- *Corresponding Author:
- S. Yu
Department of Neurology, The First Medical Center, Chinese People's Liberation Army General Hospital, Haidia, Beijing 100853, China
E-mail: yusy1963@126.com
| This article was originally published in a special issue, “Transformative Discoveries in Biomedical and Pharmaceutical Research” |
| Indian J Pharm Sci 2023:85(4) Spl Issue “20-28” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Migraine is a common disabling brain dysfunction disease. Increasing evidence shows that the braingut axis can regulate brain dysfunction disease. However, the mechanism of interaction between the gut and brain in migraine patients is still unclear. In this study, we evaluated the effect of sodium propionate treatment on intestinal microbiota, metabolites and pain behavior of the migraine model induced by inflammation soup in rats. We administered 100 mg/kg sodium propionate to rats by gavage, placed a catheter on the dura mater and stimulated them with chronic inflammation decoction. Migraine-like headache was evaluated by behavioral tests. The rat feces were extracted for microbial community and metabolic pathway analysis. Our results show that sodium propionate treatment can help rats with inflammation decoction sodium propionate induced facial hyperalgesia, and inflammation decoction sodium propionate induced pain stimulation can make the body produce higher levels of some tryptophan metabolites to fight pain and sodium propionate treatment will restore the metabolites to normal levels. These results indicate that sodium propionate alleviates inflammation decoction sodium propionate induced migraine-like headaches and intestinal metabolic disorders by regulating the changes in intestinal bacterial community composition and metabolites, and short-chain fatty acids may play an important role in the regulation and prevention of migraine in the future.
Keywords
Abnormal intestinal flora, short-chain fatty acid metabolomics, migraine, sodium propionate
Migraine is a common primary brain dysfunction disease affected by genetic and environmental factors. The International Classification of Headache Disorders standard (3rd edition) defines migraine as a moderate to severe headache that lasts for 4 to 72 h, accompanied by nausea, vomiting, photophobia and repeated attacks[1]. Based on the global burden of headache reports in 2016, it was estimated that approximately 14 % of the adult population worldwide suffer from migraine[2]. The exact etiology and pathogenesis of migraine are still unclear. At present, the recognized pathogenesis includes trigeminal neurovascular system activation, calcitonin generelated peptide release, cortical spreading depression and central sensitization. Migraine patients are often accompanied by gastrointestinal symptoms and co-occur with gastrointestinal diseases such as irritable bowel syndrome, gastric retention, gastroesophageal reflux and celiac disease. At the same time, the prevalence of headaches will increase in patients with functional gastrointestinal diseases[3]. It can be seen that intestinal flora plays a certain role in the occurrence and development of migraine.
A large number of previous preclinical studies have shown that there are at least three parallel and interactive channels between intestinal microorganisms and the central nervous system, involving neural, endocrine and immune signaling mechanisms. The brain can affect the community structure and function of intestinal flora through the autonomic nervous system and intestinal flora can affect the development, immune state and function of the central nervous system through its own metabolites[4]. This two-way communication system, which is mediated by hormones, immunity and neural signals and used to connect the gastrointestinal tract and the central nervous system, is the "brain-gut axis". Microorganisms in the intestinal microbiota may release metabolites and molecules which can trigger the activation of the inflammatory cascade of the central nervous system through the gut-brain axis, thereby interfering with the function and homeostasis of the central nervous system[5]. Previous studies have shown that intestinal microbiota not only plays a key role in visceral pain but also participates in the regulation of chronic pain.
Short-Chain Fatty Acids (SCFA) are the main metabolites produced by bacteria fermenting dietary fiber in the intestine. SCFA includes acetate, propionate and butyrate. SCFA plays an important role in maintaining immune function besides intestinal homeostasis and the morphology and function of intestinal epithelial cells. In the previous work of our team, the intestinal microbiome of migraine patients was transplanted into healthy mice and it was found that compared with the control group, the mice in the experimental group were seriously sensitized to Nitroglycerin (NTG)-induced hyperalgesia. Our work further highlights the role of intestinal microbial homeostasis in the pathogenesis of migraine[6]. Recent studies have shown that after treatment with SCFA such as propionic acid and butyric acid, the head and face hyperalgesia and photophobia of the mouse migraine model mediated by NTG have been significantly improved. At the same time, SCFA treatment can also reduce the injury of neurons in the trigeminal ganglion, intestinal mucosal injury, and the release of intestinal neurotransmitters[7]. But the formation of migraine models induced by intraperitoneal injection of NTG will cause damage to intestinal cells themselves. It will have an inevitable impact on the study of intestinal microbial homeostasis. Therefore, our research group used a repeated dural injection of inflammatory decoction to simulate neurogenic inflammation and expression of calcitonin gene-related peptides in a rodent model of chronic migraine, episodic dual stimulation in acute rates; a model for recurrent headache; at the same time, SCFA was given for intervention to observe the changes of related behaviors of rats, and SCFA and tryptophan metabolites were quantitatively analyzed by Gas Chromatography-tandem Mass Spectrometry (GC-MS/MS) and Liquid Chromatography-tandem Mass Spectrometry (LC-MS/MS).
Materials and Methods
Animals:
Specific Pathogen Free (SPF) Sprague-Dawley rats (males, 200 to 220 g, SiPeiFu Biotechnology Co., Ltd., Beijing, China) were housed in a controlled environment (23±2°, 55±15 % relative humidity, a 12:12 h light/dark cycle). Standard diet and tap water were available ad libitum. The sample size was calculated by G*Power (ver. 3.1.9.7) based on the repeated measures design (power=0.85). The experimental procedures were approved by the Institutional Animal Care and Use Committee, Chinese People’s Liberation Army (PLA) General Hospital, following the Regulations for the Administration of Affairs Concerning Experimental Animals.
Migraine model induction:
After 3 d of behavioral adaptation training for head and face pain in rats, the cannula was surgically implanted into the skull of each rat and fixed well. The specific operation methods were as follows; 12 h of fasting water was started at night the day before the operation. The rats were anesthetized by intraperitoneal injection of 50 mg/kg pentobarbital sodium. The rats were placed on the stereotactic instrument and the skull top and ground of the rats were kept level. An incision about 1.0 cm long was made at the top of the rat skull along the median line to passively separate the soft tissue at the top of the skull and fully expose the skull surface. A burr hole, 1.5 mm lateral to the midline and 1.5 mm posterior to bregma, was drilled to expose the dura mater adjacent to the superior sagittal sinus. A stainless steel inner cannula (O.D. 0.64 mm, M3.5), with a matched cap (O.D. 0.40 mm, M3.5), was implanted into the hole and affixed to the skull with tissue adhesive (3 M Vetbond). Cover the base of the sleeve with dental cement for further fixation. Carefully suture the incision and gently turn back the casing cap, and disinfect the wound with Allium iodine (fig. 1B). After the operation, the rats were fed separately for the follow-up experiment.
Experimental groups:
The overall animal grouping and experimental outline are shown in fig. 1. The experimental animals were randomly divided into three groups including control group; inflammation soup group; sodium propionate treatment group, sodium propionate or equal amount of double distilled water was given to the stomach (control group and inflammation Decoction group) every day from the 1st d for 13 consecutive d. Adaptive facial mechanical pain threshold measurement and behavioral measurement were performed on the rats of the three groups on the 5th-7th d. From the 8th d, 10 ml of Inflammation decoction, Sodium propionate treatment (IS) group or Phosphate Buffered Saline (PBS) (control group) were injected into the epidural space of the experimental animals 5 min after the end of the facial mechanical pain threshold measurement. After the end of the epidural injection, the behavior was measured. After the end of the video recording, the experimental animals were intragastric administered with propionic acid or PBS for 7 consecutive d.
The IS contained bradykinin (2 mM), histamine (2 mM), 5-HT (2 mM), prostaglandin E2 (0.2 mM), and PBS as a vehicle for solubilization. According to the study of other related SCFA, the optimal concentration of sodium propionate for intragastric administration in rats is 100 mg/kg.
Measurement of facial mechanical pain threshold:
As mentioned above, the facial mechanical pain threshold was measured in three groups of rats before each epidural injection. In this process, we selected two points of the forehead and inner canthus for measurement. First, the experimental animals were conditioned in a cured plexiglass box for 10 min before the measurement. When the rats were in a quiet state, von Frey filaments (aesthesio, Ugo Basile, Italy) were placed in the inner canthus and forehead of the rats for measurement. According to manufacturer calibrated forced values, the intensity of mechanical stimulation was increased from low to high. When the rats use their forepaws to comb their faces quickly, the head retracts quickly or the head shakes quickly, they are all recorded as positive reactions. When a strong positive reaction occurs, the value of von Frey filament is reduced and the measurement is performed again. When the von Frey filament under a certain value is positive in 3 of the 5 stimuli to rats, the value is defined as the pain threshold of the stimulation site. The von Frey tests were performed by an investigator blind to the treatment groups.
Behavioral recording and analysis:
Immediately after the end of each epidural injection, the three groups of rats were videotaped for 15 min and analyzed. From the 5th d to the 14th d, a total of 10 consecutive behavioral tests were conducted, of which the first 3 d were adaptive tests. The rats were placed in a black opaque box of 90 cm×90 cm, and the position and movement track of the rats in the box were recorded by the camera located in the center of the top of the box. The behavioral data were collected by CinePlex Studio V3 (Plexon Inc., TX, USA). The moving distance and trajectory map of rats can be automatically obtained through cineplex studio V3 and the speed threshold of moving is 0.1 cm/s. Static time was preliminarily detected and screened by the freezing detector module of cineplex editor V3 (Plexon Inc., TX, USA). Then, relevant personnel will manually analyze and check the automatically generated video clips twice. The behavioral data were analyzed by an investigator blind to the treatment groups.
Bacterial 16S rRNA V3-V4 region sequencing:
After the last dural injection to the experimental animals, the rats were quickly killed under isoflurane induction anesthesia and 7 % chloral hydrate intraperitoneal injection anesthesia within 3-4 h. Fecal samples of experimental animals were collected in sterile centrifuge tubes and stored at -80°. Bacterial genomic DNA was extracted from stool samples using a QIAamp PowerFecal Kit (Qiagen, Hilden, Germany) following the manufacturer’s manual. The V3-4 hypervariable region of the bacterial 16S rRNA gene was amplified with the primers 338F (5’-ACTCCTACGGGAGGCAGCAG-3’) and 806R (5’-GGACTACNNGGGTATCTAAT-3’). Polymerase Chain Reaction (PCR) was carried out on a Mastercycler Gradient (Eppendorf, Germany), and deep sequencing was performed on an Illumina MiSeq platform. After removing low-quality sequences from raw data, qualified reads were clustered into Operational Taxonomic Units (OTU) at a similarity level of 97 % using the UPARSE algorithm of Vsearch (ver 2.7.1). The OTUs were assigned to taxa based on the Silva 138 bacterial database for further analysis by Qiime (Version 1.8.0).
GC-MS/MS:
The levels of SCFAs were detected by GC-MS/MS in both fecal and serum samples, an efficient and sensitive assay to analyze volatile compounds. Fecal samples were obtained as described above and blood samples were collected using the abdominal aortic method before transcardial perfusion of the rats. The serous samples were obtained after centrifugation (4200 rpm, 10 min) and stored at -80°. All the samples were prepared following standard procedures and analyzed in Multiple Reaction Monitoring (MRM) mode on an Agilent 7890B gas chromatograph coupled to a 7000D mass spectrometer system (Agilent, CA). The MRM parameters for each targeted analyte were optimized by flow injection analysis. Agilent MassHunter (B.08.00, Agilent Technologies) was used for data acquisition and processing.
LC-MS/MS:
Levels of tryptophan and its metabolites were detected by LC-MS/MS in both feces and sera. Fecal and serous samples were obtained as described above. The prepared samples were analyzed using an LCESI- MS/MS system, including ultra-performance liquid chromatography (UPLC, ExionLC AD) and tandem mass spectrometry (MS/MS, QTRAP 6500+). Quantitative analysis of tryptophan metabolites was performed by MRM. Analyst (ver 1.6.3, Sciex) and Multiquant (ver 3.0.3, Sciex) were used for data acquisition and analyte quantification, respectively.
Statistical analysis:
The R software (v 4.0.1; R Foundation for Statistical Computing, Vienna, Austria) with the ggplot2 (v 3.3.2) package was used for data visualization[8]. Permutational multivariate Analysis of Variance (ANOVA) (PERMANOVA, Adonis test of vegan v 2.5-6) was performed for statistical analysis of beta diversity. One-way ANOVA and Kruskal-Wallis H nonparametric tests compared the three groups. A p value or FDR≤0.05 was considered statistically significant.
Results and Discussion
In collecting behavioral data, we divided the mechanical pain threshold into the forehead and inner canthus. According to the statistical results from the 6th to 10th d of the sodium propionate gavage treatment (after the third injection of IS), the mechanical pain threshold of the forehead of the rats in the IS group was significantly lower than that of the other two groups (IS vs. SP, p<0.05; IS vs. CON, p<0.05). On the 6th, 8th, and 10th d the pain threshold of the inner canthus in the CON group was significantly lower than in the IS and SP groups (fig. 1C-fig. 1H). These results indicate that repeated dural injection of IS can induce abnormal pain in the head and face of rats, which can be avoided by prophylactic intragastric administration of sodium propionate.
According to statistics, there is no significant difference in the total distance traveled by the CON and SP groups. However, in the statistics of freezing time, there was a substantial difference between IS and CON/SP groups on the 7th d (IS vs. SP, p<0.05; IS vs. CON, p<0.05, respectively, fig. 1I- fig. 1K).
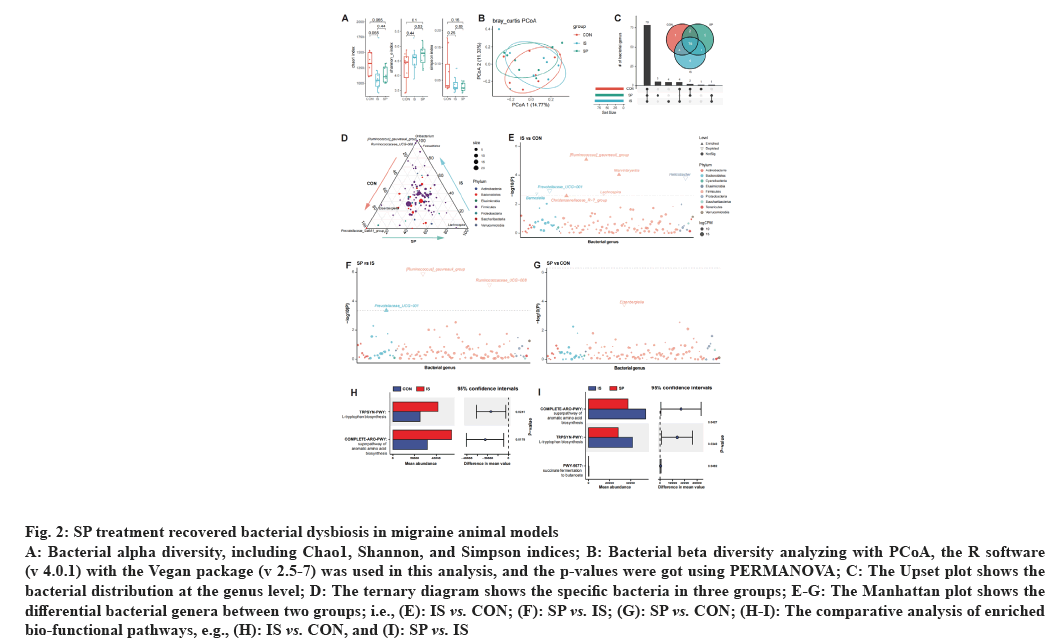
As mentioned above, the gut-brain axis highlights the gut microbial role in the pathological process of migraine[9]. To investigate this bacterial role, we performed a 16S rRNA sequencing to profile the bacterial community and the functional alteration of the migraine model mice; also, we tested the relationship between sodium propionate treatment efficacy and gut microbiota. Data showed no significant alteration in bacterial alpha diversity, including migraine model mice (IS vs. CON, p>0.05) and Sodium propionate treated mice (SP vs. IS, p>0.05), when compared with CON and IS groups, respectively (fig. 2A). Principal Coordinates Analysis (PCoA) was used to test the differentiation in the bacterial community; our test data also showed no significant variations among the three groups (Adonis p=0.58 and 0.24 for IS and SP vs. CON, and 0.21 for SP vs. IS, respectively, fig. 2A).
Fig. 2: SP treatment recovered bacterial dysbiosis in migraine animal models
A: Bacterial alpha diversity, including Chao1, Shannon, and Simpson indices; B: Bacterial beta diversity analyzing with PCoA, the R software
(v 4.0.1) with the Vegan package (v 2.5-7) was used in this analysis, and the p-values were got using PERMANOVA; C: The Upset plot shows the
bacterial distribution at the genus level; D: The ternary diagram shows the specific bacteria in three groups; E-G: The Manhattan plot shows the
differential bacterial genera between two groups; i.e., (E): IS vs. CON; (F): SP vs. IS; (G): SP vs. CON; (H-I): The comparative analysis of enriched
bio-functional pathways, e.g., (H): IS vs. CON, and (I): SP vs. IS
These data indicated that the migraine modeling with Sodium propionate treatment had no dramatic influence on the bacterial community. We then tested the difference in bacterial contents. We found that all three groups of mice shared the majority of bacterial genera; however, Prevotellaceae_Ga6A1_group was specifically colonized in the CON group (fig. 2C). The IS and SP groups also have group-specific bacterial genera, such as Ruminococcus gauvreauii group, Oribacterium, Corynebacterium_1, Anaerofilum in IS group, and Elusimicrobium, Lachnospira, Candidatus stoquefichus, Acetatifactor, Enterococcus in SP group (fig. 2C); composition mapping data also confirmed these results (fig. 2D).
Although there was no marked alteration in the gut microbial community, we still found a group of bacterial genera significantly changed in the migraine model mice; i.e. Ruminococcus gauvreauii group, Marvinbryantia, Christensenellaceae R-7 group were enriched and Barnesiella, Prevotellaceae_UCG-001, Lachnospira, and Helicobacter were depleted in the IS group (vs. CON, fig. 2E). Interestingly, compared with the IS group, treating with Sodium propionate, Ruminococcus gauvreauii group and Prevotellaceae_ UCG-008 were decreased, and Prevotellaceae_UCG- 001 was increased in the SP group (fig. 2F). There were only a few bacteria with significant differences between the SP and the CON groups (fig. 2G). These data suggested that the abundance of specific bacterial genera was altered with migraine modeling; however, SP treatment recovered the dysbiotic gut microbiome.
Furthermore, we also investigated the bio-functional enrichment in different groups of mice with PICRUSt2. As reported previously, L-tryptophan and aromatic amino acids were involved in the serotonin system; intestinal flora can stimulate the secretion of 5-HT by intestinal chromaffin cells by ingesting exogenous tryptophan[10]. We found that L-tryptophan biosynthesis and aromatic amino acid biosynthesis were enriched in the IS group (p=0.0241 and 0.0178, respectively, fig. 2H); notably, these pathways were reversed with SP treatment (p=0.0427 and 0.0349, respectively; fig. 2I). These data were paralleled with the tendency of bacterial fluctuation; which confirmed the shaping role of migraine modeling and SP treatment on the bacterial community.
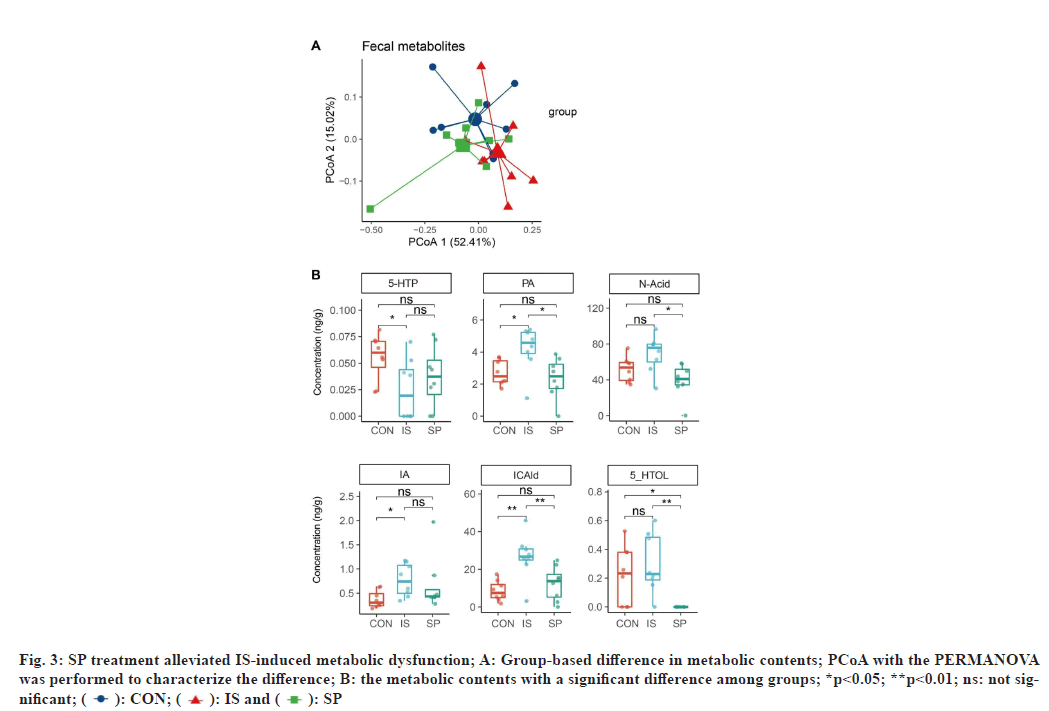
We further analyzed the fecal metabolites involved in the tryptophan and SCFA metabolic pathways. Data showed that the migraine modeling partially drove the bacterial metabolic dysfunction (Adonis p=0.098 for IS vs. CON, fig. 3A); however, SP treatment restored the metabolic homeostasis (Adonis p=0.032 for SP vs. IS, and 0.595 for SP vs. CON). We found that the tryptophan-associated metabolites, including PA, IA, and ICAId were significantly increased in the IS group compared with the CON group (p=0.020, 0.028, and 0.007, respectively; fig. 3B); however, SP treatment decreased the concentrations of these metabolites (p=0.014 for PA, 0.194 for IA, and 0.007 for ICAId in SP vs. IS, fig. 3B). There was also an increasing trend for N-Acid and 5-HTOL in rats with migraine modeling (p=0.065 and 0.597 for N-Acid and 5-HTOL in IS vs. CON, respectively; fig. 3B); the richness of these metabolites was also reversed in the SP group (p=0.014 and 0.001 for N-Acid and 5-HTOL in SP vs. IS, respectively; fig. 3B). 5-HTP decreased markedly in the IS group (p=0.030, vs. CON; fig. 3B) without restoration in the SP-treated rats (p=0.419, vs. IS; fig. 3B). Interestingly, no SCFAassociated fecal metabolites varied with modeling and SP treatment. Besides we also analyzed the serum tryptophan and SCFA metabolites, but no statistical difference was found in our analysis. These data indicated a close association between migraine and fecal tryptophan-associated metabolites rather than those in serum.
Fig. 3: SP treatment alleviated IS-induced metabolic dysfunction; A: Group-based difference in metabolic contents; PCoA with the PERMANOVA
was performed to characterize the difference; B: the metabolic contents with a significant difference among groups; *p<0.05; **p<0.01; ns: not significant;
( ): CON; (
): CON; ( ): IS and (
): IS and (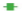 ): SP
): SP
In this paper, we used the chronic migraine model stimulated by repeated inflammatory soup to study and found that the IS-induced chronic migraine model, compared with the healthy control group, had changes in head and face mechanical hyperalgesia, headache-related behavior, intestinal microbial community, and intestinal tryptophan metabolic pathway and product level. After treatment, these changes can be reversed by SCFAs represented by sodium propionate.
Migraine, a brain dysfunction disease, is characterized by unilateral, pulsatile, moderate to severe pulsatile headache, which will be aggravated after exercise or daily activities. The activation of the trigeminal neurovascular system is a critical theory in its pathogenesis. Some people also believe that it is a series of reactions triggered by Cortical Spreading Depression (CSD) of focal origin, which leads to local neurogenic inflammation of the meninges[10], then activates the injury receptors around the meningeal vessels, and finally activates the brainstem trigeminal nerve neck complex, which is uploaded to the sensory cortex to cause migraine[11]. In our experimental study, chronic migraine was caused by repeatedly using IS to stimulate the dura mater of rats[12]. The rats would have pain sensitivity and decreased activity in the head and face, and using sodium propionate could significantly change this phenomenon[13].
Gut-brain axis has the function of regulating the intestine and central nervous system. Maintain homeostasis in three ways: Systemic horizontal path, Cellular immune path, and Neuronal path. In recent years, with the emergence of research on intestinal flora and intestinal microecology, the intestinal microbiome has been considered an essential factor in regulating brain-gut signal transduction. The Microbiota-Gut-Brain Axis was also established[14]. The Microbiota-Gut-Brain Axis is deemed to be a two-way pathway. While brain dysfunction affects intestinal microecology, intestinal microecology abnormality can also cause brain dysfunction. In recent years, the vagus nerve, as a critical two-way pathway of brain-gut connection (many scholars believe that the vagus nerve has an internal relationship with the Central Nervous System (ENS)), has been focused on research. Still, there is no direct evidence to prove the mechanism of this interaction and the pathway from the vagus nerve signal to the ENS to the central nervous system.
Some experimental studies have shown that the metabolites of intestinal flora can affect the vagus nerve; intestinal bacteria can produce SCFA metabolites (such as propionic acid, butyric acid, acetic acid, etc.) and regulate intestinal movement, secretion, inflammation, and other functions by acting on its homologous Free Fatty Acid Receptor (FFAR) [9]. Chromaffin cells in the intestine contain more than 90 % of human 5-HT, while the synthesis and release of 5-HT in Entero-Chromaffin Cells (ECCs) are regulated by SCFAs[15]. The low function of the 5-HT system is an integral part of migraine etiology. In addition, propionate and butyrate can restore the morphological defects of microglia in germ-free mice[16] and reduce the persistent changes caused by repeated psychological stress[17]. Administration of propionate and butyrate to neuron-like cells in vitro can increase the expression of Tyrosine Hydroxylase (TH), and TH is a crucial enzyme involved in catecholamine synthesis. Some studies used sodium propionate and butyrate to treat the mouse model of paroxysmal migraine induced by NTG by gavage.
The results showed that compared with the untreated mice, the head and face hyperalgesia of the mice in the SCFAs treatment group was reversed, the mast cell infiltration and inflammatory reaction in the intestine were significantly reduced, and the intestinal wall permeability was improved[7,18]. A recent research result of our research group also shows that in the IS-induced chronic migraine rat model, intestinal microbiota and tryptophan metabolism pathway will also be affected, and the disorder of intestinal microbiota and metabolism has also been corrected to some extent after the use of drugs to treat migraine[19]. At the same time, intestinal microorganisms can also enhance the stimulation of ECCs by improving the utilization of tryptophan[10]. Our study found that the IS group had significant changes in the microbial community, different from the CON group and SP group. These microbial community changes may impact the intestinal microecology, thus aggravating migraine, or it may be the adaptive changes in the intestinal flora caused by long-term headache stimulation. Because intestinal microflora is affected by many factors such as age, diet, feeding conditions, climate and temperature of experimental animals, it is impossible to determine the species of intestinal microorganisms involved. In our study, we also found that the IS group, compared with the CON group and SP group, had abnormalities in the metabolic pathways of tryptophan and SCFAs, which means that the IS-induced chronic migraine model had metabolic dysfunction of tryptophan and aromatic amino acids. After sodium propionate treatment, these metabolic functions and bacterial flora abnormalities were corrected to a certain extent. However, the common problem in current research is that the amount of SCFAs applied in the experiment is far greater than the amount produced by intestinal microorganisms under physiological conditions.
This study found differences in ICAld, N-Acid, and PA between the CON and IS groups. N-Acid is a compound in Niacin (vitamin B3, vitamin PP), and the other is nicotinamide. N-Acid is the precursor of Nicotinamide Adenine Dinucleotide (NAD) and the coenzyme of various key enzymes (core enzymes regulating sugar metabolism, fatty acid oxidation, and synthesis). It is vital in maintaining intracellular homeostasis and basic cellular metabolic activities. N-Acid plays a crucial role in maintaining mitochondrial function and normal oxidative phosphorylation process[20,21]. An older woman with a 40 y history of migraine was recorded in a case report. She had a general reaction to triptans. However, the response to oral nicotinic acid was good, and migraine was completely controlled after oral nicotinic acid[22]. Others believe that niacin acted as a negative feedback regulator on the kynurenine pathway of tryptophan metabolism, directing it to the serotonin pathway, of which low levels are reported in migraineurs[23]. However, there is no standard randomized controlled trial to evaluate niacin's feasibility and specific mechanism in treating migraine. Some researchers suggested that according to the role of nicotinic acid in expanding peripheral blood vessels, it may be possible to expand central blood vessels, which may play a role in the pathogenesis of migraine[24,25]. PA can play a protective role in many neurodegenerative diseases by blocking cholinergic toxicity, neuronal loss, and glial cell proliferation. Because it can chelate metal ions and effectively inhibit the neurotoxicity of N-methyl-D-aspartate receptors, it is considered an endogenous neuroprotective compound in the central nervous system. In one study, PA levels were significantly lower in migraine patients with aura and without aura than in healthy controls during the interictal period. At the beginning of the attack, the pica level was slightly increased[26]. The effect of ICAld on migraine and pain has not been reported, but its role in headaches cannot be denied entirely .
Therefore, in our study, in the chronic migraine model induced by repeated IS stimulation, PA and N-Acid were significantly increased. This shows that different from the change of metabolite level of paroxysmal migraine, in chronic migraine, such neuroprotective tryptophan metabolites maintain a high level under the stimulation of long-term chronic pain, which may be the adaptive change of the body in response to chronic stimulation.
In conclusion, our research shows that repeated dural stimulation can improve head and face hyperalgesia, reduced movement, flora difference, and tryptophan metabolism disorder by giving sodium propionate to the stomach. Moreover, tryptophan metabolites such as PA, ICAld, and N-Acid may be adaptive metabolic changes intestinal flora produce to adapt to long-term chronic pain stimulation, which can be used as specific biomarkers of chronic migraine. In future research, it is necessary to verify further these metabolites' role and particular mechanism in the pathogenesis and prevention of migraine. These metabolites are expected to be part of the future migraine prevention and treatment program.
Conflict of interests:
The authors declared no conflict of interests.
References
- The international classification of headache disorders. 3rd ed (beta version). Cephalalgia: 2013;33(9):629-808.
- Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet: 2017;390(10100):1211-59.
- Silberstein SD. Migraine. Lancet 2004;363(9406):381-91.
- Holzer P, Farzi A. Neuropeptides and the microbiota-gut-brain axis. Adv Exp Med Biol 2014:195-219.
[Crossref] [Google Scholar] [PubMed]
- Aguilera M, Rossini V, Hickey A, Simnica D, Grady F, Felice VD, et al. Inflammasome signaling regulates the microbial–Neuroimmune axis and visceral pain in mice. Int J Mol Sci 2021;22(15):8336.
[Crossref] [Google Scholar] [PubMed]
- Kang L, Tang W, Zhang Y, Zhang M, Liu J, Li Y, et al. The gut microbiome modulates nitroglycerin-induced migraine-related hyperalgesia in mice. Cephalalgia 2022;42(6):490-9.
[Crossref] [Google Scholar] [PubMed]
- Lanza M, Filippone A, Casili G, Giuffrè L, Scuderi SA, Paterniti I, et al. Supplementation with SCFAs Re-establishes microbiota composition and attenuates hyperalgesia and pain in a mouse model of NTG-induced migraine. Int J Mol Sci 2022;23(9):4847.
[Crossref] [Google Scholar] [PubMed]
- Villanueva, Ram, Chen ZJ. ggplot2: Elegant graphics for data analysis: 2nd ed. Measurement-interdisciplinary research and perspectives: 2019;17(3):160-7.
- Bonaz B, Bazin T, Pellissier S. The vagus nerve at the interface of the microbiota-gut-brain axis. Front Neurosci 2018;12:49.
[Crossref] [Google Scholar] [PubMed]
- Agus A, Planchais J, Sokol H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe 2018;23(6):716-24.
[Crossref] [Google Scholar] [PubMed]
- Noseda R, Constandil L, Bourgeais L, Chalus M, Villanueva L. Changes of meningeal excitability mediated by corticotrigeminal networks: A link for the endogenous modulation of migraine pain. J Neurosci 2010;30(43):14420-9.
[Crossref] [Google Scholar] [PubMed]
- Oshinsky ML, Gomonchareonsiri S. Episodic dural stimulation in awake rats: A model for recurrent headache. Headache 2007;47(7):1026-36.
[Crossref] [Google Scholar] [PubMed]
- Melo-Carrillo A, Lopez-Avila A. A chronic animal model of migraine, induced by repeated meningeal nociception, characterized by a behavioral and pharmacological approach. Cephalalgia 2013;33(13):1096-105.
[Crossref] [Google Scholar] [PubMed]
- Margolis KG, Cryan JF, Mayer EA. The microbiota-gut-brain axis: From motility to mood. Gastroenterology 2021;160(5):1486-501.
[Crossref] [Google Scholar] [PubMed]
- Reigstad CS, Salmonson CE, Rainey III JF, Szurszewski JH, Linden DR, Sonnenburg JL, et al. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J 2015;29(4):1395.
[Crossref] [Google Scholar] [PubMed]
- Erny D, Hrabě de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci 2015;18(7):965-77.
[Crossref] [Google Scholar] [PubMed]
- van de Wouw M, Boehme M, Lyte JM, Wiley N, Strain C, O'Sullivan O, et al. Short‐chain fatty acids: microbial metabolites that alleviate stress‐induced brain–gut axis alterations. J Physiol 2018;596(20):4923-44.
[Crossref] [Google Scholar] [PubMed]
- Lanza M, Filippone A, Ardizzone A, Casili G, Paterniti I, Esposito E, et al. Scfa treatment alleviates pathological signs of migraine and related intestinal alterations in a mouse model of ntg-induced migraine. Cells 2021;10(10):2756.
[Crossref] [Google Scholar] [PubMed]
- Miao S, Tang W, Li H, Li B, Yang C, Xie W, et al. Repeated inflammatory dural stimulation-induced cephalic allodynia causes alteration of gut microbial composition in rats. J Headache and Pain 2022;23(1):71.
- Meyer-Ficca M, Kirkland JB. Niacin. Adv Nutr 2016;7(3):556-8.
- Kirkland JB, Meyer-Ficca ML. Niacin. Adv Food Nutr Res 2018;83:83-149.
[Crossref] [Google Scholar] [PubMed]
- Velling DA, Dodick DW, Muir JJ. Sustained-release niacin for prevention of migraine headache. Mayo Clin Proc 2003;(78)6:770-1.
[Crossref] [Google Scholar] [PubMed]
- Giniatullin R. 5‐hydroxytryptamine in migraine: The puzzling role of ionotropic 5‐HT3 receptor in the context of established therapeutic effect of metabotropic 5‐HT1 subtypes. Br J Pharmacol 2022;179(3):400-15.
[Crossref] [Google Scholar] [PubMed]
- Mason BN, Russo AF. Vascular contributions to migraine: Time to revisit? Front Cell Neurosci 2018;12:233.
[Crossref] [Google Scholar] [PubMed]
- Fila M, Chojnacki C, Chojnacki J, Blasiak J. Nutrients to improve mitochondrial function to reduce brain energy deficit and oxidative stress in migraine. Nutrients 2021;13(12):4433.
[Crossref] [Google Scholar] [PubMed]
- Tuka B, Nyári A, Cseh EK, Körtési T, Veréb D, Tömösi F, et al. Clinical relevance of depressed kynurenine pathway in episodic migraine patients: Potential prognostic markers in the peripheral plasma during the interictal period. J Headache Pain 2021;22(1):1-9.
 ): CON; (
): CON; ( ): IS and (
): IS and ( ): SP
): SP