- *Corresponding Author:
- I. Kuźmicka
Institute of Chemistry, University of Białystok, Hurtowa 1, Białystok 15-399, Poland
E-mail: kuzmicka@uwb.edu.pl
| Date of Submission | 21 September 2007 |
| Date of Revision | 06 August 2008 |
| Date of Acceptance | 12 January, 2009 |
| Indian J Pharm Sci,2009, 71 (1): 8-18 |
Abstract
Psychotropic drugs are an important family of compounds from a medical point of view. Their application in therapy requires methods for the determination in pharmaceutical dosage forms and body fluids. Several methods for their analysis have been reported in the literature. Among the methods, spectrophotometric and electrochemical are very useful for the determination of the drugs. Some of the spectrophotometric methods are based on the formation of the binary and ternary compounds with complexes of metals. The formed compounds are sparingly soluble in water, but quantitatively extracted from aqueous phase into organic solvents and the extracts are intensely colored and stable for a few days. These complexes have been employed in pharmaceutical analysis. The electrochemical procedures are very useful in determination of the psychotropic substances in pharmaceutical preparations.
Keywords
Psychotropic drugs, spectrophotometric and electrochemical methods, analysis
Introduction
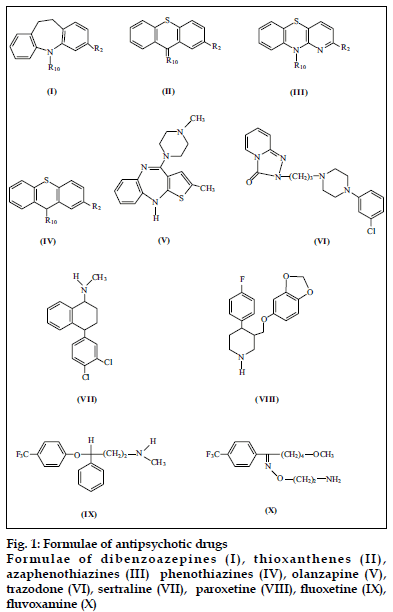
Psychotropic drugs belong to a large group of organic compounds. They exhibit high activity and manysided pharmacological actions. Owing to these properties, they have been subject of extensive pharmacological studies. Typical psychotropic drugs are often prescribed in severe cases of schizophrenia or depression, when new generation neuroleptics and SSRI medications do not work. Typical tricyclic psychotropic drugs are characterized by a tricyclic rings and presence of sulfur and nitrogen atoms. Structures of select psychotropic drugs of both typical and atypical type are presented in fig. 1.
Tricyclic psychotropic drugs due to their characteristic structure – the presence of chemically active sulfur and nitrogen atoms and substituents react with oxidants (e.g. Os(VIII), Cr(VI), V(V), Ce(IV), Au(III), Fe(III)), platinum metals (e.g. Pt(IV), Rh(IV), Ru(III), Pd(II)), thiocyanate or halide complexes of metals and some organic substances (e.g. picric acid, alizarin S, dipicrylamine) [1-6]. The above mentioned reactions are very important from an analytical point of view.
The liability of phenothiazines, thioxantenes and dibenzoazepines to the oxidation in acid medium by K2Cr2O7, NH4VO3, Ce(SO4)2 has been exploited as indicators in various redox titrations [5,7] and as reagents for the spectrophotometric determination of the drugs [8-10].
The new generation psychotropic drugs, e.g., fluoxetine, fluvoxamine and trazodone react with some organic compounds such as chrome azurol S, eriochrome cyanine R, bromophenol blue, methyl orange, bromocresol green and thymol blue to form ion-association compounds [11-16]. These properties have been exploited for the development of spectrophotometric methods for the determination of the psychotropic drugs.
Atypical psychotropic drugs can also easily reduced on mercury electrode [17,18]. Mechanism of electrochemical reactions of these compounds was investigated using different electrochemical methods, e.g. cyclic voltammetry and differential pulse voltammetry [19]. Electrochemical behaviour of these substances can be successfully employed for elaborate simple, rapid and sensitive procedures for the determination of new psychotropic drugs in pharmaceutical preparations and biological fluids.
In the presented review the analytical applications of the reactions of psychotropic drugs with organic substances and thiocyanate complexes of metals for the spectrophotometric determination of the drugs have been described. This review is also devoted to the analytical application of the electrochemical methods for their determination.
Binary and ternary complexes of psychotropic drugs
Psychotropic substances, e.g. phenothiazines, dibenzocycloheptadienes and thioxantenes react with organic substances, which occur as anions in aqueous solutions forming colored ion-association binary compounds [20]. These compounds are insoluble into organic solvents, e.g. chloroform, butanol and benzene. The extracts are intensely colored and very stable (1-3 days) [21]. These properties were applied successfully for the spectrophotometric determination of psychotropic drugs [21-41] (Table 1).
| Psychotropic drugs | Organic reagents | Organic solvent | λ [nm] | ε [l·-mol-1cm-1] | Range of determination [ppm] | Ref. |
|---|---|---|---|---|---|---|
| Perphenazine | Dipicrylamine | chloroform | 420 | 1.09·104 | 8 – 60 | 21 |
| Chlorpromazine | chloroform | 420 | 3.22·104 | 0.7 - 7 | 22 | |
| Thioproperazine | 435 | 1.51·104 | 1.6-16 | |||
| Chlorpromazine | Alizarin S | chloroform | 420 | 8.00·103 | 7 – 70 | 23 |
| Promethazine | chloroform | 420 | 8.50·103 | 7 - 70 | 24 | |
| Chlorpromazine | Brilliant blue | chloroform | 620 | 2.21·104 | 1 – 10 | 25 |
| Fluphenazine | 1.02·104 | 2 - 10 | ||||
| Thioridazine | 2.78·104 | 1 - 10 | ||||
| Levomepromazine | Bromophenol blue | chloroform | 409 | - | 5 - 25 | 26 |
| Fluoxetine | Chrome | chloroform | 500 | 1.02·104 | 5-50 | 12 |
| Fluvoxamine | Azurol S | chloroform- | 502 | 9.05·103 | 7-100 | |
| butanol (3:1) | ||||||
| Chlorpromazine | chloroform | 510 | 1.48·104 | 2 – 20 | 27 | |
| Promethazine | 2.04·104 | 1 – 12 | ||||
| Thioproperazine | 460 | 1.57·104 | 2 – 28 | 28 | ||
| Trifluopromazine | 2.12·104 | 1 – 12 | ||||
| Promethazine | Methyl orange | chloroform | - | - | 20–100 | 29 |
| Imipramine | Eriochrome | butanol | 520 | 4.80·103 | 10 - 80 | 30 |
| Cyanine R | ||||||
| Chlorpromazine | Pyrocatechol | chloroform - | 445 | 1.04·104 | 3.5 – 35 | 31 |
| Chlorprothixene | violet | butanol (5:1) | ||||
| chloroform – | 445 | 1.40·104 | 3.5 - 32 | 32 | ||
| butanol (5:1) | ||||||
| Chlorpromazine | Flavianic acid | benzene | 390 | 9.60·103 | 7– 70 | 33 |
| Thioridazine | 385 | - | 5 – 20 | 34 | ||
| Promazine | Picramic acid | chloroform | 500 | 2.10·103 | 8 – 80 | 35 |
| Thioproperazine | - | 16 – 160 | 36 | |||
| Thioridazine | Picric acid | benzene | 405 | - | 20 – 70 | 34 |
| Thioproperazine | 406 | 7.30·103 | 10 - 80 | 33 | ||
| Trifluoperazine | 406 | 6.70·103 | 10 – 100 | 37 | ||
| Perphenazine | 407 | 7.60·103 | 4-80 | 21 | ||
| Promazine | 405 | - | 10-60 | 38 | ||
| Methopromazine | ||||||
| Promethazine | Orange II | dichloro-methane | 485 | - | 5 – 20 | 25 |
| Fluphenazine | 1.02·104 | 3 – 25 | ||||
| Prochlorpromazine | chloroform | 495 | 5.40·104 | 30-130 | ||
| Trifluoperazine | 1.14·104 | 3 – 25 | 39 | |||
| Nortriptyline | chloroform | 490 | - | - | ||
| Chlorpromazine | Bromocresol green | chloroform | 420 | 2.63·104 | 2 – 8 | 40 |
| Trifluorpromazine | 2.02·104 | 2 -10 | ||||
| Thioproperazine | 2.65·104 | 2 -12 | ||||
| Thioridazine | 2.13·104 | 2 -18 | ||||
| Chlorpromazine | Titanium yellow | ethyl acetate | 405 | - | 10 - 60 | 41 |
| Fluoxetine | Eriochrome | butanol | 520 | 1.7·104 | 2-30 | 13 |
| Fluvoxamine | cyanine R | 518 | 6.5·103 | 2-40 | ||
| Trazodone | Bromophenol blue | chloroform | 414 | - | 3.75-14 | 11 |
Line denotes for lack reference
Table1: Determinationofpsychotropicdrugs in binarysystems
Significant advantages of the spectrophotometric methods are that they can be applied to the determination of individual components in a multicomponent mixture. This aspect of spectrophotometric analysis is of major interest in pharmacy, since it offers distinct possibilities in the assay of a particular component in a complex dosage formulation. For example in the spectrophotometric method elaborated by Basavaiah [40], the commonly used additives and excipients in the dosage forms of active compounds, such as starch, lactose, glucose, sugar, talc, gelatin, magnesium stearate, sodium lauryl sulphate, sodium sulphite, sodium chloride, calcium chloride, ethanol, formaldehyde and sodium salt of EDTA did not interfere in the analysis.
It has been pointed out in our previous papers [2,4,42] that active substances of psychotropic drugs (PS), which occur in an aqueous solution as large cation, PS·HCl⇄(PS·H)++Cl-, or base, PS+H+⇄(PS·H)+, react with some thiocyanate and halide complexes of metals forming ion-association compounds, (m-n) (PS·H)++ [MeXn](m-n)-⇄(PS·H)(m-n) [MeXn], where, Me denotes metal ion with an n-oxidation state (e.g. Co(II), Cd(II), Hg(II), Pd(II), Fe(III), Cr(III), Ti(IV), Pt(IV), Re(IV), Nb(V), Mo(V), W(V), U(VI)); X- SCN- or halide ion. These compounds exhibit a number of properties very important from the analytical view-point, i.e, a well-defined composition and high molecular weight. They are hardly soluble in water but fairly soluble in acetone, methanol, ethanol. They can be extracted from aqueous phase with chloroform and other organic solvents. The extracts are intensely colored and stable for a few days [2]. These properties have been used for the development of the spectrophotometric methods for the determination of psychotropic drugs.
Structures of binary and ternary complexes of psychotropic drugs
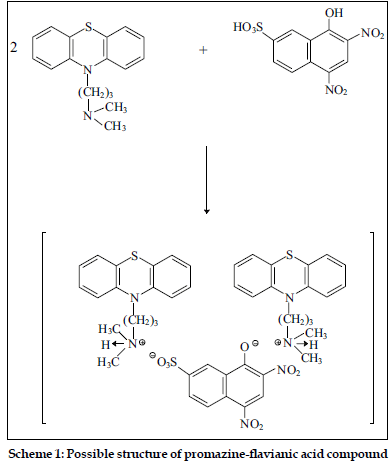
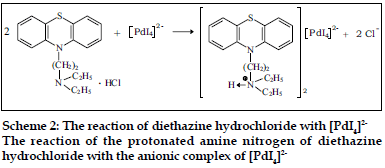
The compositions of binary and ternary complexes of psychotropic drugs, e.g., dibenzoazepines, dibenzocycloheptadienes, thioxanthenes, phenothiazines were established by Job`s continuous variation method and by spectrophotometric titration. These compositions are described in the literature [2,4,43,44]. The absorption spectra of the compounds obtained in the UV/VIS and IR-region have been recorded. It was found the main absorption bands of the components are observed in UV-VIS spectra of the compounds [2,43,45]. Infrared spectra of the compounds studied were recorded (KBrdisc) in the region of 400-4000 cm-1 [2,27,45,46]. Significant changes in the spectra were observed in the region of 2300-3700 cm-1. For example, the wide bands, appearing in the phenothiazines spectra in the region of 2300-2700 cm-1 and characteristic for vibration of ≡NH+ group, were shifted (~350 cm-1) towards higher frequencies in the spectra of the compounds. On the basis of the data obtained, it has been established that the compounds studied are ionassociates. For example, the structure of the compound promazine with flavianic acid and compound obtained in Pd(II) - I- - DT system can be presented as follows (Schemes 1 and 2).
Spectrophotometric methods
Reviews of the methods for the determination of phenothiazines presented by Blažek [47], Fairbrother [48] and Puzanowska-Tarasiewicz [49] show that spectrophotometric methods are very useful for the determination of psychotropic substances in pharmaceuticals and body fluids [50-56]. They are summarized in Table 2.
| Psychotropicsubstance [ppm] | Systemsolvent | Organic [nm] | λ [l·mol-1cm-1] | Εdetermination | Range of | Ref. |
|---|---|---|---|---|---|---|
| Levomepromazine (LPZ) | LPZ- [Cr(NH3 )2 (SCN)4 ] | acetone | 525 | 9.12·10-4 | 53 – 427 | 45 |
| Chlorpromazine (CPZ) | CPZ- [Cr(NH3 )2 (SCN)4 ] | acetone | 520 | 9.12·10-4 | 156 – 689 | 50 |
| Chlorpromazine (CPZ) | Fe(III)-SCN--CPZ | chloroform | 490 | - | 120 - 300 | 51 |
| Levomepromazine (LPZ) | Fe(III)-SCN-- LPZ | 140 - 400 | ||||
| Promethazine (PMT) | Fe(III)-SCN-- PMT | 160 - 550 | ||||
| Chlorpromazine (CPZ) | Co(II)-SCN--CPZ | ether | 620 | - | 100 - 600 | 52 |
| Chloracizine (CRZ) | Co(II)-SCN--CRZ | 100 - 900 | ||||
| Chlorpromazine (CPZ) | Ge(IV)-PCV-CPZ | cyclohexa-none | 580 | 6.8 ·103 | Jul-70 | 53 |
| Chlorpromazine (CPZ) | Sn(IV)-PCV-CPZ | butanol | 580 | - | 20-Feb | 31 |
| Desipramine (DE) | Ti(IV)- SCN- - DE | chloroform | 355 | 5.85 ·10-4 | 5 – 200 | 54 |
| Thioridazine (TR) | Ti(IV) – SCN-- TR | chloroform - | 417 | - | 20 - 160 | |
| Perazine (PZ) | Ti(IV) – SCN-- PZ | butanol (4:1) | 360 | 8.96·103 | 20 – 170 | 55 |
| Amitriptyline (AM) | Ti(IV) – SCN-- AM | 3 – 60 | ||||
| Promazine (PM) | Nb(V)-SCN--PM | trichloro-ethylene | 400 | - | 20 – 200 | 56 |
| Imipramine (IM) | Nb(V)- SCN- - IM | butanol-chloroform (1:9) | 350 | 6.67∙10-4 | 0.8 - 8 | 43 |
| Doxepin (DX) | Ti(IV)- SCN- - DX | butanol-chloroform (2:3) | 400 | 7.12· 103 | 5 – 50 | 44 |
| Chlorprothixene (CX) | Nb(V)- SCN- - CX | butanol | 362 | 8·103 | Sep-50 | 46 |
Line denotes for lack reference
Table2: Detrmination of psychotropic drugs in ternary systems.
Materials and Methods
Recently Misiuk [44] studied the ion association compounds of doxepin (DX) with thiocyanate complexes of titanium (IV) and iron (III). The produced compounds were insoluble in water, but well soluble in some organic solvents. They were quantitatively extracted with a mixture of butanolchloroform (2:3) and (1:4) using titanium (IV) and iron (III) thiocyanates, respectively. The mentioned properties were applied for the elaboration of new spectrophotometric methods for the determination of doxepin in DX-Ti-SCN- and DX-Fe-SCN- systems, respectively. The proposed methods have been successfully applied for the determination of the main active ingredient in different dosage forms. The method can also be used for the determination of doxepin in the presence of its degradation product, dibenzo [b,e]oxepin-11-(6H)-one. The dibenzo [b,e] oxepin-11-(6H)–one was examined by TLC, UV and IR techniques. The precision, accuracy and reproducibility of the methods were good and RSD values were low.
It has been found that imipramine and chlorprothixene react with thiocyanate complexes of niobium (V) forming yellow sparingly soluble in water compound in a molar ratio of 1:2 of Nb (V):each drug [43,46]. These compounds can be quantitatively extracted with chloroform-butanol (1:9) or butanol alone. The spectrophotometric methods have been developed for the determination of imipramine and chlorprothixene in the ranges of 0.8-8 ppm and 9-50 ppm, respectively.
Desipramine forms a compound of the ion pair type with thiocyanate complexes of titanium (IV) [54] in acid medium, which can be quantitatively extracted with chloroform. The properties have been used for the spectrophotometric determination of desipramine in the range of 5 - 200 ppm.
Chlorpromazine (CPZ) forms a yellow sparingly soluble in water compound of molar ratio of 1:1 (λmax = 445 nm) with pyrocatechol violet (PCV) in an acid medium [31]. The drug also reacts with tin(IV) ions in the presence of pyrocatechol violet, in aqueous phase at the molar ratio Sn(IV):PCV:CPZ = 1:2:2, and in the organic phase at the ratio 1:2:4 (λmax = 580 nm). These compounds can be quantitatively extracted from aqueous solutions with chloroform-butanol (5:1) or butanol alone. Taking advantage of these properties, the spectrophotometric methods for the determination of chlorpromazine have been developed. The methods proved suitable for assaying chlorpromazine in the range of its concentrations from 3.5 ppm to 35 ppm and from 2 ppm to 20 ppm, respectively. The reaction in the systems chlorpromazine-chrome azurol S [27] and germanium (IV)-pyrocatechol violet-chlorpromazine [53] were applied for CPZ determination in the concentration range 2-20 ppm and 7-70 ppm, respectively.
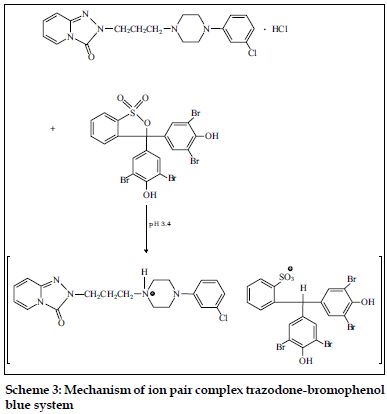
Fluoxetine and fluvoxamine were determined spectrophotometrically using chrome azurol S and eriochrome cyanine R [12,13]. These dyes react in aqueous media with studied psychotropic drugs forming colored, sparingly soluble in water complexes. These complexes can be quantitatively extracted with chloroform or chloroform-buthanol (3:1). Gindy et al. [11] described the methods for the determination of trazodone in pharmaceutical preparations. The spectrophotometric and spectrofluorimetric methods were based on the formation of yellow ion pair complex between the basic nitrogen of the drug and bromophenol blue. The formed complex was extracted with chloroform and the absorbance was measured at 414 nm. The suggested mechanism of trazodone – bromophenol blue ion pair complex is presented in Scheme 3.
Electrochemical methods
Psychotropic active substances are easily oxidized (e.g., dibenzoazepines, thioxanthenes, phenothiazines) or reduced (e.g., trazodone, sertraline, paroxetine), electrochemically. The first step in the electrochemical oxidation of phenothiazine and azaphenothiazine derivatives occurs at the sulphur atom, while the second wave is attributed to the transformation of the radical cation into a dication [5]. The mechanism of the oxidation of thioxanthenes is not fully understood, but the potentials and peaks shapes of the thioxanthene derivatives are similar to those of the phenothiazine derivatives, the first oxidation wave involving two-electron oxidation to the sulphoxide [1].
The dibenzoazepines are the most easily oxidized, the first electron is removed from the monomer nitrogen and the radical can then exist in a number of resonance forms. The monocation rapidly dimerises or reacts with an unoxidised molecule. The dimerisation is accompanied by the loss of two protons per dimer. The dimer is more easily oxidized than the monomer [57].
Electrochemical oxidation of some active substances is exploited for using these substances in polarographic and voltammetric analysis. Several electrochemical techniques have been applied for the determination of psychotropic substances in drugs preparations and differential biological samples [58-60]. Oelschlager [59] reviewed the polarographic methods reported for psychotropic drugs including phenothiazine and azaphenothiazine derivatives. Temsamani et al. [61] have analyzed chlorpromazine in plasma by using cyclic voltammetry and modified gold electrode. Chlorpromazine has been determined in urine samples of patients by adsorptive stripping voltammetry in the presence of Triton X-100 [62]. Fluphenazine and trifluoperazine were determined by differential pulse voltammetry after pre-concentration at a wax-impregnated graphite electrode. For plasma, the electrode was covered with a membrane to prevent fouling by proteins [63]. Alternatively, promazine, chlorpromazine and promethazine spiked in urine samples were oxidized by nitrous acid into the corresponding sulphoxides, which were polarographically active. They produce well-defined diffusion-controlled cathodic wave [58].
The dibenzoazepine derivatives, e.g. imipramine, clomipramine and trimipramine were determined in drugs preparations and plasma samples with adsorptive stripping voltammetry [64,65]. Imipramine and desipramine were analysed by cyclic voltammetry at glassy carbon and boron-doped diamond electrode [66]. Wang et al. [67] determined imipramine and trimipramine in urine sample by using cyclic voltammetry and differential pulse voltammetry. Among thioxanthene derivatives, zuclopenthixol was determined quantitatively by measuring the height of voltammetric peaks. The oxidative voltammetric behaviour of zuclopenthixol at a glassy carbon electrode has been studied using cyclic, linear sweep and differential pulse voltammetry [68]. Tuzhi et al. [69] reported an adsorptive pre-concentration method for the voltammetric measurement of trace levels of chlorprothixene. Alternatively, chlorprothixene and thiothixene were determined polarographically through the formation of their bromo-derivatives, which manifest well defined cathodic waves in select supporting electrolytes [70].
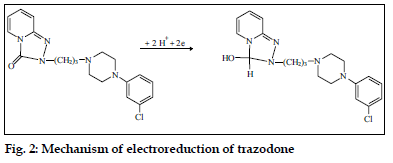
Trazodone, which is a triazolopyridine derivative, unlike the tricyclic antidepressants was studied using direct current, differential pulse and alternating current polarography [71]. It was concluded that, only the carbonyl group was involved in the reduction process according to the mechanism given in fig. 2. The proposed method was successfully applied to the determination of the trazodone in pure form and in formulations and the biological fluids (human urine and plasma).
A review of the electrochemical methods for the determination of some psychotropic drugs (e.g. phenothiazines, azaphenothiazines, dibenzoazepines, thioxanthenes) [68,72-89] is presented in Table 3 and the electrochemical methods for the determination of new atypical psychotropic drugs (e.g. olanzapine, sertraline, trazodone) [90-92] are given in Table 4.
| Psychotropic drug | Medium | Method | Working electrode |
Range of determination [mol/I] |
LOD [mol/l] |
Practical application |
Ref. |
|---|---|---|---|---|---|---|---|
| Chlorpromazine, | Britton's buffer, pH=2-7 | DPP | Hg | 6·10-6 - 1·10-4 | 3·10-7 | Drug | 58 |
| Promazine, | 5·10-6 - 8·10-5 | 3·10-7 | preparations | ||||
| Promethazine | 8·10-6 - 1·10-4 | 4·10-7 | Urine | ||||
| Chlorpromazine | 0.2 M H2S04, | LSV | Ru | 2·10-4 - 8·10-4 | - | Drugs preparations | 72 |
| Thioridazine | 0.2 M H2S04, | CV | Pt, | 5·10-5 - 1·10-3 | - | Drugs preparations | 73 |
| Ru, | 6·10-4 - 1·10-2 | ||||||
| GC | 1·10-4 - 1·10-3 | ||||||
| Fluphenazine | 0.5 MI-1,504, | CV | Pt, | 4·10-4 - 1·10-2 | - | Drugs preparations | 74 |
| Phosphate buffer, pH=6.2 | 2·10-4 - 4·10-3 | ||||||
| 0.5 M H250, | GC | 2·10-5 - 8·10-4 | |||||
| Promazine, | 0.1MHCl | LSV | CPE, | 2.5·10-5- 5·10-3 | - | Drugs preparations | 75 |
| Promethazine, | SCPE | 2.5·10-5- 5·10-3 | |||||
| Levomepromazine | GC | 6.2·10-5-1.2·10-3 | |||||
| Chlorpromazine, Thioridazine | 0.1 M IIaCl0, in acetonitrile |
DPV | Pt | 7·10-7 -1.4·10-5 | 4·10-7 | Drugs preparations | 76 |
| Chlorpromazine, | Britton's buffer, pH=7.0 (in the presence of Triton X-100) |
AdSV | Hg | 2·10-8 -5·10-6 | 4.2·10-9 | Drugs preparations Urine |
62 |
| Ethopropazine | 0.05 M Phthalate buffer, pH=3.5 |
CV | Au | 4·10-7 - 4·10-6 | 77 | ||
| (in the presence of SDS) | - | ||||||
| Promethiazine, | Phosphate buffer, pH=7 | DPV | WIGE | 5·10-7 – 1·10-4 | 5·10-8 | Urine, plasma | 63 |
| Diethazine, | - | ||||||
| Trifluoperazine, | |||||||
| Fluphenazine | |||||||
| Chlorpromazine | Phosphate buffer, pH=7 | DPV | WIGE | 4.8·10-8 – 2.4·10-4 | 5·10-9 | Urine | 78 |
| Chlorpromazine, | 0.1 M Phosphate | AdSV | CPE | 8.3·10-8 – 2·10-6 | 1·10-9 | Urine, | 79, |
| Perphenazine, | buffer, pH=7.4 | DPV | blood samples | 80 | |||
| Promazine | |||||||
| Chlorpromazine | 0.1 M Phosphate | AdSV | CPE | 1.5·10-6 – 9·10-6 | 1·10-7 | Urine | 81 |
| buffer, pH=7.4 | DPV | ||||||
| Chlorpromazine, | Britton's buffer, pH=9 | AdSV | GC | 1.5·10-7 – 3.4·10-6 | 1.3·10-7 | Blood | 82 |
| Promethiazine | DPV | 3·10-7 – 3·10-6 | 1.2·10-7 | samples | |||
| Phenothiazine, | Acetate buffer, | CV | MCPE | 2·10-8 – 3·10-7 | 1.2·10-8 | Drugs' | 83 |
| Chlorpromazine, promethiazine | pH=5.0 | 7·10-9 5·10-9 |
preparations | ||||
| Thioridazine, | Acetate buffer, | DPV | MCPE | 1·10-7 - 1·10-6 | 4.5·10-8 | Drugs' | 84 |
| Prochlorperazine, | pH=5 | 1.2·10-8 | preparations | ||||
| Chlorpromazine | |||||||
| Chlorpromazine, | Phosphate | DPV | MCPE | 1.96·10-7 | 1·10-7 | Model | 64 |
| Thioridazine, | buffer, pH=7.4 | 2.75·10-6 | 7·10-8 | serum | |||
| Prochlorperazine, | 4·10-8 | ||||||
| Levomepromazine | 8·10-8 | ||||||
| Thioridazine | Phosphate buffer, pH=6.6 |
AdSV CV, DPV |
MCPE | 1·10-8 - 1·10-7 | 7·10-9 | Drugs' preparations |
65 |
| Fluphenazine | 0.05 M HCOOH- HCOOHa buffer, pH=3.5 |
CV | MAu | 5·10-8 - 1.5·10-5 | 1·10-8 | Drugs' preparations |
85 |
| Perphenazine | 0.05 M borate | CV | MAu | 5·10-7 - 5·10-6 | Drugs' | 86 | |
| buffer, pH=10.0 | 6·10-6 - 5·10-5 | - | preparations | ||||
| Chlorpromazine | 0.05 M Phosphate buffer, pH=9 |
CV | MAu | 2·10-7 - 3·10-5 | - | Biological fluids |
61 |
| Promazine, Promethiazine, | Acetate buffer, pH=4.7 | CV, A |
CPE, SCPE |
2·10-7 - 3·10-5 | down to 1·10-8 | Drugs' preparations |
87 |
| Trifluoperazine, | |||||||
| Chlorpromazine, | |||||||
| Thioridazine | |||||||
| Prothipendyl | Britton's buffer, pH=3.5 |
DPP | Hg | - | Blood, plasma, urine |
59 | |
| Imipramine | 0.1 M H2504, Phosphate buffer, pH=7.4 |
CV | MCPE | 2·10-7 - 3·10-5 | - | Drugs' preparations |
60 |
| Imipramine, Trimipramine | Phosphate buffer, pH=6.6 |
AdSV CV, DPV |
MCPE | 1.10'-1.10° | 2·10-8 | Drugs' preparations |
65 |
| Imipramine, | Phosphate buffer, | DPV | MCPE | 6·10-5 - 8·10-4 | 1·10-7 | Plasma | 64 |
| Trimipramine, | pH=7.4 | 1.1·10-7 | |||||
| Clomipramine | 0.5·10-7 | ||||||
| Imipramine | Phosphate buffer, pH=9 |
LSV | MCPE | 1·10-7 - 8·10-6 | Drugs' preparations, urine |
88 | |
| Imipramine, | Acetate buffer, | DPV | 1·10-7 - 8·10-6 | 8.5 ·10-8 | Drugs' | 84 | |
| MCPE | |||||||
| Clomipramine, | pH=5 | 9.2 ·10-8 | preparations | ||||
| Trimipramine | 9.0 ·10-8 | ||||||
| pH=9 | preparations, urine |
||||||
| Imipramine, | Acetate buffer, | DPV | MCPE | 1·10-7 - 1·10-6 | 8.5 ·10-8 | Drugs' | 84 |
| Clomipramine, | pH=5 | 9.2 ·10-8 | preparations | ||||
| Trimipramine | 9.0 ·10-8 | ||||||
| Imipramine, | Phosphate buffer, | CV, | CPE, | 2·10-7 - 6·10-7 | 1.5·10-8 | Urine | 67 |
| Desipramine, | pH=9 | DPV | GC | 2·10-7 - 6·10-7 | 1.7·10-8 | ||
| Trimipramine | 2·10-7 - 1.6·10-6 | 1.4·10-8 | |||||
| Clomipramine | Britton's buffer, phosphate buffer |
SWP | Hg | - | - | Drugs' preparations | 89 |
| Imipramine, Clomipramine, | Phosphate buffer, pH=6.9 |
CV | GC, BDD |
- | - | Plasma | 66 |
| Dezipramine | |||||||
| Imipramine, Clomipramine, | 0.1 E1250,, | ISV, CV |
Pt, Au |
- | - | 57 | |
| Dezipramine, | |||||||
| Trimipramine | |||||||
| Zuclopenthixot | 0.1 M H,S0, Britton's buffer, pH=2.0 - 11.5, phosphate buffer, pH=5.2 - 8.3 |
CV, ISV, DPV |
GC | 8·10-7 - 2·10-4 | 2.2·10-7 | Drugs' preparations | 68 |
| Chlorprothixene | Britton's buffer, pH=8.2 |
CV, DPV | GC | 0.1 - 1 μg/ml time of condition =30s 0.01 - 1 μg/ml time of condition = 120 s |
- | Urine | 69 |
| Chlorprothixene, Thiothixene | 0.1 M HCl, Britton's buffer, |
dc-P | Hg | 2.7·10-5 - 1·10-4 | - | Drugs' preparations | 70 |
| pH=10.13 | |||||||
AdSV=adsorptive stripping voltammetry, CV= cyclic voltammetry, DPV= differential pulse voltammetry, LSV= linear sweep voltammetry, SWP = square wave polarography, dc-P=direct current polarography, ac-P=alternating current polarography, DPP=differential pulse polarography, A=amperometry, CPE= carbon paste electrode, SCPE= solid carbon paste electrode, GC=glassy carbon electrode, MCPE= modified carbon paste electrode, WIGE= wax-impregnated graphite electrode, BDD= boron-doped diamond, Ru= ruthenium electrode, Pt= platinum electrode, Au= gold electrode, MAu= modified gold electrode, RDE= rotating disc electrode, SDS= sodium dodecylsulfate.
Table 3: Voltammetric Analysis Of Psychotropic Drugs
| Psychotropic drug | Medium | Method | Working electrode | Range of determination [mol/l] | LOD [mol/l] | Practical application | Ref. |
|---|---|---|---|---|---|---|---|
| Olanzapine | Phosphate buffer, pH=2.5 | LSV | GC | 1.97·10-5-1.59·10-4 | 9.54·10-6 | Drugs preparations | 90 |
| Fluoxetine | Ringer buffer, pH=12 | AdSV | Hg | - | 91 | ||
| CV, | - | - | |||||
| DPV | - | - | |||||
| SWV | 5.2·10-5 -5.2·10-5 | 3.9·10-8 | |||||
| Paroxetine | Borate buffer,pH=8.8 | AdSV, | Hg | 3·10-6 - 1.7·10-5 | 4.8·10-7 | Drugs preparations | 17 |
| SWV, | |||||||
| Trazodone | Britton’s buffer,pH=10 | ac-P, | Hg | - | - | Urine, plasma | 71 |
| dc-P, DPV | 9.8·10-6 -7.8·10-5 | 7.69·10-7 | |||||
| 1.9·10-6 -5.8·10-5 | 2.54·10-7 | ||||||
| Sertraline | Borate buffer,pH=8.2 | FIA | Hg | 2·10-7 -1.2·10-6 | 1.5·10-7 | Drugs preparations | 18 |
| AdSV | |||||||
| SWV | |||||||
| Sertraline | Borate buffer,pH=8.2 | AdSV | Hg | 2.33·10-7 - 3.15·10-6 | 1.98·10-7 | Drugs preparations | 92 |
| SWV |
Table 4: Voltammetric Analysis Of Atypical Psychotropic Drugs
Conclusions
Psychotropic drugs, e.g. dibenzoazepines, dibenzocycloheptadienes, thioxanthenes, and phenothiazines, and new generation drugs, e.g. fluoxetine, fluvoxamine, and trazodone form cations which react with some organic substances (picric acid, flavianic acid, alizarin S, brilliant blue, and triphenylmethane dyes) and thiocyanate or halide anionic complexes (e.g. Co(II), Pd(II), Fe(III), Cr(III), Au(III), Ti(IV), Pt(IV), Mo(V), W(V), U(VI) forming ion-association compounds. The compounds get precipitated from aqueous solutions and can be quantitatively extracted into organic solvents (e.g. chloroform, dichloromethane and butanol). The extracts are intensely colored and stable for 1-3 days. These properties have been applied for the determination of above - mentioned metal ions and active compounds in pharmaceutical preparations.
As mentioned previously, the official compendia [93] recommends determination of psychotropic active substances in bulk or in pharmaceutical forms by measurement of the absorbance at selected wavelengths, or titration in a non–aqueous medium with potentiometric or visual indication at the endpoint. The proposed pharmacopoeial procedures require intensive isolation and purification steps in the case of the assay of the studied psychotropic substances in their pharmaceutical dosage forms. The main disadvantage of direct UV spectrophotometry is the sensitivity to excipients usually presented in pharmaceutical formulations.
In the presented review, methods based on the complexation reactions are discussed as alternative methods. The absorbance of colored ion association complexes of psychotropic drugs are less liable to spectral interferences from other ingredients of pharmaceuticals. The reviewed methods offer advantages of their simplicity, rapidity and common access to instrumentation. The analytical methods for the determination of psychotropic drugs are characterized with good precision, sensitivity and reproducibility.
Electrochemical methods have also been used for the study and determination of some psychotropic drugs. Among the methods, mainly used are voltammetry - cyclic voltammetry and differential pulse voltammetry, after pre-concentration of studied substances at several kinds of bare surfaces of electrodes, e.g. glassy carbon electrode, carbon paste electrodes or using different modified electrodes. Procedures have been described in literature for the determination of dibenzoazepines, dibenzocycloheptadienes, thioxanthenes, phenothiazines and new generation psychotropic drugs such as olanzapine, fluoxetine, paroxetine, trazodone, sertraline. Those are projected to be simple, fast and sensitive, which can be applied successfully to determine active substances, their metabolites in pharmaceutical formulations and biological fluids.
References
- Gupta RR editor. Bioactive Molecules. Vol. IV. Phenothiazines and 1,4-Benzothiazines. Chapter XVI, Analytical Application of Phenothiazines, Amsterdam: Elsevier; 1998. p. 861-98.
- Kojło A, Karpińska J, Kuźmicka L, Misiuk W, Puzanowska-TarasiewiczH,Tarasiewicz M. Analytical study of the reaction of phenothiazineswith some oxidants, metal ions, and organic substances. J TraceMicroprobe Tech 2001;19:45-70.
- Puzanowska-TarasiewiczH, TarasiewiczM,KarpińskaJ, Kojło A,WołyniecE. KleszczewskaE, Analytical applicationof the reactions of 2- and 10-disubstituted phenothiazines with some oxidizing agents. Chem Anal (Warsaw) 1998;43:159-78.
- Tarasiewicz M,Puzanowska-TarasiewiczH,Misiuk W,KojłoA,Grudniewska A, Starczewska B. Analytical applications of the reactionsof 2- and 10-disubstituted phenothiazines with some metal ions. ChemAnal (Warsaw) 1999;44:137-55.
- Karpińska J, Starczewska B, Puzanowska-TarasiewiczH. Analyticalproperties of 2- and 10-disubstituted phenothiazines derivatives. AnalSci 1996;12:161-70.
- Belal F, Hefnawy MM, Aly FA. Analysis of pharmaceutically-important thioxanthene derivatives. J Pharm Biomed Anal 1997;16:369-76.
- Misiuk W,Puzanowska-TarasiewiczH,Kuźmicka L, MielechK.Application of the reaction of promazine hydrochloride with chromium(VI) in volumetricand spectrophotometric determination. J TraceMicroprobe Tech 2002;20:305-16.
- Belokov VG, Moiseeva GF. Pharmaceutical analysis of phenothiazine derivatives by oxidation reactions. Farmatsiya (Moscow) 1979;36:87-92.
- Basavaiah K, Swamy JM, KrishnamurthyG. Quantitationof pharmaceutically important phenothiazines by oxidimetry. Farmaco2000;55:87-92.
- Basavaiah K, Swamy JM. Applicationof potassium dichromate and iron-thiocyanate in the spectrophotometric investigations of phenothiazines. Farmaco 2001;56:579-85.
- El-Gindy A, El-Zeany B, Award T,Shabana MM. Spectrophotometric,spectrofluorimetric andLC determinationof trazodone hydrochloride.J Pharm Biomed Anal 2001;26:211-7.
- Starczewska B, Mielech K. Application of chrome azurol S for the extractive spectrophotometric determinationof fluoxetine andfluvoxamine. J Pharm Biomed Anal 2000;23:243-7.
- Starczewska B,Puzanowska-TarasiewiczH, Baranowska,K.Investigation and analytical application of the reactions of eriochrome cyanine R with fluvoxamine and fluoxetine. J Pharm Biomed Anal2000;23:477-81.
- Prabhakar AH, Patel VB, Giridhar R. Spectrophotometric determinationof fluoxetine hydrochloride in bulk and in pharmaceutical formulations.J Pharm Biomed Anal 1999;20:427-32.
- Mohamed GG, Nour El-Dien FA, Khalil SM, Mohamed NA.Spectrophotometricdetermination of trazodone, amineptine and amitriptyline hydrochloridesthrough ion-pair formation witH of reinecke salt and alizarin S for the determination of promazine. J Pharm Biomed Anal 2001;26:1-5.
- Bhongade SL, Kasture AV. Extractive spectrophotometric determination of some phenothiazine derivatives in pharmaceuticalpreparations. Talanta 1993;40:87-91.
- Kedar-Hackman E,PradoMS, Santoro MI.Determination of methotrimeprazine in pharmaceutical preparationsby visible spectrophotometry. Drug Develop Ind Pharm 2000;26:261-6.
- Starczewska B, Puzanowska-TarasiewiczH.Application of chrome azurol S to extractive spectrophotometricdetermination of chlorpromazine. Farm Polska 1993;49:34-9.
- Basavaiah K, Swamy JM, KrishnamurthyG. Use of extractive- spectrophotometry and ion-pair formation with chrome azurol S for the assay of some phenothiazine antipsychotropic drugs in pharmaceutical formulations. Chem Anal (Warsaw) 1999;44:1049-54.
- Pinjżang Ch, Wanghzen Z. Determination of promethazine in Phenergan cough syrups. Chem Abst 1983;98:40662.
- Starczewska B. Spectrophotometricstudies and applicationof imipramine–eriochromecyanine R system for determinationof imipramine in pharmaceuticals. J Pharm Biomed Anal 2000;23:383-6.
- Puzanowska -TarasiewiczH, MyszczyńskaB. Application ofpyrocatechol violet andtin(IV) ionstothe extractivespectrophotometric determination of chlorpromazine. Acta Pol Pharm1988;45:311-7.
- Starczewska B, KarpińskaJ. Spectrophotometricdeterminationofchlorprothixene hydrochloride by pyrocatechol violet and ceric (IV)ions. J Trace Microprobe Tech 2002;20:317-25.
- Tarasiewicz M, StaniszewskaE, Puzanowska-TarasiewiczH.Application ofpicric and flavianic acids forthe extractive spectrophotometricdetermination of thioproperazine.Pharmazie1983;38:203-5.
- Tarasiewicz M,KuźmickaL. Extractivespectrophotometricdeterminationof some phenothiazineswith picricandflavianic acids.Anal Lett 1996;26:929-36.
- Puzanowska-Tarasiewicz H, Regulska E, Kleszczewska E. Extractive spectrophotometric determination of phenothiazines with picramic acid. Acta Pol Pharm 1990;47:1-4.
- Puzanowska-TarasiewiczH,WołyniecE, PółtorakJ. Determination of phenothiazines. VIII. Spectrophotometric determination of perazinemolybdenum and thiocyanate. Spectrochim Acta 2006;65:1221-6.
- Nour El-Dien FA, Mohamed GG, Mohamed NA. Spectrophotometricand tioproperazine by meas CuSO1989;46:55-62.
- Nouws HPA, Delerue-MatosC, Barros AA,Rodriques JA.Electroanalytical determination of paroxetine in pharmaceuticals. J Pharm Biomed Anal 2006;42:341-6.
- Nouws HPA, Delerue-MatosC, Barros AA,Rodriques JA.Electroanalytical study of the antidepressant sertraline. J PharmBiomed Anal 2005;39:290-3.
- Mielech-ŁukasiewiczK, Puzanowska-TarasiewiczH, Panuszko A.Electrochemical oxidation of phenothiazine derivatives at glassy carbo electrode and their differential pulse and square-wave voltammetric determination in pharmaceuticals. Anal Lett 2008;41:789-805.
- Tarasiewicz, M,WołyniecE, Puzanowska-TarasiewiczH. Analyticalapplication of the reaction of 2,10-disubstituted phenothiazines withorganic substances. Pharmazie 1998;53:151-5.
- Regulska E, Tarasiewicz M, Puzanowska-TarasiewiczH. Extractive spectrophotometricdeterminationof some phenothiazineswith dipicrylamine and picric acid. J Pharm Biomed Anal 2002;27:335-40.
- Regulska E,Puzanowska-Tarasiewicz H.Extractive- spectrophotometric determination of some 2- and 10-disubstituted phenothiazines with dipicrylamine. Acta Pol Pharm 2001;58:151-5.
- Tarasiewicz M, Staniszewska E, Puzanowska-Tarasiewicz H. Extractive spectrophotometric determination of chlorpromazine using alizarin S. Chem Anal (Warsaw) 1980;25:591-7.
- Puzanowska-Tarasiewicz H, Kuźmicka L, Kleszczewska E. Applicationdetermination of trazodone, amineptine and amitriptyline hydrochlorides through ion-pair formation using methyl orange and bromocresol green reagents. Spectrochim Acta 2006;65:20-6
- Puzanowska-Tarasiewicz H, Staniszewska E, Tarasiewicz M. Extractive spectrophotometric determination of trifluperazine with picric acid. Farm. Polska 1981;37:495-8.
- Tarasiewicz M, Puzanowska-Tarasiewicz H. Application of picric acid to the extraction-colorimetric determination of phenothiazine derivatives. Microchim Acta 1973;721-8.
- Bhongade SI, Kasture AV. Extractive spectrophotometric determinations of nortriptyline hydrochloride. Indian J Pharm Sci 1993;55:155-7.
- Basavaiah K, KrishnamurthyG. Extractivespectrophotometric determination of some phenothiazine derivatives in pharmaceutical preparations. Talanta 1998;46:665-70.
- Puzanowska-Tarasiewicz H, Tarasiewicz M.Application of titanium yellow for the extractivespectrophotometricdetermination of chlorpromazine. Farm Polska 1973;29:1009-13.
- Regulska E, The reactions of phenothiazines with some aromatic nitro- compounds and organic oxidants and their analytical applications. In Thesis Ph D, Bialystok University, Bialystok 2003.
- Misiuk W, KleszczewskaE, Karpinska J. Spectrophotometric determination of imipraminehydrochlorideusing ammonium peroxidisulfate and niobium (V) thiocyanate complex. Anal Lett2001;34:201-9.
- Misiuk W. Extractive spectrophotometric methods for the determination of doxepin hydrochloride in pharmaceutical preparations using titanium (IV) and iron (III) thiocyanate complexes. Farmaco 2005;60:61-9.
- Szydłowska -CzerniakA. Newmethods forthe determinationofphenothiazine, thioxantene,5H-dibenzoazepine, 5H-dibenzocycloheptadiene derivatives and their analytical applications. InPh. D. Thesis,Nicolas Copernicus University, Toruń 2000.
- Misiuk W.Extractive spectrophotometricdetermination ofchlorprothixene hydrochloride. Anal Lett 2000;33:1281-91.
- Blažek J,KrečmarJ. Analytickastudielečiv zeskupinyfenothiazinovych derivatu. IV. Spectrofotometricka studie v ultrafialoveoblasti. Českoslov Farm 1967;16:437-46.
- Fairbrother JE. Analysis of phenothiazine drugs. Pharm J 1979;31:271-5.
- Puzanowska -TarasiewiczH, KarpińskaJ. Determinationofphenothiazines in drugs. Pharmazie 1992;47:887-92.
- Dembiński B. Studies of physicochemical properties of phenothiazine,5 H-dibenzo(b,f) azepine and 5 H-dibenzocycloheptadiene(a,d)derivatives with the complexes of metals and picric acid, and their analytical application. In; D. Sc. Thesis, Nicolas Copernicus University,Toruń 1987.
- Tarasiewicz M. Determination of phenothiazines. V. The application ofiron - thiocyanate complexes for the spectrophotometric determination of chlorpromazine, levomepromazine and promethazine. Acta Pol Pharm1972;29:578-84.
- Tarasiewicz M. Determination of phenothiazines. VI. The application of cobalt - thiocyanate complexes for the extractive spectrophotometric determination of chlorpromazineand chloracizine. Acta Pol Pharm1974;31:201-5.
- Puzanowska-Tarasiewicz H, Myszczyńska B. Application of the reactionin the system germanium-(IV)-pyrocatechol violet–chlorpromazine.Farm Polska 1989;45:87-91.
- Misiuk W. Spectrophotometricdetermination of desipramine using ammonium peroxidisulfate and titanium (IV) thiocyanate complex. J Trace Microprobe Tech 1999;17:425-31.
- Misiuk W, Tarasiewicz M. Application of thiocyanate complex of titanium (IV) in extractive spectrophotometric determination of some phenothiazines. Acta Pol Pharm 1997;54:115-8.\
- Misiuk W, Tarasiewicz M. Application of thiocyanate complex of niobium (V) in spectrophotometricinvestigationsof promazine hydrochloride in pure form and in pharmaceutical preparations. Acta Pol Pharm 1995;52:373-8.
- Bishop E, Hussein W. Electroanalytical studies of phenothiazine neuroleptics at gold and platinum electrodes. Analyst 1984;109:229-34.
- Belal F, El-Ashry L, Shehata I, El-Sherbeny MA, El-Sherbeny DT.Differential pulse polarographic determination of some N-substituted phenothiazine derivatives in dosage forms and urine through treatment with nitrous acid. Mikrochim Acta 2000;135:147-54.
- Oelschlager H, Polarographicanalysisof psychotrophicdrugs.Bioelectrochem Bioenergetics 1983;10:25-36.
- Biryol I, Uslu B, Küçükyavuz Z. Voltammetricdeterminationof imipramine hydrochlorideand amitriptyline hydrochlorideusing apolymer-modified carbonpaste electrode. J Pharm Anal 1996;15:371-81.
- Temsamani K, Fahmi T, Bouchta D, Kaifer A. Cyclic voltammetricstudies of the antipsychotic chlorpromazine using an alkylthiol/phospholipids–modified gold electrode. J Solid State Electrochem1997;1:143-7.
- Zhang Z, Chen Z, Yang Z, Zhang H. Adsorptivevoltammetric determination of chlorpromazine in the presence of Triton X-100. Microchem J 1996;53:282-9.
- Jarbawi T. Preconcentration of tranquilizers by adsorption/extraction at a wax-impregnated graphite electrode. Anal Chim Acta 1986;186:11-9.
- Ferancova A, Korgova E, Buzinkaiova T, Kutner W, Stepanek I, Labuda J. Electrochemical sensors using screen-printed carbon electrode assemblies modified with the b-cyclodextrin or carboxymethylated b-cyclodextrinpolimer films fordetermination oftricyclic antydepressive drugs. Anal Chim Acta 2001;447:47-54.
- Ferancova A, Korgova E, Miko R, Labuda J. Determination oftricyclic antidepresantsusinga carbonpaste electrodemodified withb-cyclodextrin. J Electroanal Chem 2000; 492:74-7.
- Ivandini TA, Sarada BA, Terashima C, Rao TN, Tryk DA, IshiguroH, Kubota Y, Fujishima A. Electrochemicaldetection of tricyclicantidepressant drugs by HPLC using highly boron-doped diamond electrodes. J Electroanal Chem 2002;521:117-26.
- Wang J, Bonakdar M, Morgan C. Voltammetricmeasurement of tricyclic antidepressants following interfacial accumulation at carbon electrodes. Anal Chem 1986;58:1024-8.
- Sentürk Z, Özkan SA, Özkan Y, Aboul-Enein HY. Voltammetric investigation of oxidation of zuclopenthixol and application to its determination in dosage forms and in drug dissolution studies. J Pharm Biomed Anal 2000;22:315-23.
- Tuzhi P, Zhongping Y, Huiping L. Adsorptive preconcentration for voltammetric measurements of trace levels of chlorprothixene. Analyst1991;116:727-30.
- Walash ML, Rizk M,Belal F, El-Brashy A.Direct current polarographic determination of chlorprothixene and thiothixene after bromination. Microchem J 1988;38:300-6.
- El-Ennany N, Belal F, Rizk MS. Voltammetric analysis of trazodoneHCl in pharmaceutical and biological fluids. J Pharm Biomed Anal2002;30:219-26.
- Dermis S, Biryol I. Voltammetric determination of chlorpromazine hydrochloride. Analyst 1989;114:525-6.
- Biryol I, Dermis S. Voltammetricdetermination of thioridazine hydrochloride.Turkish J Chem 1998;22:325-33.
- Senturk Z, Ozkan SA, Uslu B, Biryol I. Anodic voltammetry of fluphenazine at different solid electrodes. J Pharm Biomed Anal1996;15:365-70.
- Sandulescu R, Mirel S,Oprean R,Lotrean S. Comparative electrochemical study of some phenothiazines with carbon paste, solid carbon paste and glass-like carbon electrodes. Collect Czech Chem Commun 2000;65:1014-28.
- Zimova N, Nemec I, Zima J. Determination of chlorpromazine and thioridazine by differential pulse voltammetry in acetonitrile medium. Talanta 1986;33:67-70.
- Huang L, Bu L, Zhao F, Zeng B. Voltammetricbehavior of ethopropazine and the influence of sodium dodecylsulfate on its accumulationon gold electrodes. J Solid State Electrochem 2004;8:976-81.
- Jarbawi T, Heineman R. Preconcentration of chlorpromazine at a wax- impregnated graphite electrode. Anal Chim Acta 1982;135:359-62.
- Wang J, Freiha BA, Deshmukh BK. Adsorptive/extractivestripping voltammetry of phenothiazine compounds at carbon paste electrodes. Biolectrochem Bioenerg 1985;14:457-67
- Wang J, Freiha B. Substractive differential pulse voltammetry following adsorptive accumulation of organic compounds. Talanta 1983;30:837-40.
- Wang J, Freiha B. Selective voltammetric detection based on adsorptive preconcentration for flow injection analysis. Anal Chem 1983;55: 1285-8.
- Ni Y, Wang L, Kokot S. Voltammetric determination of chlorpromazine hydrochloride and promethazine hydrochloridewith the use of multivariate calibration. Anal Chim Acta 2001;439:159-68.
- Wang J, Rivas G, Cai X, Shiraishi H, Farias PAM, Dontha N, Luo D.Accumulation and trace measurements of phenothiazine drugs at DNA-modified electrodes. Anal Chim Acta 1996;332:139-44.
- Vanickova M, Buckova M, Labuda J. Voltammetric determination ofazepine and phenothiazine drugs with DNA biosensors. Chem Anal(Warsaw) 2000;45:125-33.
- Zeng B, Huang F. Electrochemicalbehavior and determination of fluphenazine at multi-walled carbon nanotubes/(3-mercaptopropyl) trimethoxysilane bilayer modified gold electrodes. Talanta 2004;64:380-6.
- Zeng B, Yang Y, Ding X, Zhao F. Electrochemical study and detectionof perphenazine using a gold electrode modified with decanethiol SAM.Talanta 2003;61:819-27.
- Petit C, Murakami K, Erdem A,Kilinc E, Borondo G, Liegeois J, Kauffmann J. Horseradishperoxidase immobilized electrode for phenothiazine analysis. Electroanalysis 1998;18:1241-8.
- Khodari M, Mansour H, Salah El- Din H.Preconcentration and determination of the tricyclic antidepressant drug–imipramine atmodifiedcarbon paste electrode.Anal Lett 1997;30:1909-21.
- Brunt K. The polarographic behaviour of the antidepressant drugclorimipramine. Anal Chim Acta 1978;98:93-9.
- Raggi MA, CasamentiG, Mondrioli R, Izzo G, Kenndler E.Quantitation of olanzapine in tablets by HPLC, CZE, derivative spectrometryand linear voltammetry.J Pharm Biomed Anal2000;23:973-81.
- Roque da Silva AMS, Lima JC, Teles MT, Brett MA. Electrochemical studies and square wave adsorptivestripping voltammetryof theantidepressant fluoxetine.Talanta1999;49:611-7.
- Vela MH, Quinaz Garcia MB,Montenegro MCBS. Electrochemicalbehaviour of sertraline at a hanging mercury drop electrode and itsdetermination in pharmaceutical products. Fresenius’ J Anal Chem2001;369:563-6.
- European Pharmacopoeia, Fifth ed., Council of Europe, Strasbourg2005.