- *Corresponding Author:
- I. Singhvi
Department of Pharmacy, Mohan Lal Sukhadia University, Udaipur-313 001, India.
E-mail: indrajeet_s@yahoo.com
| Date of Submission | 5-Feb-2005 |
| Date of Decision | 20-June-2005 |
| Date of Acceptance | 10-Feb-2006 |
| Indian J Pharm Sci, 2006, 68 (1): 72-75 |
Abstract
Two simple, accurate, economical and reproducible UV spectrophotometric and one HPLC method for simultaneous estimation of two-component drug mixture of pseudoephedrine hydrochloride and loratadine in combined tablet dosage form have been developed. The first developed method employs multiwavelength spectroscopy using seven mixed standards and 257.0 nm and 283.0 nm as two wavelengths for estimation. The second method involves first derivative spectroscopy using 308.6 nm and 263.0 nm as zero crossing points for pseudoephedrine hydrochloride and loratadine respectively. For both spectrophotometric methods, 0.2 M hydrochloric acid was used as solvent. Linearity was observed in concentration range of 0-40 mg/ml of loratadine and 0-800 mg/ml of pseudoephedrine hydrochloride. Developed HPLC method is reverse-phase chromatographic method using Inertsil C 18 column and methanol:ammonium acetate buffer in ratio of 80:20 pH 7.5 as mobile phase. Nimesulide was used as internal standard for HPLC method. For HPLC method, linearity was observed in concentration range of 0-200 mg/ml of loratadine and 100-2000 mg/ml of pseudoephedrine hydrochloride. Results of analysis were validated statistically and by recovery studies.
Introduction
Pseudoephedrine hydrochloride, chemically 2- methylamino-1-phenyl-1-propanol hydrochloride, has sympathomimetic activity[1] and is official in the United States Pharmacopoeia[2], British Pharmacopoeia[3] and Indian Pharmacopoeia[4]. All the three pharmacopoeia describe HPLC method for estimation of pseudoephedrine hydrochloride from tablet formulation. Several chromatographic methods are reported for estimation of pseudoephedrine hydrochloride[5-9] from pharmaceutical formulation. Loratadine, chemically 4-(8-chloro-5,6-dihydro- 11-H-benzo-[5,6]cyclohept-al[1,2-b]pyridin-11-ylidene)-1- piperidine carboxylic acid ethyl ester, is an antihistaminic agent[10] and is yet not official in any of the pharmacopoeia. Several methods are reported for estimation of loratadine[11-18] from pharmaceutical formulations; however, none of the method is reported for simultaneous estimation of two drugs.
Developed spectrophotometric and HPLC methods are simultaneous methods of analysis of pseudoephedrine hydrochloride and loratadine from combined tablet dosage form. The developed methods were found to be simple, rapid, accurate, reproducible and economical. These methods can be used successfully for quality control testing of the drugs from combined tablet dosage form.
Materials and Methods
Standard bulk drug sample of pseudoephedrine hydrochloride, loratadine and nimesulide were provided by Ranbaxy Laboratories Ltd., Dewas. Tablets of combined dosage form were procured from the local market. All other reagents used were of analytical grade for spectrophotometric methods and of HPLC grade for HPLC method.
Shimadzu UV/V is spectrophotometer (model-160 A) with 1 cm matched quartz cells was used for spectrophotometric methods. Spectra’s were recorded using specific program of apparatus, having specifications as follows: spectral bandwidth 3 nm, wavelength accuracy ±0.5 nm, wavelength readability 0.1 nm increments. For HPLC method, Shimadzu delivery module LC-10AD with UV SPD-10A detector and Chromatopac C-R7A integrator were used.
Method I—Multiwavelength spectroscopy
Using the overlain spectra of pseudoephedrine hydrochloride and loratadine in 0.2 M HCl, the wavelength maxima of both drugs, i.e., 257.0 and 283.0 nm, were selected as two sampling wavelengths for this method. Seven mixed standards of two drugs in 0.2 M HCl were prepared so as to contain 0-600 μg/ml of pseudoephedrine hydrochloride and 0-25 μg/ml of loratadine. All mixed standard solutions were scanned over the range of 400 nm to 220 nm in multicomponent mode of spectrophotometer using 257.0 nm and 283.0 nm as two sampling wavelengths. The spectral data from these scan were used to determine the concentration of two drugs in the sample solution.
Analysis of commercial formulation
Twenty tablets were accurately weighed and average weight per tablet determined. Tablets were grounded to fine powder, and weighed tablet powder equivalent to 120 mg pseudoephedrine hydrochloride was transferred to 100 ml volumetric flask. The powder was extracted four times with 20 ml portions of methanol. The extract was collected in 100 ml volumetric flask after filtration through Whatman filter paper no. 41. The filter paper was washed with 20 ml methanol, and the washing was added to the filtrate. The methanol was evaporated on water bath and the residue was dissolved in 75 ml of 0.2 M HCl and again filtered through Whatman filter paper No. 41. The filter paper was washed with 20 ml of 0.2 M HCl and the washing was added to the filtrate. The volume of filtrate was made to 100 ml mark with 0.2 M HCl. In 10 ml volumetric flask, 2.5 ml of filtrate was taken and diluted to the mark with 0.2 M HCl.
The sample solution was scanned over the range of 400 nm to 220 nm in multicomponent mode and concentration of each component was estimated by analysis of spectral data of sample solution with respect to that of mixed standards by the instrument. Results of analysis are reported in Table 1.
| Method | Label Claim (mg/tablet) | % of Label Claim Estimated* | Standard Deviation | |||
|---|---|---|---|---|---|---|
| PS | LR | PS | LR | PS | LR | |
| Method I | 120 | 05 | 101.06 | 99.54 | 0.140 | 1.128 |
| Method II | 120 | 05 | 100.73 | 98.06 | 1.120 | 0.785 |
| Method III | 120 | 05 | 101.79 | 98.90 | 0.155 | 0.376 |
Table 1: Results Of Analysis Of Commercial Formulation
Method II—First derivative spectroscopy
From first derivative spectra of pseudoephedrine hydrochloride and loratadine in 0.2 M HCl zero crossing points, 308.6 nm and 263.0 nm were selected for simultaneous estimation of two drugs. Accurately weighed pure drug sample of pseudoephedrine hydrochloride and loratadine were dissolved in 0.2 M HCl and were diluted with same so as to give several dilutions in the range of 0-800 μg/ml of pseudoephedrine hydrochloride and 0-30 μg/ml of loratadine. The absorbance of these dilutions were recorded in first derivative mode at 308.6 nm for estimation of loratadine, and 263.0 nm for estimation of pseudoephedrine hydrochloride. Respective calibration curves were prepared. Validity of proposed method was checked by preparing five mixed standards containing different concentrations of pure drug sample of pseudoephedrine hydrochloride and loratadine. Absorbance was measured at respective selected zero crossing points, and concentration of two drugs, using respective calibration curve, was determined. Validation studies gave satisfactory results.
Analysis of commercial formulation
Tablet sample was prepared in similar manner as for Method I. Absorbance of final dilution of sample was recorded at 308.6 nm and 263.0 nm from first derivative spectra of sample and amount of two drugs calculated using respective calibration curve. Results of analysis are reported in Table 1.
Method III—High-performance liquid chromatographic method
HPLC method was developed using Inertsil C18 ODS 3V(5μ) 250 × 4.6 mm column. Mobile phase selected for this method contained 20 parts of ammonium acetate buffer (3.85 g/l) and 80 parts of methanol adjusted to pH 7.5 with sodium hydroxide that was filtered through 0.2 micron membrane filter. Flow rate employed was 1.0 ml/min. Detection of eluent was carried out at 250 nm and AUFS was set at 0.032. Method was developed using nimesulide as internal standard.
Standard stock solution
Standard stock solutions of pure drugs were made separately in mobile phase containing 2000 μg/ml of pseudoephedrine hydrochloride, 200 μg/ml of loratadine and 100 μg/ml of nimesulide and filtered through a 0.2 micron membrane filter.
Preparation of calibration curve
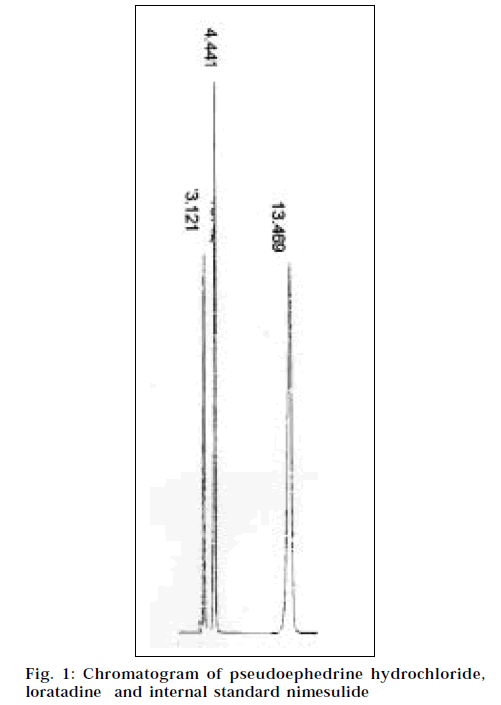
For preparation of the drug solutions for the calibration curves, in a series of 10 ml volumetric flasks, 1, 2, 3, 4 and 5 ml of the pure drug standard stock solutions of pseudoephedrine hydrochloride and 0.5, 1.0, 1.5, 2.0 and 2.5 ml of the pure drug standard stock solutions of loratadine were transferred. In each flask, 2.0 ml of stock pure drug solution of nimesulide was added and the volume was made up to the mark with the mobile phase. Each solution was injected and a chromatogram was recorded. Mean retention time for pseudoephedrine hydrochloride was found to be 3.158 min, for loratadine 13.464 min, and for nimesulide 4.440 min.
The peak area ratios of pseudoephedrine hydrochloride to nimesulide and loratadine to nimesulide were calculated and respective calibration curves were plotted against concentration of drug and peak area ratio of drug to internal standard.
Procedure for analysis of formulations
Twenty tablets of the formulation were weighed and the average weight per tablet was calculated. Twenty tablets were crushed and ground to a fine powder. Powder equivalent to 240 mg of pseudoephedrine hydrochloride was weighed and transferred to a 100 ml volumetric flask containing about 75 ml mobile phase. To this volumetric flask, accurately weighed 10 mg of pure standard nimesulide was added. The powder mixture was dissolved in the mobile phase with the aid of ultrasonication. The solution was filtered through Whatman filter paper no. 41 into another 100 ml volumetric flask. The filter paper was washed with mobile phase and washings were added to the filtrate. Volume of filtrate was made up to the mark with the mobile phase. To another 10 ml volumetric flask, 2.0 ml of this solution was transferred and the volume was made up to the mark with the mobile phase. This solution was filtered through a 0.2 m membrane filter.
After setting the chromatographic conditions and stabilizing the instrument to obtain a steady baseline, the tablet sample solution was loaded in the 20 μl fixedsample loop of the injection port. The solution was injected and a chromatogram was recorded. The injections were repeated five times and the peak areas were recorded. A representative chromatogram has been given in Fig. 1. The peak area ratios of each of the drugs to the internal standard were calculated and the amount of each drug present per tablet was estimated from the respective calibration curves. The results of analysis are presented in Table 1.
Recovery studies
To study the accuracy, reproducibility and precision of the above methods, recovery studies were carried out by addition of standard drug solution to pre-analyzed sample at three different levels. Results of recovery studies were found to be satisfactory and are reported in Table 2.
| Method | Conc. Added (mg/ml) | % Conc. Recovered* | ||
|---|---|---|---|---|
| PS | LR | PS | LR | |
| 100 | 05 | 98.80 | 101.61 | |
| Method I | 200 | 10 | 101.42 | 100.67 |
| 300 | 15 | 100.88 | 98.69 | |
| 100 | 05 | 98.65 | 99.68 | |
| Method II | 200 | 10 | 98.79 | 100.23 |
| 300 | 15 | 99.76 | 98.26 | |
| 100 | 10 | 99.98 | 97.44 | |
| Method III | 200 | 20 | 98.87 | 100.78 |
| 300 | 30 | 100.80 | 98.08 |
Table 2: Results Of Recovery Studies
Results and Discussion
The proposed methods for simultaneous estimation of pseudoephedrine hydrochloride and loratadine in combined tablet dosage form were found to be simple, accurate, rapid and economical. The values of coefficient of variance were satisfactorily low and recovery was close to 100%, indicating reproducibility of the methods.
First method involving multiwavelength spectroscopy is specific to instrument having software for provision of such determination. Selection of proper sampling wavelengths and concentration of components in mixed standard is critical. Since calculations are done by the instrument itself, chances of manual error are nil; furthermore, the method is quite rapid.
Second method involving first derivative ultraviolet spectroscopy requires recording spectrophotometer with data processing mode. Proper selection of zero crossing point in derivative spectra completely eliminates the interference of unwanted component, and thus, concentration of two components can be easily calculated without prior separation of components from combined dosage form.
Third developed method for simultaneous estimation of two drugs from combined dosage form is reverse phase chromatographic method utilising C18 column and methanol ammonium acetate as mobile phase. Detection of eluent was carried out using UV detector. The method was developed using nimesulide as internal standard. The run time per sample is just 15 min. The excipients in the formulation did not interfere in the accurate estimation of pseudoephedrine hydrochloride and loratadine.
Since none of the methods is reported for simultaneous estimation of pseudoephedrine hydrochloride and loratadine from combined dosage form, these developed methods can be used for routine analysis of two components without prior separation.
Acknowledgements
The authors would like to acknowledge Ranbaxy Laboratories Ltd., Dewas, India, for providing pure drug samples of pseudoephedrine hydrochloride, loratadine and nimesulide. Acknowledgment is further extended to Core Health Care Ltd., Rajpur, India, for providing facilities to carry out HPLC work.
References
- Budavari, S., Eds., In; The Merck Index, 12th Edn., Merck and Co., Inc., Whitehouse Station, NJ, 1996, 3647.
- United States Pharmacopoeia, 18th Edn., USP Convention Inc., Rockville, MD, 1995, 1339.
- British Pharmacopoeia, Vol. II, Her Majesty’s Stationary office, British Pharmacopoeia commission, London,1993,1085.
- Indian Pharmacopoeia, Vol. II, Government of India, The controller of Publication, New Delhi, 1996, 641.
- Dash, A.K., J. Pharm. Biomed. Anal., 1998, 121, 601.
- Fong, G.W. and Eickhoff, W.M., Int. J. Pharm., 1989, 53, 91.
- Maierdan Wang, J.H., Yan, F.Z., Jin, Y., Cui, Y.Q. and Sun, D.J., Yaowu Fenxi Zazhi, 1994, 14, 27.
- Noggle, F.T. and Clark, C.R., J. Assoc. Off. Anal. Chem., 1984, 67, 198.
- Paeeran, M.T., Baravani, G.S. and Bhalla, V.K., Indian Drugs, 1988, 25, 242.
- Budavari, S., Eds., In; The Merck Index, 12th Edn., Merck and Co., Inc., Whitehouse Station, NJ, 1996, 953.
- El-Ragehy, N.A., Badaway, A.M. and Khateeb, S.Z., through Anal.Abstr., 1995, 28, 2363.
- Kovas, E.M., J. Chromatogr., 1995, 692, 103.
- Lin, Z.H., Yaowu Fenxi Zazhi, 1996, 16, 53.
- Rajput, S.J. and Vyas, A.G., Indian Drugs, 1998, 35, 352.
- Squella, J.A., Sturm, J.C., Diaz, M.A., Pessoa, H. and Nenuz-Vergora, L.J., Talenta, 1996, 43, 2029.
- Vyas, A.G. and Rajput, S.J., Indian J. Pharm. Sci., 1997, 59, 186.
- Zhang, D. and Blume, H., Pharmazie, 1994, 49, 736.
- Zhong, D. and Blume, H., Pharmazie, 1994, 49, 730.