- *Corresponding Author:
- S. Ponnusankar
Department of Pharmacy Practice, JSS College of Pharmacy, JSS Academy of Higher Education and Research, Ooty, Tamilnadu 643001, India
E-mail: ponnusankarsivas@gmail.com
| Date of Received | 02 August 2022 |
| Date of Revision | 29 June 2023 |
| Date of Acceptance | 29 March 2024 |
| Indian J Pharm Sci 2024;86(2):420-432 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The rise in comorbid conditions with type 2 diabetes requires novel drug targets which benefits not only in glycaemic management but provide beneficial effects to other co-existing conditions too. One such drug available in market is the sodium glucose cotransport-2 inhibitor which is known for its pleiotropic effects. Keyword based comprehensive search was made in PubMed, Scopus and Google Scholar. Critical appraisal of all the collected literature was done and the key findings were used. The novel class of oral anti-diabetics act by reducing hyperglycaemia by rising urinary glucose excretion independently of insulin secretion or action. Clinical trials have shown its effects not only in controlling glycaemic levels but other effects such as reducing hypertension, providing cardiovascular benefits and positive renal outcomes too. Despite the positive outcomes, a segment of adverse effects such as lower limb amputations, risk of genital infections has set as drawbacks to bringing the drug into extensive clinical practice. This review provides an overview of the current evidence available so far on the therapeutic potential of the sodium glucose cotransporter-2 inhibitors for the treatment of type 2 diabetes mellitus and suggests the use of drug into practice to obtain evidences at a large level.
Keywords
Sodium glucose cotransporter-2 inhibitors, type 2 diabetes, glycaemic control, efficacy, pleiotropic effects, clinical trials
Inadequate glycemic control is one of the major risk factors leading to diabetic complications. The etiology of Type 2 Diabetes Mellitus (T2DM) being intricate and multifaceted makes the management a difficult process. Apart from the complexity of the disease, individuals are affected due to varying degrees to changes in insulin resistance, insulin deficiency and genetic variances[1]. There have been advancements and newer targets to treat the disease worldwide but a standard therapy is not yet in picture. An addition to surge in diabetes cases, a growing prevalence of overweight and obesity is not to be forgotten which worsens the disease condition[2] and has now become a serious health concern leading to several other complications[3]. The physicians of this decade are focusing on the management of obesity which is a key strategy in controlling diabetes. The commonly used oral anti-diabetic agents are metformin, insulin secretagogues (sulfonylureas and meglitinides), alpha-glucosidase inhibitors, thiazolidinedione’s, dipeptidyl peptidase-4 inhibitors and injectables like glucagon-like peptide-1 receptor agonists and insulin preparations are used worldwide to treat the disease[4].
Most of the hyperglycemic agents such as sulfonylurea, insulin, thiazolidinedione’s and glinides possess an adverse effect of causing gain in weight[5,6]. Modern practices in managing the condition involves a combination of lifestyle and pharmacological interventions which are aimed towards preventing and managing glycemic levels by ensuring sufficient glucose delivery to targeted tissues, improving insulin resistance, augmenting insulin secretion and blocking the recovery of glucose from nephrons[7]. In kidneys, glucose transport is mediated by a family of proteins called Sodium Glucose co-Transporters (SGLT). The role of SGLTs in maintaining glucose homeostasis has been established by studies worldwide thereby marking it as an important target to management of diabetes[8]. SGLT-2 inhibitors (SGLT2i) act through a novel mechanism of reducing renal tubular glucose reabsorption thereby producing a reduction in blood glucose without stimulating release of insulin. This drug proves to be advantageous due to its ability to lower weight and blood pressure and is said to posess reno-protective and cardioprotective effects[9,10]. SGLT2i with their insulin-independent action regulate blood glucose by increasing the excretion of urinary glucose[11]. The SGLT2i have pleiotropic effects other than the effect on T2DM which makes it a novel class of drugs[5].
Here, we aim to review the various aspects of SGLT2 inhibitors therapy in T2DM. This literature review aims at exploring the overall effects of SGLT-2i from its evolution to present day clinical practice.
Evidence Acquisition
A keyword based comprehensive desktop search was conducted to gather the relevant literature for the review from search databases such as PubMed, Scopus and Google scholar. Articles published between January 1998-December 2020 were considered and analysed for the review writing. Search terms like “SGLT2i”, “pharmacokinetic”, “efficacy”, “cardiovascular safety” and generic names of the SGLT2 is that are commercialized worldwide (“Canagliflozin”, “Dapagliflozin”, “Empagliflozin”, “Ertugliflozin”) were included. Literature published in languages other than English was excluded. All the collected literature was critically appraised. Further, reference list of the included studies and key reviews in the area were scanned for additional relevant studies. Electronic database searches were completed with reference list and citation check.
Evolution and Development of SGLT Inhibitor
In 1835, a natural product called phlorizin was extracted from the bark of Malus pumila (apple), which possessed SGLT inhibitory activity[12]. The bitter flavor of phlorizin reminded the chemists of cinchona, which was a reasonable candidate to treat fever, infectious diseases and malaria. Later, in the 1900s, the trials revealed that phlorizin caused glycosuria which when administered to the canine model produced symptoms of human diabetes (glycosuria and weight loss) which lead to the development of phlorizin-induced diabetes animal model[13]. In the following decades, the effect of phlorizin on renal physiology was studied through clinical trials. In the 1970s, it was revealed that phlorizin had a comparatively much higher binding affinity than that of its analogs[14]. Most in vivo studies incorporating animal diabetic models revealed that phlorizin significantly enhanced insulin sensitivity[15-21]. Further it was revealed that SGLT2 comparatively has low affinity than the SGLT1 receptor, Phlorizin inhibits both SGLT1 and SGLT2[22-24]. Phlorizin's catalyzed hydrolytic metabolites B-glycosidases, actively inhibit Glucose Transporter 1 (GLUT1), which can then hinder the absorption of glucose in various tissues. Because of its primary involvement in the small intestine, SGLT1 inhibition can cause many side effects of the gastrointestinal tract, such as diarrhea, dehydration and malabsorption[13,25-27]. Table 1 summarizes the evolution and development of SGLT inhibitors.
| History | Development |
|---|---|
| 1836 | Isolation of phlorizin from bark of apple tree[12] |
| 1933 | Found to block intestinal glucose absorption[15] |
| 1938 | Clear explanation on renal glucose kinetics[16,17] |
| 1970 | Transport systems of phlorizin concept proposed |
| 1987 | Cloning of SGLT 1 inhibitor[25] |
| Phlorizin reversed the symptoms of diabetes in rat model | |
| 1992 | Cloning of SGLT 2 inhibitor |
| 1995 | National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) published first report of treatment potential of selective SGLT2 inhibitor |
| 2013 | Approval of canaglifozin (SGLT2 inhibitor) in type 2 diabetes by FDA |
Table 1: History and Development of Sglt-2 Inhibitors
The limitations of phlorizin led researchers to discover an orally administrable drug that does not require the use of prodrugs, leading to the development of the novel class of “SGLT2 inhibitors”[28]. C-glucosides, owing to their potent and precise inhibition of SGLT2, gained prominence in the healthcare industry and were called gliflozins[29]. United States of America has currently licensed four SGLT2 inhibitors, namely canagliflozin, dapagliflozin, empagliflozin and ertugliflozin for treating type 2 diabetes[30,31]. Additional SGLT2 inhibitors, including sotagliflozine and bexagliflozin, are in the late development stage, while ipragliflozin, luseogliflozin and tofogliflozin are licensed only in Japan[32,33].
Location and Mechanism of the SGLT Receptors
SGLT1 is located on the lumina of the small intestine with smaller amounts in other parts of the gastrointestinal tract of humans[34,35]. SGLT1 in the gut transports glucose and galactose from the gut lumen across the gut wall[34]. SGLT1 is also located in the late proximal tubule of the nephrons of the kidney. SGLT2 is located in the early proximal tubule[35,36].
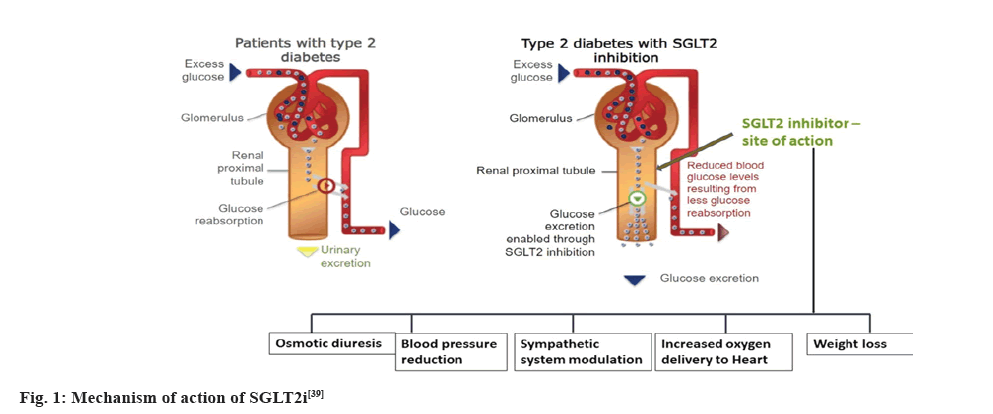
SGLT2 proteins are expressed in proximal convoluted tubule and in normal humans, reabsorb about 90 % of the filtered glucose[37]. The first stage in the mechanism is the transport of glucose across the apical membrane of the proximal tubule which leads to glucose accumulation within the epithelium. The concentration gradient of glucose between the cell and the plasma results in the second stage of mechanism which is the net passive exit of glucose through the basolateral membrane towards the plasma, via GLUT-2. Thus, the two-stage process, along with the absorption of glomerular fluid, results in complete absorption of glucose before the filtrate reaches the end of the proximal tubule[38]. In patients with diabetes, the renal threshold goes above normal and SGLT- 2i can reduce the threshold thereby being a renal protectant. Fig. 1 provides a graphical illustration of the mechanism of action of SGLT2i[39].
Fig. 1: Mechanism of action of SGLT2i[39]
Pharmacokinetic Considerations of SGLT2 Inhibitors
SGLT2i have a decent pharmacokinetic profile and are characterized by an excellent bioavailability, a long t1/2 which enables the drug to be used as a once-daily dose, low accumulation index following repeated administration, negligible renal clearance and no interactions with other hypoglycaemics[40]. The pharmacokinetic properties of some of the commonly used “gliflozins” are summarized in the Table 2[41-45].
| Characteristics | Dapagliflozin | Canagliflozin | Empagliflozin | Ertugliflozin |
|---|---|---|---|---|
| Affinity to receptors | 1.2×103 folds affinity towards SGLT2 than SGLT1[31] | 2.5×102 folds affinity towards SGLT2 over SGLT1[31] | 2.5×103 fold selectivity towards SGLT2 over SGLT1[31] | 2×103 folds towards SGLT2 over SGLT1[15] |
| Initial dose and uptitration dose bioavailability | 5-10mg [31] | 100-300 mg[42] | 10-25 mg[42] | 5-15 mg[42] |
| 78 %[42] | 65 % (300 mg)[42] | 75 %[42] | 90 %[43] | |
| After or before food | Not altered by high-fat meals. Can be administered irrespective of food[41] | Before the first meal of the day[40] | In the morning with or without food[41] | In the morning with or without food[43] |
| Tmax | 1-1.5 h[31] | 1-2 h[31] | 1.5h[31] | 0.5-1.5 h[41] |
| Protein binding | 91 %[40] | 99 %[44] | 86 %[44] | 94 %[44] |
| Interactions with other antidiabetic drugs | No interactions[42] | No interactions[44] | No interactions[6] | No interactions[44] |
| Use in hepatic impairment | Start as 5mg once daily[43] | Not recommended[6] | Not recommended[6] | Not recommended[44] |
| Use in pregnancy and breastfeeding excretion | Not recommended[43] | Category C in pregnancy[42] | Not recommended[44] | Not recommended[44] |
| Renal (75 %) | Renal (33 %) | Renal (55 %) | Renal (50 %) | |
| Fecal (21 %)[6] | Fecal (41.5 %)[6] | Fecal (40 %)[6] | Fecal (41 %)[6] |
Table 2: Pharmacokinetic Summary of Sglt-2 Inhibitors
Major Clinical Trails on SGLT2 Inhibitors
All the available anti-diabetic drugs worldwide have data supporting the micro and macrovascular complications while SGLT2i was an exception as stated by American Diabetes Association (ADA). ADA considered the discovery of SGLT- 2i as serendipitous due to its extended positive cardiovascular outcomes[45]. After this, several clinical trials were conducted to support the data regarding the same and many trials are still ongoing to explore the pleiotropic effects of SGLT2 inhibitors. Major clinical trials conducted on the drugs belonging to SGLT2i class have been summarized in Table 3.
| Trial name | Drug used | Objective | Sample size | Study duration | Primary outcome |
|---|---|---|---|---|---|
| EMPA-REG Outcome[46] | Empaglifozin 10 mg and 25 mg | To investigate the safety of Empaglifozin in patients with type 2 diabetes mellitus and with high cardiovascular risk | 7028 | 3.1 y | Significant reduction of cardiovascular mortality, 32 % reduction of general mortality, in addition to a 35 % reduction of hospitalization due to heart failure |
| Canvas[47] | Canaglifozin 100 mg and 300 mg | To evaluate canaglifozin in type 2 diabetes mellitus with regards to cardiovascular risk for major adverse cardiac events | 1 0142 | 3.6 y | Benefits of reduced risk of a composite outcome of major adverse cardiovascular events, Cardiovascular death, nonfatal Myocardial Infarction (MI), or nonfatal stroke), greater risk of lower-limb amputation |
| Credence[48] | Canaglifozin 100 mg | To assess the effects of the SGLT2 inhibitor canagliflozin on renal outcomes in patients with type 2 diabetes and albuminuric chronic kidney disease | 4401 | 2.6 y | 30 % relatively lower risk of renal failure and death due to kidney disease |
| Declare TIMI 58[49] | Dapaglifozin 10 mg | To assess the Cardiovascular (CV) safety of dapagliflozin in patients with type Diabetes Mellitus (DM2) and either established Cardiovascular Disease (CVD) or multiple risk factors | 1 7160 | 6 y | Dapaglifozin reduces the relative risk of major cardiovascular event by 16 % reduced the hospitalisation |
| CVD-REAL (comparative effectiveness of cardiovascular outcomes in new users of SGLT-2 inhibitors[50] | Any of these: Dapaglifozin, empagliflozin, ipragliflozin, canagliflozin, tofogliflozin or luseogliflozin | To determine the association between initiation of SGLT-2i therapy and HF or death in patients with and without CVD | >400 0000 | 3 y | Compared with therapy using other anti-diabetics, initiation of an SGLT-2i was associated with lower risk of death in patients with and without CVD. Lower risk of Heart failure was associated with the use of SGLTi |
Table 3: Major Clinical Trials Indicating Study Types/Design, Population, Sample Size and Major Outcomes
EMPA-REG OUTCOME is a cardiovascular outcome trial on empagliflozin on patients with T2DM prone to cardiovascular complications. It demonstrated a decrease in the rate of heart failure hospitalization and a reduction in cardiovascular deaths and provides supportive data regarding the use of empagliflozin as a long term therapy. It also reported that genital infections were more common in the subjects who were on the drug. As far as renal safety is concerned, the number of patients with acute kidney injury was lower when compared to the placebo groups and hence it was found to maintain renal function[46].
CANVAS was commenced to assess the cardiovascular efficacy and safety of canagliflozin to assess the renal protective effects of canagliflozin another trial called the CANVAS-R (CANVAS-Renal) was commenced which was then later joined with the CANVAS trial to form the CANVAS program to detect the overall cardiovascular and renal efficacy and safety of canagliflozin. The patients of this trial had a reduction in the cardiovascular deaths and also a greater risk of amputation particularly with the CANVAS group and not in CANVAS-R group. The renal protective effects of canagliflozin could not be studied thoroughly by the CANVAS program, hence it paved the way for a more specific trial of canagliflozin on patients with kidney disease- CREDENCE trial[47].
CREDENCE was conducted on T2DM patients with kidney disease. This research shows a massive reduction in renal complications with a reduced risk of end-stage renal failure. It was also found to be safe with cardiovascular outcomes and hence, it can be prescribed in patients with both renal and cardiac disease. Since the trial was stopped prematurely after 2.6 y, the study of the effect of drugs on secondary outcomes might have been limited. But later it was suggested that the limitations were unlikely to have a major effect on their findings[48].
DECLARE TIMI 58 assessed the cardiovascular outcomes of dapagliflozin in patients with T2DM. The results revealed that it reduced the cardiovascular complications and hospitalization for cardiac failure. The risk of stroke, amputation, bladder cancer and fracture associated with the drug was low when compared to the placebo. But the risk of diabetic ketoacidosis and genital infections was high with the drug than with the placebo. The renal protective effects of dapagliflozin are yet to be studied in detail[49].
Comparision of SGLT2 Inhibitors with Other Anti-Diabetic Drugs
SGLT2 inhibitors are compared with other classes of antidiabetics which are summarized in Table 4[50-61]. There is only a slight variation in the pharmacokinetics between metformin and SGLT2 inhibitors. Although metformin has been ruling, studies have shown that many patients will not achieve their target HbA1c with metformin therapy alone due to varied inter-individual responses. Hence, metformin could be combined with other antidiabetics and SGLT2i can be safe for combining with metformin or SGLT2i alone as a monotherapy are efficacious in people intolerant to metformin[56]. Both the SGLT2i and Dipeptidyl Peptidase 4 (DPP4) inhibitors have varying risks and benefits making it difficult to decide which class is the best. But better glycaemic control has been achieved when both the drugs are combined and many fixed-dose combinations of DPP4 inhibitors and SGLT2 inhibitors are available nowa- days. But the synergistic effect of SGLT2i in combination with DPP4 inhibitors are debated[51]. Glucagon-Like Peptide-1 (GLP-1) agonists are very efficacious when compared to the other class of drugs mentioned here but they offer some serious disadvantages like lack of availability of an orally bioavailable form due to rapid metabolism by proteases of the mucosa of the intestine, the variability in its chemical stability by time and increase in pH and temperature, immunogenic reactions associated with this drug, side effects such as nausea and vomiting and above all, the cost associated with it making patient compliance an issue[52]. Hence the SGLT2i can be preferred over GLP-1 peptide agonist drugs. Sulfonylureas on the other hand are less efficacious in terms of glycaemic control, half-life and cardiovascular safety when compared to the SGLT2i. Therefore SGLT2i are superior in all aspects when compared to sulfonylureas[4].
| Characteristics | Metformin vs. SGLT2 inhibitors | DPP4 inhibitors vs. SGLT2 inhibitors | GLP-1 peptide agonists vs. SGLT2 inhibitors | Sulfonyl ureas vs. SGLT2 inhibitors |
|---|---|---|---|---|
| Glycaemic control | Metformin reduces HbA1c by 1.5 % and fasting blood glucose by 20 % whereas SGLT2 inhibitors reduce HbA1c by 1.0 %-1.7 % which indicates a very little overlap in pharmacokinetic parameter between the both[53] | DPP4 inhibitors have shown to reduce HbA1c by approximately 0.7 % which is lesser than SGLT-2 inhibitors | GLP-1 peptide agonists reduce HbA1c by approximately 1.20 %-1.88 % offering much more effect than SGLT2 inhibitors. It also shows a reduction in Fasting plasma glucose (-25.6 mg/dL.). From the baseline as monotherapy and offers much more reduction in combination with other antidiabetics. It also shows a reduction in postprandial plasma glucose (-48.6mg /dL) from the baseline in combination with rosiglitazone and metformin[52] | Sulfonyl ureas reduce HbA1c by 1.0 %-1.5 % which is almost similar to SGLT-2 inhibitors |
| Pharmacokinetics | Metformin does not bind to plasma proteins whereas SGLT2 binds extensively from 86.2 %-99 %. Therefore, metformin has a large volume of distribution when compared to SGLT2 inhibitors. Metabolism of metformin is not through the liver whereas SGLT2 is metabolized by hepatic glucuronidation. Metformin is eliminated unchanged in urine through active tubular secretion whereas SGLT2 inhibitors are eliminated in the faeces and urine[4] | The DPP4 inhibitors have a lower protein binding effect when compared to SGLT2 inhibitors with an exception of linagliptin having a protein binding of 75 %-99 % and the volume of distribution of the DPP4 inhibitors are modest and they are usually less than total body water[46,64] . Much larger amount of DPP4i drugs are excreted unchanged in urine when compared to the SGLT2 inhibitor[55] | These drugs are metabolized in the body by dipeptidyl peptidase-4 enzyme and neutral endopeptidase. These drugs are not excreted in the urine or the faeces, and lower levels of metabolites are found in plasma which indicates that the drug is completely decomposed into amino acids, peptides, and fatty acid fragments in the body[53,58] | The SGLT2 inhibitors have long half-lives when compared to the sulfonylureas. The protein binding of both classes is almost the same. Both the drugs are metabolized by the liver. Only metabolites of sulfonylureas are eliminated in the urine in significant amounts[4] |
| Cardiovascular safety | Metformin prevents micro and macrovascular complications and some lipid-lowering effects. SGLT2 are beneficial in cardio protection by reducing the deaths due to cv causes, reduction in body weight and reducing systolic and diastolic blood pressure[4] | Saxagliptin and Alogliptin are known to increase cardiovascular risk whereas the SGLT2 inhibitors are comparatively cardioprotective since they have reduced the rate of HF hospitalizations and rates of death due to CV causes[52] | The risk of cardiovascular diseases is low with this class of drugs[50] | Increased risk of cardiovascular deaths is observed with sulfonylureas[58,63] whereas SGLT2 inhibitors are cardioprotective and have not reported deaths due to cardiovascular causes |
| Adverse effects | Metformin is associated with nausea, diarrhoea, and abdominal discomfort. Metformin carries the black box warning of lactic acidosis[55]. SGLT2 inhibitors carry a black box warning for diabetic ketoacidosis. It also has other side effects like genitourinary tract infections, amputations, hypotension, particularly breast and bladder cancer with dapagliflozin[46] | DPP4 inhibitors are associated with fatal haemorrhagic or necrotizing pancreatitis and they also pose a risk of pancreatic cancer. They are contraindicated in patients with pancreatitis[56,59]. SGLT2 inhibitors are associated with genitourinary tract infections, DKA, amputations, orthostatic hypotension, and particularly breast and bladder cancer with dapagliflozin[46] | Since the GLP-1 receptors are situated in the stomach and the intestines, reduction in gastric emptying, nausea, and vomiting are observed with such high doses. The levels of amylase and lipase increases in patients on Liraglutide treatment posing the risk of pancreatitis and pancreatic cancer[48] | The main adverse effects of sulfonylureas are weight gain and hypoglycaemia[53,55] whereas SGLT2 inhibitors are not associated with the risk of hypoglycaemia and they are known to reduce body weight |
| Other effects | Both metformin and SGLT2 inhibitors reduce body weight[55,60] | DPP4 inhibitors are weight neutral whereas the SGLT2 inhibitors are known to reduce the body weight by 2-3 kg57,60 SGLT2 inhibitors are known to reduce systolic and diastolic pressure[62,63] | GLP-1 agonists reduce body weight by 2.0-2.5 kg due to its action on the central body visceral adipose tissue[52]. SGLT2 inhibitors also reduce body weight substantially | Nil |
Table 4: Comparison of Sglt2 Inhibitors with Other Antidiabetic Drugs
Efficacy of SGLT2 Inhibitors
Glycemic control:
Data from trials on the drug class have shown that reduction in a larger extent that is as an additional improvement. SGLT2i can be prescribed as monotherapy or as add on therapy especially in patients who are on metformin.
This is due to the similar safety profile of both the drugs, it is said to exhibit lowering the HbA1C which can be observed in patients who were recently diagnosed with diabetes, lower age and high body mass index within 5 mo (average) of taking the drug[62,63]. Of the other drugs belonging to the class canagliflozin at a higher dose is said reduce HbA1c and exhibit almost nil drug-drug interactions and a higher reduction in HbA1c levels (0.3 %-0.5 %) can be noted[54].
Weight loss:
SGLT2 inhibitors causes weight loss due to glycosuria induced energy loss and is said to sustain for a long time[63], despite monotherapy or combination therapy. Evidences showed that the weight loss are maintained upto 4 y unlike other anti-diabetic class of drugs[64,65]. It is also said that the adiposity is reduced in rodent models which is to be explored in human body.
Blood pressure reduction:
Dapagliflozin is said to possess blood pressure lowering effects due to an anticipated diuretic like mechanism in addition to glycemic control but needs to be explored more[66].
Cardiovascular outcomes:
Empagliflozin and canagliflozin have shown to significantly reduce cardiovascular events in individuals having cardiovascular disease which has been established in EMPA-REG OUTCOME trial. SGLT2 inhibitors reduced the risk of the composite cardiovascular outcome, hospitalizations related to cardiovascular condition such as fatal heart failure and myocardial infarction and no significant effect on stroke or cardiovascular death. Thus far have been a safe drug to be administered in patients at risk of developing cardiac disease[67,68].
Adverse Effects
Risk of hypoglycemia:
The risk of hypoglycemia is most common and frightening with any class of glucose-lowering agents. The insulin-independent mechanism of SGLT2i usually results in a decreased risk of hypoglycemia[69]. The incidence of SGLT2-related hypoglycemia is low, except if given in combination with insulin or insulin secretagogues[70-74].
The incidence of hypoglycemia was not observed with dapagliflozin, canagliflozin, or empagliflozin respectively as monotherapy but hypoglycemia was observed when any of these three drugs was administered with insulin or sulphonylureas[75-79]. As far as luseogliflozin is concerned, hypoglycemia was not observed with both monotherapy or in combination[77].
Risk of genital mycotic infections:
Vulvovaginitis caused by Candida species is the most commonly occurring infection in women, particularly in diabetic women who are at a risk of 80 % when compared to non-diabetic individuals[80].
The genital candidiasis in diabetics consuming SGLT2i is observed because of the excretion of sugars through urine, which promotes mycotic colonization and facilitates a favorable environment for the growth of bacteria[81]. The risk of this infection is more in women but only a few rare events of men contracting this infection have been observed[72]. The risk of infection was higher even in dapagliflozin either as monotherapy or in combination[82]. These infections may usually occur within the first months; however risk of infection may persist during ongoing treatment[82,83]. These infections are of mild to moderate severity[84]. Individuals with a history of genitourinary infections tend to contract this infection while undergoing SGLT2 therapy[85]. The risk of genital mycotic infection can be mitigated by hydration and maintaining good perineal hygiene[71].
Urinary Tract Infections (UTI):
The results from a systematic analysis[72] stated that UTI was observed in patients consuming canagliflozin but the meta-analyses reports of the same do not state an incidence of UTI[86]. Dapagliflozin meta-analyses stated an increase in UTI when compared to the placebo[87,88]. But no incidence of UTI was observed with empagliflozin[89,90]. Uncommon incident cases of pyelonephritis and urosepsis with SGLT2i were reported to the Food and Drug Administration (FDA) and their causal relationship remains a mystery[20]. Patients with a history of recurrent UTI, neurogenic bladder, indwelling catheters and paraparesis should be considered before starting SGLT2 therapy[81,90-92].
Hypotension:
The inhibition of SGLT2 receptors in the kidney by the “flozins” results in a decrease of tubular reabsorption of glucose resulting in increased urinary glucose excretion[93,94]. This inhibition also increases the excretion of sodium[95]. The risk of hypotension with SGLT2i was found to be high when compared to other hypoglycemics[96]. Dosage adjustment of “flozins” may be required in volumedepleted patients who are on loop diuretic therapy or thiazide therapy. Contrarily, if the patients are not volume depleted due to other factors; SGLT2i can be safely co-administered with diuretics as it causes only a little synergistic effect in decreasing blood pressure[97,98]. To prevent symptoms of hypotension, acute kidney injury and falls due to volume depletion, it should be assessed whether appropriate doses of hypotensive medications affecting the Renin-Angiotensin-Aldosterone System (RAAS) are prescribed[96].
Risk of cancer:
FDA in 2011 published an advisory about the use of dapagliflozin and its correlation with an augmented risk for cancer (breast and bladder)[99]. Females were observed with breast cancer and males with bladder cancer[100]. The risk of cancer was not observed in phase 2-3 randomized controlled trials with canagliflozin or empagliflozin[101-103].
Bone safety:
Dapagliflozin seems to have no impact on bone development. After 50 and 102 w of use, no substantial differences in bone mineral density of the lumbar spine, total hip and femoral neck were observed. However, an elevated incidence of fracture was seen in diabetic patients with significant renal impairment[98-99]. A warning was published by the FDA in 2015 regarding the risk of fracture associated with canagliflozin. The physicians must consider the risk for fractures before prescribing elderly people with canagliflozin[59,104-106].
Several studies propose the following mechanisms on the effect of T2DM on bone metabolism which stated elderly diabetic patients are at higher risk of falling correlated with peripheral neuropathy[101]. Increased urinary pentosidine, is linked to an elevated risk of lacerations in patients with T2DM. This indicates that the aggregation of complex glycation byproducts that stiffen bone collagen can reduce bone strength in T2DM[107]. The chronic hyperglycemia linked to single-gene mutations in the leptin gene or its receptor causes severe cytolipidemia-induced osteopenia[99].
Amputations:
The risk of amputation is linked with diabetes due to chronic hyperglycemia and microvascular complications such as Peripheral Artery Disease (PAD), peripheral neuropathy, or the risk of infections[107]. Likewise, in the Announce analysis, there was no amputation risk with dapagliflozin. This risk was not observed with dapagliflozin and empagliflozin[108,109].
The publication of the CANVAS program elucidated the risk of amputation associated with canagliflozin for which a boxed warning was published by the FDA. From the program, it was evident that canagliflozin was mainly associated with lower limb amputation the toe. The patients with the following disease conditions were at risk of amputation, neuropathy, ulcers and peripheral arterial disease and previous history of amputations. But patients without the above disease conditions also were at risk of amputations while consuming canagliflozin[110].
Conflicting results have been found in numerous observational studies. A review of more than 900 000 individuals showed that new users of SGLT2- sulphonylurea, metformin or thiazolidinedione’s had a two-fold risk of amputations, and a marginal rise in incidence in contrast with DPP or GLP-1 agonists were seen[111].
Since the risk of amputation is not evident or limited to canagliflozin alone, it is not recommended to use SGLT2i in patients with ischemic extremities, peripheral vascular disease and history of amputation is not recommended. The physicians must consider the risk before prescribing patients with these drugs[112].
Diabetic ketoacidosis:
Diabetic ketoacidosis may develop in patients with type 1 and type 2 diabetes that are on SGLT2i medications[110,111]. The increased levels of ketone in the serum may be due to a decrease in insulin requirement, increased fatty acid oxidation rate, decreased clearance of ketones by the kidney and stimulation of glucagon secretion.
Diabetic ketoacidosis was not observed with canagliflozin[110] or empagliflozin[112] but with dapagliflozin, the incidence was high[113]. The incidence of diabetic ketoacidosis was particularly high in T1DM patients who are on SGLT2i and comparatively less in T2DM people on SGLT2 inhibitors[114]. But T2DM people with low c-peptide levels are at a greater risk of diabetic ketoacidosis[115].
Fournier gangrene:
FDA identified 55 cases of Fournier gangrene in the period 2013 to 2019, which recommended the physicians to discontinue the gliflozins in serve cases. The patients were treated with broad-spectrum antibiotics along with surgical debridement[116].
Pharmacoeconomics of SGLT2 inhibitors:
Pharmacoeconomic analysis suggest that dapagliflozin when co-administered with metformin increases the overall quality of life, with a decrease in the rate of incidence of diabetes complications when compared to the combination of metformin+sulfonylurea or metformin+DPP4 inhibitors making it a cost-effective treatment alternative[117]. The results of meta-analyses of ipragliflozin as an add on to metformin apart from lowering blood glucose in T2DM, provided other benefits such as weight reduction, reduction in blood pressure, reduction in Triglycerides (TG) levels and this combination reduces the risk urinary tract and genital mycotic infections making it a cost-effective treatment alternative[117]. In general, SGLT2i seem to be the most cost-effective in the treatment of T2DM compared with other antidiabetics and insulin. Further studies are needed to determine the cost-effectiveness of other SGLT2i and the cost-effectiveness of these drugs should also be examined in populations with renal and hepatic dysfunction[10].
Conclusion
This article addressed the literature available on the safety and efficacy of SGLT2i by comparing these drugs with the other classes and SGLT2i were chosen since they have been gaining popularity for the treatment of type 2 diabetes. ADA has recommended the use of SGLT2i for the patient with comorbidities and history of kidney and heart disease. SGLT2i are a practicechanging development for people with type 2 diabetes and Chronic Kidney Disease (CKD) that have gone from a three-pointer to an easy lay-up. The clear and consistent benefits for kidney and CV outcomes with SGLT2i have led to revised treatment recommendations from many major international guidelines. In short, SGLT2i should be prioritized in people with type 2 diabetes and a starting eGFR of >30 ml/min/1.73 m2 to prevent the progression of kidney disease, cardiovascular events, or both, especially in those with urine albumin-to-creatinine ratio >300 mg/g (Level A recommendation).
SGLT2i have proven efficacious in lowering HbA1c and fasting blood glucose levels thus delaying the disease progression of T2DM and bringing it into control. SGLT2i with its effect on reducing body weight have become the most sought after treatment option for obese T2DM people. Surprisingly its cardioprotective effects are being discussed now making it a success. Despite several adverse events of the class being reported, only a few like genital mycotic infections, UTI and fractures are more common yet mild to moderate in intensity. Further studies on a larger population are required to confirm the other adverse effects associated with the drugs. Several fixed-dose combinations of SGLT2 inhibitors with biguanides and DPP4 inhibitors are approved by the FDA, giving this class a very prominent position in the treatment of T2DM.The inclusion of this class of drug in general practice may provide efficacious results and could bring in stringent glycemic control.
Study Recommandation
Diabetes is complicated by the presence of comorbidities-chronic conditions existing in addition to T2DM. It is observed that hypertension, cardiac diseases and obesity predominantly present as comorbid condition in patients with diabetes thereby necessitating the need for targeting treatment in a holistic way so as to prevent severity of the condition. This requires choosing of wise but minimal drugs to improve the quality of life. SGLT2i which helps control glycemic levels serves as a potent drug in controlling glycemic levels, hypertension, weight loss and cardiac protectant due to its pleiotropic effects. Bringing the drug into clinical practice as much as it is in the literature requires more studies and awareness in physicians especially in the South Asian region where the prevalence of the disease remains high. Despite the inherent limitations this narrative review possesses, we have tried to counter subjective bias by wide diversity of authorship from developed and developing nations making it non location specific and thereby makes it likely to readers of any part of the world to derive a clear understanding on the drug class and to implement the drug to clinical practice for visible results.
Acknowledgements:
Authors wish to thank JSS College of Pharmacy, Ooty and JSS Academy of Higher Education and Research, Mysuru for providing all the necessary facilities and support to write this article. We also thank physicians/consultants of Government Medical College and Hospital, Ooty for providing the necessary inputs and corrections of this manuscript.
Conflict of interest:
The authors declare that they have no competing interests.
References
- Prentki M, Nolan CJ. Islet β cell failure in type 2 diabetes. J Clin Invest 2006;116(7):1802-12.
[Crossref] [Google Scholar] [PubMed]
- Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the developing world-a growing challenge. N Engl J Med 2007;356(3):213-5.
[Crossref] [Google Scholar] [PubMed]
- Scheen AJ, Van Gaal LF. Combating the dual burden: Therapeutic targeting of common pathways in obesity and type 2 diabetes. Lancet Diabetes Endocrinol. 2014;2(11):911-22.
[Crossref] [Google Scholar] [PubMed]
- Marín-Peñalver JJ, Martín-Timón I, Sevillano-Collantes C, del Cañizo-Gómez FJ. Update on the treatment of type 2 diabetes mellitus. World J Diabetes 2016;7(17):354.
[Crossref] [Google Scholar] [PubMed]
- Marathe PH, Gao HX, Close KL. American diabetes association standards of medical care in diabetes. J Diabetes 2017;9(4):320-4.
[Crossref] [Google Scholar] [PubMed]
- Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR. Management of hyperglycaemia in type 2 diabetes, 2015: A patient-centred approach: Update to a position statement of the American diabetes association and the European association for the study of diabetes. Diabetes Care 2015;58:429-42.
[Crossref] [Google Scholar] [PubMed]
- Chaudhury A, Duvoor C, Reddy DVS, Kraleti S, Chada A, Ravilla R, et al. Clinical review of antidiabetic drugs: Implications for type 2 diabetes mellitus management. Front Endocrinol 2017;8:6.
[Crossref] [Google Scholar] [PubMed]
- Neumiller JJ, White JR, Campbell RK. Sodium-glucose co-transport inhibitors: Progress and therapeutic potential in type 2 diabetes mellitus. Drugs 2010;70:377-85.
[Crossref] [Google Scholar] [PubMed]
- Hallow KM, Greasley PJ, Helmlinger G, Chu L, Heerspink HJ, Boulton DW. Evaluation of renal and cardiovascular protection mechanisms of SGLT2 inhibitors: Model-based analysis of clinical data. Am J Physiol Renal Physiol 2018;315(5):1295-306.
[Crossref] [Google Scholar] [PubMed]
- Kuang H, Liao L, Chen H, Kang Q, Shu X, Wang Y. Therapeutic effect of sodium glucose co-transporter 2 inhibitor dapagliflozin on renal cell carcinoma. Med Sci Monit 2017;23:3737.
[Crossref] [Google Scholar] [PubMed]
- Bays H. From victim to ally: The kidney as an emerging target for the treatment of diabetes mellitus. Curr Med Res Opin 2009;25(3):671-81.
[Crossref] [Google Scholar] [PubMed]
- Ehrenkranz JR, Lewis NG, Ronald Kahn C, Roth J. Phlorizin: A review. Diabetes Metab Res Rev 2005;21(1):31-8.
[Crossref] [Google Scholar] [PubMed]
- Stiles PG, Lusk G. On the action of phlorhizin. Am J Physiol Cell Physiol 1903;10(1):67-79.
- Vick H, Diedrich DF, Baumann K. Reevaluation of renal tubular glucose transport inhibition by phlorizin analogs. Am J Physiol 1973;224(3):552-7.
[Crossref] [Google Scholar] [PubMed]
- Polidori D, Sha S, Mudaliar S, Ciaraldi TP, Ghosh A, Vaccaro N, et al. Canagliflozin lowers postprandial glucose and insulin by delaying intestinal glucose absorption in addition to increasing urinary glucose excretion: Results of a randomized, placebo-controlled study. Diabetes care 2013;36(8):2154-61.
[Crossref] [Google Scholar] [PubMed]
- Polidori D, Sha S, Ghosh A, Plum-Mörschel L, Heise T, Rothenberg P. Validation of a novel method for determining the renal threshold for glucose excretion in untreated and canagliflozin-treated subjects with type 2 diabetes mellitus. J Clin Endocrinol Metab 2013;98(5):867-71.
[Crossref] [Google Scholar] [PubMed]
- Rossetti L, Smith D, Shulman GI, Papachristou D, DeFronzo RA. Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J Clin Invest 1987;79(5):1510-5.
[Crossref] [Google Scholar] [PubMed]
- Dimitrakoudis D, Vranic M, Klip A. Effects of hyperglycemia on glucose transporters of the muscle: Use of the renal glucose reabsorption inhibitor phlorizin to control glycemia. J Am Soc Nephrol 1992;3(5):1078-91.
[Crossref] [Google Scholar] [PubMed]
- Jonas JC, Sharma A, Hasenkamp W, Ilkova H, Patane G, Laybutt R, et al. Chronic hyperglycemia triggers loss of pancreatic β cell differentiation in an animal model of diabetes. J Biol Chem 1999;274(20):14112-21.
[Crossref] [Google Scholar] [PubMed]
- Abdul-Ghani MA, DeFronzo RA. Inhibition of renal glucose reabsorption: A novel strategy for achieving glucose control in type 2 diabetes mellitus. Endocr Pract 2008;14(6):782-90.
[Crossref] [Google Scholar] [PubMed]
- Turk E, Martín MG, Wright EM. Structure of the human Na+/glucose cotransporter gene SGLT1. J Biol Chem 1994;269(21):15204-9.
[Crossref] [Google Scholar] [PubMed]
- Mackenzie B, Panayotova-Heiermann M, Loo DD, Lever JE, Wright EM. SAAT1 is a low affinity Na+/glucose cotransporter and not an amino acid transporter. A reinterpretation. J Biol Chem 1994;269(36):22488-91.
[Crossref] [Google Scholar] [PubMed]
- Panayotova-Heiermann M, Loo DD, Wright EM. Kinetics of steady-state currents and charge movements associated with the rat Na+/glucose cotransporter. J Biol Chem 1995;270(45):27099-105.
[Crossref] [Google Scholar] [PubMed]
- Wright EM, Loo DD, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev 2011;91(2):733-94.
[Crossref] [Google Scholar] [PubMed]
- Thorens B, Mueckler M. Glucose transporters in the 21st Century. Am J Physiol Endocrinol Metab 2010;298(2):E141-5.
[Crossref] [Google Scholar] [PubMed]
- Bays H. Sodium glucose co-transporter type 2 (SGLT2) inhibitors: Targeting the kidney to improve glycemic control in diabetes mellitus. Diabetes Ther 2013;4(2):195-220.
[Crossref] [Google Scholar] [PubMed]
- Larson GL. The synthesis of gliflozins. Chem Today. 2015;33:37-40.
- Satirapoj B. Sodium-glucose cotransporter 2 inhibitors with renoprotective effects. Kidney Dis 2017;3(1):24-32.
[Crossref] [Google Scholar] [PubMed]
- Bokor E, Kun S, Goyard D, Toth M, Praly JP, Vidal S, et al. C-Glycopyranosyl arenes and hetarenes: Synthetic methods and bioactivity focused on antidiabetic potential. Chem Rev 2017;117(3):1687-764.
[Crossref] [Google Scholar] [PubMed]
- Inzucchi SE, Zinman B, Wanner C, Ferrari R, Fitchett D, Hantel S, et al. SGLT-2 inhibitors and cardiovascular risk: Proposed pathways and review of ongoing outcome trials. Diab Vasc Dis Res 2015;12(2):90-100.
[Crossref] [Google Scholar] [PubMed]
- Madaan T, Akhtar M, Najmi AK. Sodium glucose CoTransporter 2 (SGLT2) inhibitors: Current status and future perspective. Eur J Pharm Sci 2016;93:244-52.
[Crossref] [Google Scholar] [PubMed]
- Cai W, Jiang L, Xie Y, Liu Y, Liu W, Zhao G. Design of SGLT2 inhibitors for the treatment of type 2 diabetes: A history driven by biology to chemistry. Med Chem 2015;11(4):317-28.
[Crossref] [Google Scholar] [PubMed]
- Thynne T, Doogue M. Sodium-glucose co-transporter inhibitors: Mechanisms of action. Aust Prescr 2014;37(1):14-6.
- Thomson SC, Vallon V. Renal effects of sodium-glucose co-transporter inhibitors. Am J Med 2019;132(10):S30-8.
- Du F, Hinke SA, Cavanaugh C, Polidori D, Wallace N, Kirchner T, et al. Potent sodium/glucose cotransporter SGLT1/2 dual inhibition improves glycemic control without marked gastrointestinal adaptation or colonic microbiota changes in rodents. J Pharmacol Exp Ther 2018;365(3):676-87.
[Crossref] [Google Scholar] [PubMed]
- Chen J, Williams S, Ho S, Loraine H, Hagan D, Whaley JM, et al. Quantitative PCR tissue expression profiling of the human SGLT2 gene and related family members. Diabetes Ther 2010:57-92.
[Crossref] [Google Scholar] [PubMed]
- Ghezzi C, Loo DD, Wright EM. Physiology of renal glucose handling via SGLT1, SGLT2 and GLUT2. Diabetologia 2018;61:2087-97.
[Crossref] [Google Scholar] [PubMed]
- Washburn WN. Development of the renal glucose reabsorption inhibitors: A new mechanism for the pharmacotherapy of diabetes mellitus type 2. J Med Chem 2009;52(7):1785-94.
[Crossref] [Google Scholar] [PubMed]
- Scheen AJ. Evaluating SGLT2 inhibitors for type 2 diabetes: Pharmacokinetic and toxicological considerations. Expert Opin Drug Metab Toxicol 2014;10(5):647-63.
[Crossref] [Google Scholar] [PubMed]
- Ferrannini E, Solini A. SGLT2 inhibition in diabetes mellitus: Rationale and clinical prospects. Nat Rev Endocrinol 2012;8(8):495-502.
[Crossref] [Google Scholar] [PubMed]
- Ni L, Yuan C, Chen G, Zhang C, Wu X. SGLT2i: Beyond the glucose-lowering effect. Cardiovasc Diabetol 2020;19:1-0.
[Crossref] [Google Scholar] [PubMed]
- Miao Z, Nucci G, Amin N, Sharma R, Mascitti V, Tugnait M, et al. Pharmacokinetics, metabolism, and excretion of the antidiabetic agent ertugliflozin (PF-04971729) in healthy male subjects. Drug Metabolism and Disposition. 2013;41(2):445-56.
[Crossref] [Google Scholar] [PubMed]
- Hasan FM, Alsahli M, Gerich JE. SGLT2 inhibitors in the treatment of type 2 diabetes. Diabetes Res Clin Pract 2014;104(3):297-322.
[Crossref] [Google Scholar] [PubMed]
- Garcia-Ropero A, Badimon JJ, Santos-Gallego CG. The pharmacokinetics and pharmacodynamics of SGLT2 inhibitors for type 2 diabetes mellitus: The latest developments. Expert Opin Drug Metab Toxicol 2018;14(12):1287-302.
[Crossref] [Google Scholar] [PubMed]
- Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373(22):2117-28.
[Crossref] [Google Scholar] [PubMed]
- Neal B, Perkovic V, Mahaffey KW, De Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377(7):644-57.
[Crossref] [Google Scholar] [PubMed]
- Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019;380(4):347-57.
[Crossref] [Google Scholar] [PubMed]
- Anker SD, Butler J, Filippatos GS, Jamal W, Salsali A, Schnee J, et al. Evaluation of the effects of sodium-glucose co‐transporter 2 inhibition with empagliflozin on morbidity and mortality in patients with chronic heart failure and a preserved ejection fraction: Rationale for and design of the EMPEROR‐Preserved Trial. Eur J Heart Fail 2019;21(10):1279-87.
[Crossref] [Google Scholar] [PubMed]
- Cavender MA, Norhammar A, Birkeland KI, Jørgensen ME, Wilding JP, Khunti K, et al. SGLT-2 inhibitors and cardiovascular risk: An analysis of CVD-REAL. J Am Coll Cardiol 2018;71(22):2497-506.
[Crossref] [Google Scholar] [PubMed]
- Abdul-Ghani M. Where does combination therapy with an SGLT2 inhibitor plus a DPP-4 inhibitor fit in the management of type 2 diabetes?. Diabetes Care 2015;38(3):373-5.
[Crossref] [Google Scholar] [PubMed]
- Bode B. An overview of the pharmacokinetics, efficacy and safety of liraglutide. Diabetes Res Clin Pract 2012;97(1):27-42.
[Crossref] [Google Scholar] [PubMed]
- Balant L. Clinical pharmacokinetics of sulphonylurea hypoglycaemic drugs. Clin Pharmacokinet 1981;6(3):215-41.
[Crossref] [Google Scholar] [PubMed]
- Donnan K, Segar L. SGLT2 inhibitors and metformin: Dual antihyperglycemic therapy and the risk of metabolic acidosis in type 2 diabetes. Eur J Pharmacol 2019;846:23-9.
[Crossref] [Google Scholar] [PubMed]
- Gu N, Park SI, Chung H, Jin X, Lee S, Kim TE. Possibility of pharmacokinetic drug interaction between a DPP-4 inhibitor and a SGLT2 inhibitor. Transl Clin Pharmacol 2020;28(1):17.
[Crossref] [Google Scholar] [PubMed]
- Jacobsen LV, Flint A, Olsen AK, Ingwersen SH. Liraglutide in type 2 diabetes mellitus: Clinical pharmacokinetics and pharmacodynamics. Clin Pharmacokinet 2016;55:657-72.
[Crossref] [Google Scholar] [PubMed]
- Mentlein R. Therapeutic assessment of glucagon-like peptide-1 agonists compared with dipeptidyl peptidase IV inhibitors as potential antidiabetic drugs. Expert Opin Investig Drugs 2005;14(1):57-64.
[Crossref] [Google Scholar] [PubMed]
- Xourgia E, Papazafiropoulou AK, Karampousli E, Melidonis A. DPP-4 Inhibitors vs. SGLT-2 inhibitors; cons and pros. J Renal Med 2017;1(2):7.
- Mazidi M, Rezaie P, Gao HK, Kengne AP. Effect of sodium‐glucose cotransport‐2 inhibitors on blood pressure in people with type 2 diabetes mellitus: A systematic review and meta‐analysis of 43 randomized control trials with 22 528 patients. J Am Heart Assoc 2017;6(6):e004007.
[Crossref] [Google Scholar] [PubMed]
- Rajeev SP, Cuthbertson DJ, Wilding JP. Energy balance and metabolic changes with sodium‐glucose co‐transporter 2 inhibition. Diabetes Obes Metab 2016;18(2):125-34.
[Crossref] [Google Scholar] [PubMed]
- Tentolouris A, Vlachakis P, Tzeravini E, Eleftheriadou I, Tentolouris N. SGLT2 inhibitors: A review of their antidiabetic and cardioprotective effects. Int J Environ Res Public Health 2019;16(16):2965.
- Monami M, Nardini C, Mannucci E. Efficacy and safety of sodium glucose co‐transport‐2 inhibitors in type 2 diabetes: A meta‐analysis of randomized clinical trials. Diabetes Obes Metab 2014;16(5):457-66.
[Crossref] [Google Scholar] [PubMed]
- Pinto LC, Rados DV, Remonti LR, Kramer CK, Leitao CB, Gross JL. Efficacy of SGLT2 inhibitors in glycemic control, weight loss and blood pressure reduction: A systematic review and meta-analysis. Diabetol Metab Syndr 2015;7(1):1-2.
- Bolinder J, Ljunggren Ö, Johansson L, Wilding J, Langkilde AM, Sjöström CD, et al. Dapagliflozin maintains glycaemic control while reducing weight and body fat mass over 2 years in patients with type 2 diabetes mellitus inadequately controlled on metformin. Diabetes Obes Metab 2014;16(2):159-69.
[Crossref] [Google Scholar] [PubMed]
- Del Prato S, Nauck M, Duran‐Garcia S, Maffei L, Rohwedder K, Theuerkauf A, et al. Long‐term glycaemic response and tolerability of dapagliflozin versus a sulphonylurea as add‐on therapy to metformin in patients with type 2 diabetes: 4‐year data. Diabetes Obes Metab 2015;17(6):581-90.
[Crossref] [Google Scholar] [PubMed]
- Zhang L, Feng Y, List J, Kasichayanula S, Pfister M. Dapagliflozin treatment in patients with different stages of type 2 diabetes mellitus: Effects on glycaemic control and body weight. Diabetes Obes Metab 2010;12(6):510-6.
[Crossref] [Google Scholar] [PubMed]
- Wanner C, Lachin JM, Inzucchi SE, Fitchett D, Mattheus M, George J, et al. Empagliflozin and clinical outcomes in patients with type 2 diabetes mellitus, established cardiovascular disease, and chronic kidney disease. Circulation 2018;137(2):119-29.
[Crossref] [Google Scholar] [PubMed]
- Bell RM, Yellon DM. SGLT2 inhibitors: Hypotheses on the mechanism of cardiovascular protection. Lancet Diabetes Endocrinol 2018;6(6):435-7.
[Crossref] [Google Scholar] [PubMed]
- Jia X, Mehta PB, Ye Y, Alam M, Birnbaum Y, Bajaj M. SGLT2 inhibitors and cardiovascular outcomes: Current perspectives and future potentials. Curr Diab Rep 2018;18:1-8.
[Crossref] [Google Scholar] [PubMed]
- Cryer PE. The barrier of hypoglycemia in diabetes. Diabetes 2008;57(12):3169.
[Crossref] [Google Scholar] [PubMed]
- Scheen AJ. SGLT2 inhibition: Efficacy and safety in type 2 diabetes treatment. Expert Opin Drug Saf 2015;14(12):1879-904.
[Crossref] [Google Scholar] [PubMed]
- Yang XP, Lai D, Zhong XY, Shen HP, Huang YL. Efficacy and safety of canagliflozin in subjects with type 2 diabetes: systematic review and meta-analysis. Eur J Clin Pharmacol 2014;70:1149-58.
[Crossref] [Google Scholar] [PubMed]
- Zhang M, Zhang L, Wu B, Song H, An Z, Li S. Dapagliflozin treatment for type 2 diabetes: A systematic review and meta‐analysis of randomized controlled trials. Diabetes Metab Res Rev 2014;30(3):204-21.
[Crossref] [Google Scholar] [PubMed]
- Liakos A, Karagiannis T, Athanasiadou E, Sarigianni M, Mainou M, Papatheodorou K, et al. Efficacy and safety of empagliflozin for type 2 diabetes: A systematic review and meta‐analysis. Diabetes Obes Metab 2014;16(10):984-93.
[Crossref] [Google Scholar] [PubMed]
- Wilding JP, Woo V, Soler NG, Pahor A, Sugg J, Rohwedder K, et al. Long-term efficacy of dapagliflozin in patients with type 2 diabetes mellitus receiving high doses of insulin: A randomized trial. Ann Intern Med 2012;156(6):405-15.
[Crossref] [Google Scholar] [PubMed]
- Strojek K, Yoon KH, Hruba V, Elze M, Langkilde AM, Parikh S. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with glimepiride: A randomized, 24‐week, double‐blind, placebo‐controlled trial. Diabetes Obes Metab 2011;13(10):928-38.
[Crossref] [Google Scholar] [PubMed]
- Rosenstock J, Vico M, Wei LI, Salsali A, List JF. Effects of dapagliflozin, an SGLT2 inhibitor, on HbA1c, body weight, and hypoglycemia risk in patients with type 2 diabetes inadequately controlled on pioglitazone monotherapy. Diabetes care 2012;35(7):1473-8.
[Crossref] [Google Scholar] [PubMed]
- Wilding JP, Charpentier G, Hollander P, González‐Gálvez G, Mathieu C, Vercruysse F, et al. Efficacy and safety of canagliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin and sulphonylurea: A randomised trial. Int J Clin Pract 2013;67(12):1267-82.
[Crossref] [Google Scholar] [PubMed]
- Häring HU, Merker L, Seewaldt-Becker E, Weimer M, Meinicke T, Woerle HJ, et al. Empagliflozin as add-on to metformin plus sulfonylurea in patients with type 2 diabetes: A 24-week, randomized, double-blind, placebo-controlled trial. Diabetes care 2013;36(11):3396-404.
[Crossref] [Google Scholar] [PubMed]
- Kushner P. Benefits/risks of sodium-glucose co-transporter 2 inhibitor canagliflozin in women for the treatment of type 2 diabetes. Womens Health 2016;12(3):379-88.
[Crossref] [Google Scholar] [PubMed]
- Geerlings S, Fonseca V, Castro-Diaz D, List J, Parikh S. Genital and urinary tract infections in diabetes: Impact of pharmacologically-induced glucosuria. Diabetes Res Clin Pract 2014;103(3):373-81.
[Crossref] [Google Scholar] [PubMed]
- Nyirjesy P, Sobel JD. Genital mycotic infections in patients with diabetes. Postgrad Med 2013;125(3):33-46.
[Crossref] [Google Scholar] [PubMed]
- Dave CV, Schneeweiss S, Patorno E. Comparative risk of genital infections associated with sodium‐glucose co‐transporter‐2 inhibitors. Diabetes Obes Metab 2019;21(2):434-8.
[Crossref] [Google Scholar] [PubMed]
- Thong KY, Yadagiri M, Barnes DJ, Morris DS, Chowdhury TA, Chuah LL, et al. Clinical risk factors predicting genital fungal infections with sodium–glucose cotransporter 2 inhibitor treatment: The ABCD nationwide dapagliflozin audit. Prim Care Diabetes 2018;12(1):45-50.
[Crossref] [Google Scholar] [PubMed]
- Lipscombe L, Booth G, Butalia S, Dasgupta K, Eurich DT, Goldenberg R, et al. Pharmacologic glycemic management of type 2 diabetes in adults. Can J Diabetes 2018;42:S88-103.
[Crossref] [Google Scholar] [PubMed]
- Johnsson KM, Ptaszynska A, Schmitz B, Sugg J, Parikh SJ, List JF. Urinary tract infections in patients with diabetes treated with dapagliflozin. J Diabetes Complications 2013;27(5):473-8.
[Crossref] [Google Scholar] [PubMed]
- Ptaszynska A, Johnsson KM, Parikh SJ, De Bruin TW, Apanovitch AM, List JF. Safety profile of dapagliflozin for type 2 diabetes: Pooled analysis of clinical studies for overall safety and rare events. Drug Saf 2014;37:815-29.
[Crossref] [Google Scholar] [PubMed]
- Kohler S, Salsali A, Hantel S, Kaspers S, Woerle HJ, Kim G, et al. Safety and tolerability of empagliflozin in patients with type 2 diabetes. Clin Ther 2016;38(6):1299-313.
[Crossref] [Google Scholar] [PubMed]
- Puckrin R, Saltiel MP, Reynier P, Azoulay L, Yu OH, Filion KB. SGLT-2 inhibitors and the risk of infections: A systematic review and meta-analysis of randomized controlled trials. Acta diabetologica 2018;55:503-14.
[Crossref] [Google Scholar] [PubMed]
- Fitchett D. A safety update on sodium glucose co‐transporter 2 inhibitors. Diabetes Obes Metab 2019;21:34-42.
[Crossref] [Google Scholar] [PubMed]
- DeFronzo RA, Davidson JA, Del Prato S. The role of the kidneys in glucose homeostasis: A new path towards normalizing glycaemia. Diabetes Obes Metab 2012;14(1):5-14.
[Crossref] [Google Scholar] [PubMed]
- Scheen AJ. SGLT2 inhibitors: Benefit/risk balance. Curr Diab Rep 2016;16:1-1.
[Crossref] [Google Scholar] [PubMed]
- Vasilakou D, Karagiannis T, Athanasiadou E, Mainou M, Liakos A, Bekiari E, Sarigianni M, Matthews DR, Tsapas A. Sodium–glucose cotransporter 2 inhibitors for type 2 diabetes: A systematic review and meta-analysis. Ann Intern Med 2013;159(4):262-74.
[Crossref] [Google Scholar] [PubMed]
- Cherney DZ, Udell JA. Use of sodium glucose cotransporter 2 inhibitors in the hands of cardiologists: With great power comes great responsibility. Circulation 2016;134(24):1915-7.
[Crossref] [Google Scholar] [PubMed]
- Weber MA, Mansfield TA, Cain VA, Iqbal N, Parikh S, Ptaszynska A. Blood pressure and glycaemic effects of dapagliflozin versus placebo in patients with type 2 diabetes on combination antihypertensive therapy: A randomised, double-blind, placebo-controlled, phase 3 study. Lancet Diabetes Endocrinol 2016;4(3):211-20.
[Crossref] [Google Scholar] [PubMed]
- Heise T, Mattheus M, Woerle HJ, Broedl UC, Macha S. Assessing pharmacokinetic interactions between the sodium glucose cotransporter 2 inhibitor empagliflozin and hydrochlorothiazide or torasemide in patients with type 2 diabetes mellitus: A randomized, open-label, crossover study. Clin Ther 2015;37(4):793-803.
[Crossref] [Google Scholar] [PubMed]
- Lin HW, Tseng CH. A review on the relationship between SGLT2 inhibitors and cancer. Int J Endocrinol. 2014;2014.
[Crossref] [Google Scholar] [PubMed]
- Scott LJ. Empagliflozin: A review of its use in patients with type 2 diabetes mellitus. Drugs 2014;74:1769-84.
[Crossref] [Google Scholar] [PubMed]
- Frampton JE. Empagliflozin: A review in type 2 diabetes. Drugs 2018;78:1037-48.
[Crossref] [Google Scholar] [PubMed]
- Ljunggren Ö, Bolinder J, Johansson L, Wilding J, Langkilde AM, Sjöström CD, et al. Dapagliflozin has no effect on markers of bone formation and resorption or bone mineral density in patients with inadequately controlled type 2 diabetes mellitus on metformin. Diabetes Obes Metab 2012;14(11):990-9.
[Crossref] [Google Scholar] [PubMed]
- Kohan DE, Fioretto P, Tang W, List JF. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int 2014;85(4):962-71.
[Crossref] [Google Scholar] [PubMed]
- Taylor SI, Blau JE, Rother KI. Possible adverse effects of SGLT2 inhibitors on bone. Lancet Diabetes Endocrinol 2015;3(1):8-10.
[Crossref] [Google Scholar] [PubMed]
- Watts NB, Bilezikian JP, Usiskin K, Edwards R, Desai M, Law G, et al. Effects of canagliflozin on fracture risk in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab 2016;101(1):157-66.
[Crossref] [Google Scholar] [PubMed]
- Thrailkill KM, Bunn RC, Nyman JS, Rettiganti MR, Cockrell GE, Wahl EC, et al. SGLT2 inhibitor therapy improves blood glucose but does not prevent diabetic bone disease in diabetic DBA/2J male mice. Bone. 2016;82:101-7.
[Crossref] [Google Scholar] [PubMed]
- Kohler S, Zeller C, Iliev H, Kaspers S. Safety and tolerability of empagliflozin in patients with type 2 diabetes: Pooled analysis of phase I–III clinical trials. Adv Ther 2017;34:1707-26.
[Crossref] [Google Scholar] [PubMed]
- Schwartz AV, Garnero P, Hillier TA, Sellmeyer DE, Strotmeyer ES, Feingold KR, et al. Pentosidine and increased fracture risk in older adults with type 2 diabetes. J Clin Endocrinol Metab 2009;94(7):2380-6.
[Crossref] [Google Scholar] [PubMed]
- Garris DR, Burkemper KM, Garris BL. Influences of diabetes (db/db), obese (ob/ob) and dystrophic (dy/dy) genotype mutations on hind limb bone maturation: A morphometric, radiological and cytochemical indices analysis. Diabetes Obes Metab 2007;9(3):311-22.
- Inzucchi SE, Iliev H, Pfarr E, Zinman B. Empagliflozin and assessment of lower-limb amputations in the EMPA-REG OUTCOME trial. Diabetes Care 2018;41(1):e4-5.
[Crossref] [Google Scholar] [PubMed]
- Palmer BF, Clegg DJ, Taylor SI, Weir MR. Diabetic ketoacidosis, sodium glucose transporter-2 inhibitors and the kidney. J Diabetes Complications 2016;30(6):1162-6.
[Crossref] [Google Scholar] [PubMed]
- Umpierrez GE. Ketosis-prone type 2 diabetes: Time to revise the classification of diabetes. Diabetes care 2006;29(12):2755-7.
[Crossref] [Google Scholar] [PubMed]
- Peters AL, Buschur EO, Buse JB, Cohan P, Diner JC, Hirsch IB. Euglycemic diabetic ketoacidosis: A potential complication of treatment with sodium-glucose cotransporter 2 inhibition. Diabetes care 2015;38(9):1687-93.
[Crossref] [Google Scholar] [PubMed]
- Rosenstock J, Marquard J, Laffel LM, Neubacher D, Kaspers S, Cherney DZ, et al. Empagliflozin as adjunctive to insulin therapy in type 1 diabetes: The EASE trials. Diabetes care 2018;41(12):2560-9.
[Crossref] [Google Scholar] [PubMed]
- Henry RR, Thakkar P, Tong C, Polidori D, Alba M. Efficacy and safety of canagliflozin, a sodium–glucose cotransporter 2 inhibitor, as add-on to insulin in patients with type 1 diabetes. Diabetes care 2015;38(12):2258-65.
[Crossref] [Google Scholar] [PubMed]
- Bersoff-Matcha SJ, Chamberlain C, Cao C, Kortepeter C, Chong WH. Fournier gangrene associated with sodium-glucose cotransporter-2 inhibitors: A review of spontaneous postmarketing cases. Ann Intern Med 2019;170(11):764-9.
[Crossref] [Google Scholar] [PubMed]
- D’Elia JA, Segal AR, Bayliss GP, Weinrauch LA. Sodium–glucose cotransporter-2 inhibition and acidosis in patients with type 2 diabetes: A review of US FDA data and possible conclusions. nt J Nephrol Renovasc Dis 2017:153-8.
[Crossref] [Google Scholar] [PubMed]
- Tzanetakos C, Tentolouris N, Kourlaba G, Maniadakis N. Cost-effectiveness of dapagliflozin as add-on to metformin for the treatment of type 2 diabetes in Greece. Clin Drug Investig 2015;18(7):A606-7.
[Crossref] [Google Scholar] [PubMed]
- Wang H, Yao G, Chen X, Ouyang J, Yang J. Ipragliflozin as an add-on therapy in type 2 diabetes mellitus patients: An evidence-based pharmacoeconomics evaluation. Diabetes Res Clin Pract 2019;157:107867.
[Crossref] [Google Scholar] [PubMed]
- Rahman W, Solinsky PJ, Munir KM, Lamos EM. Pharmacoeconomic evaluation of Sodium-Glucose Transporter-2 (SGLT2) inhibitors for the treatment of type 2 diabetes. Expert Opin Pharmacother 2019;20(2):151-61.
[Crossref] [Google Scholar] [PubMed]