- *Corresponding Author:
- B. N. Suhagia
Department of Quality Assurance, L. M. College of Pharmacy, Navrangpura, Ahmedabad–380 009, India
E-mail: patelhary@rediffmail.com
| Date of Submission | 3 February 2005 |
| Date of Revision | 26 May 2005 |
| Date of Acceptance | 18 March 2006 |
| Indian J Pharm Sci, 2006, 68 (2): 247-249 |
Abstract
A simple and sensitive spectrophotometric method has been developed for the estimation of lomefloxacin hydrochloride in its marketed formulations. The method is based on the reaction between the drug and the dichlone, in the presence of crotonaldehyde in dimethylsulfoxide, which produces a blue chromogen with absorption maximum at 645 nm. The good agreement with Beer's law was found in the concentration range of 5-100 µg/ml. The optimum reaction conditions and other analytical parameters are evaluated. Statistical comparison of the results with those of reported method shows good agreement, and indicated no significant difference in precision. The proposed method was found to be simple, accurate, and reproducible for the routine analysis of the drug in pharmaceutical dosage forms.
Lomefloxacin, a DNA gyrase inhibitor, is a fluorinated 4-quinolone analogue of nalidixic acid. Chemically [1-2], it is 1-ethyl-6,8-difluro-1,4-dihydro-7-(3-methyl-1-piperazinyl)-4-oxo-3-qunoline carboxylic acid. It is used in mild to severe urinary tract infections [3]. Lomefloxacin is not official in any pharmacopoeia. Literature survey reveals that lomefloxacin is estimated in pharmaceuticals and biological fluids by Spectrophotometric [4], HPLC [5-7] . HPTLC [8], and microbiological assay methods [9]. In the present investigation, an attempt has been made to develop a simple, accurate and reproducible spectrophotometric method for estimation of lomefloxacin in pharmaceutical formulations.
A Double beam Shimadzu 160A UV/vis spectrophotometer with two matched quartz cells of 1 cm light path was employed for the spectral measurement. Lomefloxacin hydrochloride working standard was procured as a gift sample from Cadila Pharmaceuticals Limited, Ahmedabad. Other reagents used were of analytical grade, and distilled water was used during the study.
Lomefloxacin hydrochloride (125 mg) was accurately weighed and transferred to a 25 ml volumetric flask, containing a mixture of distilled water (10.0 ml) and glacial acetic acid (2.0 ml). The solution was diluted to 25 ml with acetonitrile. This stock solution (5.0 ml) was further diluted with DMSO in a 25 ml volumetric flask, to obtain the final concentration of 1000 μg/ml. Dichlone solution (1.5 % w/ v) was prepared by dissolving dichlone (1.5 g) in DMSO,and diluted to 100 ml with the same solvent.Crotonaldehyde solution (20 % v/v) was prepared by using DMSO as the solvent.
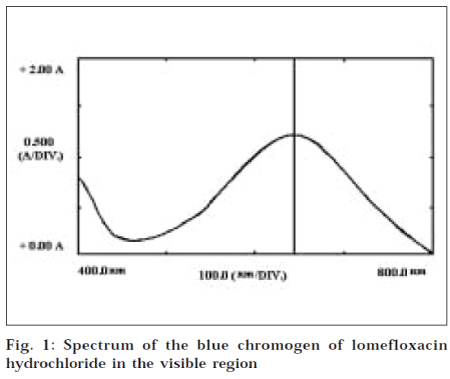
In a series of 10 ml volumetric flasks, lomefloxacin standard solution (0.05-1.0 ml, 1000 μg/ml), dichlone reagent solution (1.0 ml), and crotonaldehyde solution (1.0 ml), were pipetted out successively. The total volume was made up to 4.0 ml with DMSO. The reaction flasks were allowed to stand at 60º for 30 min. Later, the flasks were cooled to room temperature, and the final volume was adjusted to the mark with dioxane. Absorbance of the colored solution was measured on Shimadzu UV-visible spectrophotometer at 645 nm, against a reagent blank (Fig. 1).
Twenty tablets were weighed accurately, and powdered. The powder equivalent to lomefloxacin (125 mg) was transferred into a 25 ml volumetric flask, containing a mixture of distilled water (10.0 ml) and glacial acetic acid (2.0 ml); the flask was shaken for 5 min, and volume was adjusted to the mark with acetonitrile. The solution was filtered through Whatman filter paper No. 40. Rejecting the first few ml, the filtrate (5.0 ml) was diluted to 25 ml with DMSO. The diluted solution was analyzed as mentioned above.
It was known that crotonaldehyde [10] reacts with dichlone and diethylamine, to form a blue chromogen, diethylaminobutadienyl-naphthoquinone, which gives absorption maximum at 625 nm. Lomefloxacin has an amino moiety in its structure. Therefore, the above principle was used for the determination of lomefloxacin in pharmaceutical formulations. The proposed method was based on the reaction of the drug, dichlone, and crotonaldehyde in dimethylsulfoxide, to give a blue chromogen with the absorption maximum at 645 nm. The proposed method is successfully applied for the determination of lomefloxacin in pharmaceutical formulations.
The optical characteristics of lomefloxacin hydrochloride, such as Beer’s law limit, Sandell’s sensitivity, molar extinction coefficient, and percent relative standard deviation of the proposed method for the determination of lomefloxacin, are tabulated in Table 1. The Tcal and Fcal values of the proposed method were found to be 0.2034 and 0.6189, respectively (Ttab value = 4.30 and Tcal value = 18.50), which indicated no significant difference between two methods. The recovery was calculated by the addition of pure drug to the previously analyzed pharmaceutical preparations. The results of the analysis of the tablet formulations are shown in Table 2. The effective recovery confirmed the accuracy and the specificity of the proposed method, and the lack of interference from the common excipients, film coating materials, and the colorant used in the manufacture of tablets. This method is particularly useful for routine inprocess quality control for its pharmaceutical preparations.
| Parameters | Values |
|---|---|
| Wavelength for measurement (nm) | 645 |
| Beer’s Law limit (µg/ml) | 5-100 |
| Molar absoptivity(lit/mole/cm) | 4.24 × 103 |
| Sandell’s sensitivity( µg/ml/cm2/0.001 abs. unit) | 8.29 × 10-2 |
| Regression equation (Yp)Slope (b)Intercept on | |
| y-axis (a) | 0.01190.0301 |
| Correlation coefficient (r)q | 0.9956 |
| Relative standard deviation (%)s | 0.3-3.7 |
| % Recovery | 99.10-100.60% |
p means Y=a+bC, where ‘C’ is concentration in μg/ml and Y is absorbance unit, q means five replicate samples, s means five replicate samples.
Table 1: Optical characteristics of the proposed method
| Formulations | Label claim mg/tablet | % Content found by | Percent recovery by proposed methodu | |
|---|---|---|---|---|
| Proposed method | HPLC method5 | |||
| Tablet -1 | 400 | 101.90 | 99.26 | 99.10 |
| Tablet -2 | 400 | 106.60 | 106.20 | 100.12 |
| Tablet -3 | 400 | 104.60 | 103.79 | 100.60 |
u means three replicate samples, Tablet-1 stands for tablet of Torrent pharmaceuticals Ltd., Ahmedabad (brand name-Lomef 400, strength-400 mg), Tablet¬ 2 stands for tablet of IPCA, Mumbai (brand name-Lomflox, strength-400 mg) and Tablet-3 stands for tablet of Intas pharmaceuticals Ltd., Ahmedabad (brand name-Lomitas, strength-400 mg).
Table 2: Analysis of pharmaceutical formulations
References
- Reynolds, J.E.F., Eds., In; Martindale: The Extra Pharmacopoeia, 30thEdn., The Pharmaceutical Press, London, 1994, 179.
- Budavari, S. Eds., In; The Merck Index, 11th Edn., Merck and Co. Inc., Whitehouse Station, N.J.,1989, 875.
- Physicians Desk Reference, 48th Edn., Medical Economics Company Inc., Montvale, N.J., 1994, 2213.
- Rajasekaran, A., Jaykar, B., DhanaLakshmi, S., Deepalakhsmi, M. and Beulah, V.I., Indian J. Pharm. Sci., 1998, 60, 236.
- Nagashima, M., KenkyuNenpo-Tokyyo-toritsu and Eiseikenkyusho, 44, 71, 1993.
- Li, Kangle and ZhongguoYaokeXuebao, 25, 231, 1994.
- Shibl, A.M., Ashraf, A.K., Abdel-Kader, F.T. and Ahmed A.A., J.Clin. Pharmacol. Ther., 16, 353, 1991.
- Shah, S.A., Rathod, I.S., Savle, S.S. and Patel, D.R., J. Pharm.Biomed. Anal., 2002, 30, 1319.
- Chawla, J.L., Sodhi, R.A. and Sane, R.T., Indian Drugs, 1997, 34, 512.
- Buckely, D., Dunstan, S. and Henbest, H. B., J. Chem. Soc., 1957, 3032.