters throughout the 3 mo study period, regardless of whether stored at 5° or 30°. The total viable aerobic count remained low, and the total yeasts and moulds count was minimal. E. coli was consistently absent over the entire 90 d duration. These findings underscore the efficacy of the preservatives used in the extemporaneous preparation, ensuring microbial stability.
Fig. 3, illustrates the chromatogram, demonstrating the selectivity of the HPLC method employed in this study. Minimal interference from the formulation's excipients was observed. Analysis of chromatograms obtained at different intervals throughout the stability study detected no additional peaks, suggesting the absence of potential degradation compounds. This study has successfully evaluated the stability of baclofen in X-Temp® suspension in concentrations of 1 mg/ml and 10 mg/ml. The physical, chemical, and microbiological stability was assessed at refrigerated conditions (5°±1°) and at room temperature (30°±1°) for up to 3 mo. This study suggests that the suspension remained stable within this specified range and storage conditions. This indicates that X-Temp® oral suspension system has the potential to serve as a dependable vehicle for compounding various API. This study has successfully evaluated the stability of baclofen in X-Temp® suspension in concentrations of 1 mg/ml and 10 mg/ml. The physical, chemical, and microbiological stability was assessed at refrigerated conditions (5°±1°) and at room temperature room temperature (30°±1°) for up to 3 mo. This study suggests that the suspension remained stable within this specified range and storage conditions. This indicates that X-Temp® oral suspension system has the potential to serve as a dependable vehicle for compounding various API.
This study emphasizes the significant implications for paediatric formulations and compounding practices. Commercial oral formulations are often not suitable for paediatric use, so adult formulations need to be reformulated in clinical settings[20]. The World Health Organization (WHO)[21], the European Medicines Agency, United States (US) FDA[22], Health Canada[23], and the European Paediatric Formulation Initiative (EUPFI) consortium all support the development of paediatric-specific formulations[9]. This collaborative effort aims to improve the effectiveness and safety of medications for children.
Currently, compounding mainly involves liquid dosage forms, and there is limited data available on the stability and safety of solid oral formulations[14]. This study provides crucial data on the stability of baclofen in X-Temp® suspension, which is essential for ensuring safe and effective pediatric dosing. The demonstrated stability of baclofen at both low and high doses under various storage conditions provides a reliable foundation for pediatric compounding practices. This ensures that compounded formulations maintain their intended potency and safety over time, addressing the gap in commercially available paediatric formulations and supporting informed clinical decision-making in paediatric pharmacotherapy. Several studies have investigated the stability of baclofen in suspension. Polonini and colleagues[24] reported 90 d of stability for a 2.0 mg/ml extemporaneous aqueous baclofen solution compounded with SyrSpend SF pH4 at room temperature. Johnson et al.[25] and colleagues demonstrated 35 d stability of baclofen in tablet dissolved solutions in simple syrup NF, stored at 4° in amber glass. Another study indicated that a 10 mg/ml baclofen suspension, prepared in various mixtures including ORA-Sweet® and Ora-Plus® (Paddock Laboratories) and cherry syrup and stored at 25° in amber or clear polyethene terephthalate bottles, maintained stability for up to 60 d[26]. To our knowledge, this study represents the first investigation of the stability of 1 mg/ml and 10.0 mg/ml baclofen formulations prepared from commercially available baclofen tablets in X-Temp® suspension up to 30 d.
However, the dissolution and efficacy of baclofen were not investigated in this research. Additional information on the efficacy and safety of baclofen in modified dosage forms is essential. There is a need now for pharmaceutical companies to present ageappropriate development strategies for new drug formulations and make efforts to standardise dosage form changes[22]. Future research on dissolution studies and efficacy evaluations of baclofen in X-Temp® suspension is important to ensure effectiveness and safety in paediatric patients[27]. Additionally, it is vital to develop age-appropriate formulations and conduct pharmacokinetic and pharmacodynamic studies to optimize dosing for different paediatric age groups[28]. The baclofen in X-Temp® suspension, at concentrations of 1 mg/ ml and 10 mg/ml, compounded from commercially available tablets, exhibits stability when stored at both 5°±2° and 30°±2° for 90 d. This investigation provides additional evidence supporting the suitability of the X-Temp® oral suspension system as a stable medium for compounding various API.
Acknowledgements:
This study acknowledges Pharm-D Health Science (Sdn. Bhd.) for providing the baclofen tablets (Musafen, DuoPharma (M) Sdn. Bhd.) and X-Temp® oral suspension system (Pharm-D Health Science Sdn. Bhd.). This study was funded through an industrial grant awarded by Pharm-D Health Science Sdn. Bhd. (C-M010-CON-000006).
Author’s contributions:
Conceptualisation was done by Nagashekhara Molugulua, Sathiya Marana. Methodology was done by Nagashekhara Molugulua, Sathiya Marana, Usha Sundralingama. Data analysis was done by Sathiya Marana. Writing-original draft was done by Sathiya Marana. Writing-reviewing and editing was done by Sathiya Marana and Nagashekhara Molugulua.
Conflict of interests:
The authors declared no conflict of interests.
References
- Ghanavatian S, Derian A. Baclofen. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright© 2024, StatPearls Publishing LLC.;2024.
- Deon LL, Gaebler-Spira D. Assessment and treatment of movement disorders in children with cerebral palsy. Orthop Clin North Am 2010;41(4):507-17.
[Crossref] [Google Scholar] [PubMed]
- Mashkovskii MD. Drugs [in Russian]. Part II, Meditsina, Moskow. 1993;356.
- Dietz N, Wagers S, Harkema SJ, D'Amico JM. Intrathecal and oral baclofen use in adults with spinal cord injury: A systematic review of efficacy in spasticity reduction, functional changes, dosing, and adverse events. Arch Phys Med Rehabil 2023;104(1):119-31.
[Crossref] [Google Scholar] [PubMed]
- He Y, Brunstrom-Hernandez JE, Thio LL, Lackey S, Gaebler-Spira D, Kuroda MM, et al. Population pharmacokinetics of oral baclofen in pediatric patients with cerebral palsy. J Pediatr 2014;164(5):1181-8.
[Crossref] [Google Scholar] [PubMed]
- Moran LR, Cincotta T, Krishnamoorthy K, Insoft RM. The use of baclofen in full-term neonates with hypertonia. J Perinatol 2005;25(1):66-8.
[Crossref] [Google Scholar] [PubMed]
- Schulz E, Mathew OP. Is oral baclofen effective in neonatal hypertonia? J Child Neurol 2012;27(2):197-9.
[Crossref] [Google Scholar] [PubMed]
- Thean FS, Chan LT, Yeoh LS, Ng RC. Stability study of an extemporaneous isoniazid oral suspension prepared using commercially available tablets with X-Temp® Oral Suspension System. Malays J Pharm 2021;7(2):77-84.
- Ivanovska V, Rademaker CM, van Dijk L, Mantel-Teeuwisse AK. Pediatric drug formulations: A review of challenges and progress. Pediatrics 2014;134(2):361-72.
[Crossref] [Google Scholar] [PubMed]
- National Association of Pharmacy Regulatory Authorities (NAPRA). Professional competencies for Canadian pharmacists at entry to practice. 2014.
- Café’s D. Crushing and mixing pills to ease swallowing should be avoided. Here are some alternatives. In: Hanson R, editor. 2021.
- Blaszczyk A, Brandt N, Ashley J, Tuders N, Doles H, Stefanacci RG. Crushed tablet administration for patients with dysphagia and enteral feeding: Challenges and considerations. Drugs Aging 2023;40(10):895-907.
[Crossref] [Google Scholar] [PubMed]
- ASHP compounded oral liquid version 1.01 finalized list july 2017.
- Saito J, Akabane M, Ishikawa Y, Iwahashi K, Nakamura H, Yamatani A. Retrospective survey of compounded medications for children in Japan. Eur J Pharm Biopharm 2020;155:122-7.
[Crossref] [Google Scholar] [PubMed]
- Wahab MF, Amir AB, Razi SS, Ramli NB. The stability study of extemporaneous preparations prepared in the outpatient pharmacy of Tuanku Fauziah hospital stored in patient’s setting. Pharm Res Rep 2020;3(2):40.
- Abidin KN, Zunaidi NA. Cost outcomes of conversion from simple syrup to X-Temp® suspension in production of extemporaneous oral morphine solution in hospital Tengku Ampuan Afzan. Malays J Pharm 2023;9(1):1-6.
- Salim SN, Murshid MD, Gazzali AM. Stability of extemporaneous rifampicin prepared with X-Temp® oral suspension system. J Pharm 2021;1(1):54-62.
[Crossref] [Google Scholar] [PubMed]
- Cartwright AC. The British pharmacopoeia, 1864 to 2014: Medicines, international standards and the state. Ambix 2016;63(2):197.
[Crossref] [Google Scholar] [PubMed]
- Commission B. British pharmacopoeia 2015. Stationery office, London. 2014.
- Saito J, Hanawa T, Ozawa A, Matsumoto T, Yoshikawa N, Harada T, et al. Stability study of baclofen in an oral powder form compounded for pediatric patients in Japan. Children 2022;9(9):1313.
[Crossref] [Google Scholar] [PubMed]
- Gerrard SE, Walsh J, Bowers N, Salunke S, Hershenson S. Innovations in pediatric drug formulations and administration technologies for low resource settings. Pharmaceutics 2019;11(10):518.
[Crossref] [Google Scholar] [PubMed]
- Gadge PM, Kenjale PP, Pokharkar VB, Gaikwad VL. Global pediatric regulations: An overview. Ther Innov Regul Sci 2020;54(3):552-8.
[Crossref] [Google Scholar] [PubMed]
- Hepburn CM, Gilpin A, Autmizguine J, Denburg A, Dupuis LL, Finkelstein Y, et al. Improving paediatric medications: A prescription for Canadian children and youth. Paediatr Child Health 2019;24(5):333-5.
[Crossref] [Google Scholar] [PubMed]
- Polonini H, da Silva SL, Brandão MA, Bauters T, de Moerloose B, Ferreira AO. compatibility of baclofen, carvedilol, hydrochlorothiazide, mercaptopurine, methadone hydrochloride, oseltamivir phosphate, phenobarbital, propranolol hydrochloride, pyrazinamide, sotalol hydrochloride, spironolactone, tacrolimus monohydrate, ursodeoxycholic acid, and vancomycin hydrochloride oral suspensions compounded with SyrSpend SF pH4. Int J Pharm Compd 2018;22(6):516-26.
[Google Scholar] [PubMed]
- Johnson CE, Hart SM. Stability of an extemporaneously compounded baclofen oral liquid. Am J Hosp Pharm 1993;50(11):2353-5.
[Crossref] [Google Scholar] [PubMed]
- Allen LV, Erickson III MA. Stability of baclofen, captopril, diltiazem hydrochloride, dipyridamole, and flecainide acetate in extemporaneously compounded oral liquids. Am J Health Syst Pharm 1996;53(18):2179-84.
[Crossref] [Google Scholar] [PubMed]
- Lopez FL, Ernest TB, Tuleu C, Gul MO. Formulation approaches to pediatric oral drug delivery: benefits and limitations of current platforms. Expert Opin Drug Deliv 2015;12(11):1727-40.
[Crossref] [Google Scholar] [PubMed]
- Luterbach CL, Rao GG. Use of pharmacokinetic/pharmacodynamic approaches for dose optimization: A case study of plazomicin. Curr Opin Microbiol 2022;70:102204.
[Crossref] [Google Scholar] [PubMed]