- *Corresponding Author:
- Rajashree Mashru
Pharmacy Department, Faculty of Technology and Engineering, The M. S. University of Baroda, Kalabhavan, Vadodara-390 001, India.
E-mail: vsutariya@yahoo.co.in
| Date of Submission | 19 July 2004 |
| Date of Revision | 04 November 2005 |
| Date of Acceptance | 04 October 2006 |
| Indian J Pharm Sci, 2006, 68 (5): 643-645 |
Abstract
Simple UV and third derivative spectrophotometric methods in methanol have been developed for the determination of fosinopril sodium in bulk drug and its pharmaceutical formulations. The simple UV spectrum of fosinopril sodium in methanol exhibits absorption maxima (λmax) at 208 nm, whereas in the third derivative spectrum the maxima occur at 217.4 nm and the minima at 223 nm. Both the methods were found to be simple, economical, accurate, reproducible and can be adopted in routine analysis of fosinopril sodium in bulk drug and in tablet dosage form.
Keywords
Fosinopril sodium, l-proline-4-cyclohexyl-1-[[[2-methyl-1-(1-oxopropoxy)propoxy]-(4-phenylbutyl) phosphinyl] acetyl] sodium, is a new angiotensin converting enzyme inhibitor that is effective in the treatment of hypertension [1,2]. It is also effective when used alone or in combination with other medications for the treatment of high blood pressure [3]. Fosinopril sodium is also prescribed for heart failure [4].
The drug is available in tablet form and is not official in any of the pharmacopoeia. So far only spectroscopic and liquid chromatographic methods have been reported for the estimation of fosinopril sodium [5-8]. But all these methods are more time consuming and expensive than the simple spectrophotometric method. The aim of the present investigations is to develop a simpler, rapid and cost-effective analytical method for the determination of fosinopril sodium in bulk drug and in tablet dosage form. The present investigation illustrates simple, sensitive and accurate UV method and third derivative spectroscopic method for the analysis of fosinopril in bulk drug and in tablets.
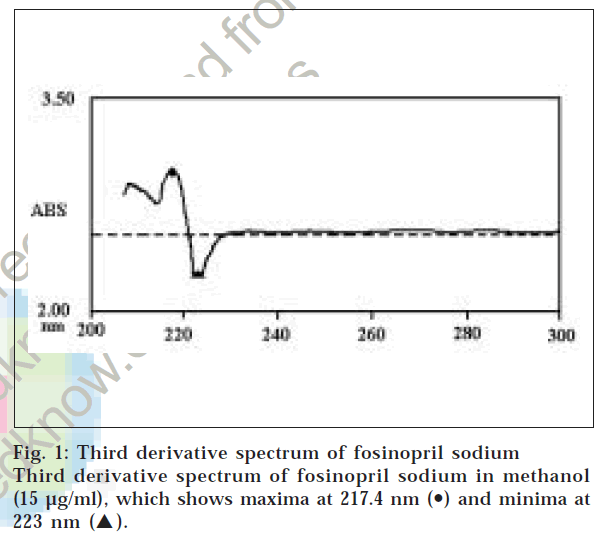
A Shimadzu UV-1601PC UV/Vis spectrophotometer was used for all absorbance measurements. Fosinopril sodium was obtained as a gift sample from Cadila Pharmaceuticals Ltd., Ahmedabad. Stock solution of fosinopril sodium (1 mg/ml) was prepared in methanol. Working solution (50 μg/ml) was prepared by appropriate dilutions of the stock solution of fosinopril sodium in methanol. In the simple UV method, aliquots of working solution of fosinopril sodium (1-8 ml, 50 μg/ml) were transferred into a series of 10 ml volumetric flasks and volume was made up to the mark with methanol. The absorbances of the resulting solutions were measured at 208 nm against a solvent blank. Calibration curve was prepared by plotting concentration versus absorbance. In the derivative spectroscopic method, aliquots of working solution of fosinopril sodium (1-6 ml, 50 μg/ml) were transferred into a series of 10 ml volumetric flasks. These solutions were diluted with methanol up to the mark and the third derivative spectra were obtained, which showed absorbance maxima at 217.4 nm and minima at 223 nm. Third derivative spectrum of fosinopril sodium in methanol (15 μg/ml) is shown in fig 1. A calibration curve was prepared by plotting the absorbance difference between maxima and minima (i.e., amplitude) versus concentration.
The optical characteristics such as Beer’s law limit, Sandell’s sensitivity, molar extinction coefficient, percent relative standard deviation (calculated from six measurements containing three-fourths of the concentration of the upper Beer’s law limit) and percentage range of error at 95% confidence limit of both methods were incorporated in Table 1.
| Parameters | Simple UV method | Third derivative method |
|---|---|---|
| Absorption maxima (λmax) | 208 nm | 217.4 nm |
| Absorption minima (λmin) | - | 223 nm |
| Beer’s law limit | 5-40 μg/ml | 5-3Molar absoptivity0 μg/ml |
| Molar absoptivity (lit/mole/cm) | 2.27×104 | 1.03×105 |
| Sandell’s sensitivity (μg/ml/cm2/0.001 abs. unit) | 2.57×10-2 | 5.67×10-3 |
| Regression equation (Y)* | ||
| Slope (b) | 0.0307 | 0.1606 |
| Intercept (a) | 0.056 | 0.0927 |
| Correlation coefficient (r) | 0.9929 | 0.9937 |
| Relative standard deviation (%)** | 0.721 | 0.589 |
| % range of error (0.05 level) |
*Y = a + bc, where c is concentration in μg/ml and Y is absorbance units.
**Six replicate samples.
Table 1: Optical Characteristics and Precision
The values obtained using the simple UV method and the third derivative method for the estimation of fosinopril sodium in marketed tablets are compared by using Student’s t-test (paired, two sided) and F-test at 95% confidence limit. The results of analysis of two different brands of marketed tablets by both the methods are shown in Table 2. For recovery study, known amounts of pure drug were added to the previously analyzed tablets and the mixtures were analyzed by proposed methods. The data of recovery studies are incorporated in Table 3. Sharp peaks were not observed in the first derivative and second derivative spectrum. There was no linearity of amplitude in first and second derivative spectroscopic method, while in the third derivative spectroscopic method, linearity was observed. In third derivative spectra, good amplitude was also observed, which is shown in fig. 1. Therefore, third derivative spectroscopy method was selected for analysis.
| Sample | Labeled value (mg) | Amount found | |||
|---|---|---|---|---|---|
| Simple UV method | Third derivative | t-value (calculated) | F-value (calculated) | ||
| (mg)* | method (mg)* | ||||
| Tablet-1 | 10 | 9.9±0.66 | 10.4±0.45 | 0.719 | 0.638 |
| Tablet-2 | 20 | 20.4±0.74 | 21.0±0.47 | 0.243 | 0.560 |
| Tablet-3 | 10 | 10.3±0.38 | 9.6±0.56 | 0.375 | 0.922 |
| Tablet-4 | 20 | 20.3±0.49 | 20.2±0.22 | 0.481 | 0.397 |
*Average ± standard deviation of six determinations. The t-value and F-value refer to compression of the simple UV method with third derivative method. Theoretical value at 95% confidence limit: t = 4.30 and F = 19.00. Tablet-1 stands for tablet of Cadila Pharmaceuticals Ltd., Ahmedabad (brand name – Fova10, strength – 10 mg); tablet-2 stands for tablet of Cadila Pharmaceuticals Ltd., Ahmedabad (brand name – Fova-20, strength –20 mg); tablet-3 stands for tablet of Cipla Ltd., Mumbai (brand name – Fosinace-10, strength –10 mg); tablet-4 stands for tablet of Cipla Ltd., Mumbai (brand name – Fosinace-20, strength – 20 mg)
Table 2: Results of Analysis of Pharmaceutical Formulation
| Sample | % recovery* | |||
|---|---|---|---|---|
| By simple UV method | By third derivative method | |||
| I | II | I | II | |
| Tablet-1 | 100.7±0.37 | 100.1±0.28 | 100.5±0.32 | 101.2±0.51 |
| Tablet-2 | 99.1±0.88 | 100.6±0.44 | 99.2±0.58 | 100.8±0.38 |
| Tablet-3 | 98.7±0.62 | 100.1±0.57 | 101.5±0.19 | 100.3±0.34 |
| Tablet-4 | 99.7±0.11 | 99.5±0.76 | 99.9±0.25 | 98.9±0.21 |
*Recovery of 4 mg added to the pharmaceutical preparation (average of six determinations). I and II indicates two replicate measurements. Tablet-1 stands for Fovas-10 from Cadila Pharmaceuticals Ltd., tablet-2 stands for Fovas-20 from Cadila, tablet-3 stands for Fosinace-10 from Cipla Ltd. and tablet-4 stands for Fosinace-20 from Cipla Ltd.
Both the proposed methods were found to be rapid, economical, accurate and precise for the determination of fosinopril sodium in bulk drug and in its tablet dosage form. In results of analysis of marketed tablets by both methods, t-calculated values and F-calculated values were less than the corresponding statistical values, indicating no significant difference in means and variances of results obtained by either of the proposed methods. Both proposed methods produce comparable results and can be used for precise and accurate analysis of fosinopril sodium in its pure and tablet dosage form. Interference studies revealed that the common excipients and other additives usually present in the dosage form did not interfere in both the proposed methods. The values of standard deviation were satisfactory and % recovery was close to 100%, indicating the reproducibility and accuracy of the methods. Any of the proposed methods can be employed as a quality control tool for the analysis of fosinopril sodium in its tablet dosage form and in bulk drug.
References
- Singhvi, S.M., Duchin, K.L., Morrison, R.A., Willard, D.A.,Everett, D.W. and Frantz, M., Brit . J . Clin . Pharmacol., 1988, 25, 9.
- Schoondyke, J.W., Mohan, R., Kelly, J.L., Ponder, M.A., Iskandar,S. and Douglas, J.E., Tenn. Med., 2002, 95, 155.
- Parodi, A., Cozzani, E., Milesi, G., Drosera, M. and Rebora, A.,Dermatology, 2002, 204, 139.
- Pahor, M., Franse, L.V., Deitcher, S.R., Cushman, W.C., Johnson,K.C., Shorr, R.I., Kottke-Marchant, K., Tracy, R.P., Somes, G.W. and Applegate, W.B., Circulation, 2002, 105, 457.
- Paraskevas, D. and Demetrius, G., Analytica . Chimica .Acta., 2003, 481, 321.
- Nevin, E., J. Pharm. Biomed. Ana., 2002, 27, 901.
- Jemal, M., Huang, M., Mao, Y., Whigan, D. and Schuster, A.,Rapid Commun. Mass Spectrom., 2000, 14, 1023.
- Jemal, M. and Mulvana, D., J. Chromatogr. B Biomed. Sci.Appl., 2000, 739, 901.
 .
.