- *Corresponding Author:
- Hui Li
Department of Anesthesiology, Suzhou Ninth Hospital Affiliated to Soochow University, Suzhou, Jiangsu Province 215200, China
E-mail: lihuibbbiiigggaaa@126.com
| This article was originally published in a special issue, “Drug Discovery and Repositioning Studies in Biopharmaceutical Sciences” |
| Indian J Pharm Sci 2024:86(4) Spl Issue “175-180” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
This paper studies the effect and mechanism of Slack channel deficiency leading to gamma-aminobutyric acid type A receptor reduction on the general anesthesia effect of isoflurane and propofol. The experimental results show that Slack gene knockout mice have reduced sensitivity to isoflurane and propofol, prolonged anesthesia induction period, shortened anesthesia duration, decreased anesthesia depth, and weakened postoperative sedation effect. Slack gene knockout mice have increased levels of adrenaline and noradrenaline during anesthesia, and decreased transcription and expression levels of gamma-aminobutyric acid type A receptors. This paper provides reference for the applicability and dosage of anesthesia scheme for patients with epilepsy or other abnormal neural activities undergoing anesthesia treatment.
Keywords
Propofol, isoflurane, Slack channel, anesthetic effect, gamma-aminobutyric acid type A receptor
Slack channels, also known as Potassium Sodium- activated channel subfamily T member 1 (KCNT1), are the most complex class of voltage-gated potassium channels, with diverse functions such as regulating neurotransmitter release, heart rate, insulin secretion, neuronal excitability and epithelial cell electrolyte transport[1,2]. This gene encodes a sodium-activated potassium channel subunit, which is thought to play a role in ion conduction and developmental signaling pathways, and its mutations may cause early-onset epilepsy, infantile malignant migrating partial seizures and autosomal dominant nocturnal frontal lobe epilepsy[3]. Studies have shown that Transmembrane Protein 16C (TMEM16C) knockout rats have downregulated Slack channel expression in dorsal root ganglia, resulting in neuronal hyper excitability and reduced pain threshold, and that in vivo gene silencing of Slack channels or knockout of TMEM16C can enhance the transmission of nociceptive stimuli[4]. This study aims to analyze the effects and mechanisms of Gamma-Aminobutyric Acid type A (GABAA) receptor agonist anesthetics propofol and isoflurane on anesthesia in normal mice and Slack knockout mice, and to provide references for the applicability and dosage of anesthesia regimens for patients with epilepsy or other neuronal hyperactivity disorders when undergoing anesthesia.
Materials and Methods
Materials:
Laboratory animal: 20 Slack gene Knockout Mice (Slack KO) and 20 male C57BL/6J mice were purchased from Changzhou Branch of Jiangsu Jicui Yaokang Biotechnology Co., Ltd., aged (6-8) w, weighing (20-22) g, animal production license number SCXK (Su) 20190009. All operations in this study complied with the 3R principle of experimental animals.
Drugs and reagents: Propofol injection, 0.2 g/20 ml, National Drug approval No: H20030115, batch No: 2020841, Sichuan Guorui Pharmaceutical Co., Ltd.; Isoflurane, 100 ml/bottle, National Drug approval No: H19980141, batch No: 20211118, Hebei Yipin Pharmaceutical Co., Ltd.; 0.9 % saline, 100 ml/bottle, National Drug approval No: H13023202, batch No: 20200423, Shijiazhuang Siyao Co., Ltd.; adrenaline Enzyme-Linked Immunosorbent Assay (ELISA) kit and noradrenaline ELISA kit were purchased from Wuhan Merck Biotechnology Co., Ltd.; GABAA Receptor Gamma-2 subunit (GABAARγ-2) antibody, batch No: 66a5897, purchased from Affinity Company in United States of America (USA); Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH) antibody, batch No: 60004-1-Ig, purchased from Wuhan Sanying Biotechnology Co., Ltd.; ChamQ Universal SYBR quantitative Polymerase Chain Reaction (qPCR) Master Mix kit, batch No: 20210813, purchased from Nanjing Novozymes Biotechnology Co., Ltd.
Instrument: YLS-6B intelligent hot plate instrument purchased from Jinan Yiyuan Technology Development Co., Ltd.
Methods:
Animal grouping and administration: Normal C57BL/6J mice were randomly divided into propofol control group and isoflurane control group, and Slack gene KO mice were randomly divided into propofol group and isoflurane group; the corresponding administration schemes were propofol control group (30 mg/kg propofol, normal C57BL/6J mice), isoflurane control group (1.5 % isoflurane, normal C57BL/6J mice), propofol group (30 mg/kg propofol, Slack KO mice) and isoflurane group (0.2 mg isoflurane, Slack KO mice), with 8 mice in each group. Propofol control group and propofol group mice were intraperitoneally injected with 30 mg/kg propofol, and isoflurane control group and isoflurane group mice were placed in an anesthesia box with 1.5 % isoflurane and oxygen for 4 h. The control group used normal mice for the experiment, while the propofol group and isoflurane group used Slack KO mice.
Time-related anesthesia detection indicators: The timing started after the anesthesia was completed, and the righting reflex was used as the judgment criterion. The disappearance time and recovery time of the righting reflex were recorded, which were the induction period, from the loss of the righting reflex to the appearance of the anesthetic state; the anesthesia period, from the appearance of the clinical manifestations of anesthesia to the recovery time; and the recovery period, from the recovery of the mice to the recovery of the righting reflex[5].
Anesthesia state detection: When the mice entered the deep anesthesia stage, the eye reflex, eyelid reflex, pedal reflex, swallowing reflex and tail pinch reflex of each group of mice were analyzed. Each reflex was scored 1 point, with a total score of 5 points. The higher the score, the worse the anesthesia effect[6].
Thermal Withdrawal Latency (TWL): Before anesthesia, 0 h and 2 h after recovery, the TWL was tested using YLS-6B intelligent hot plate instrument. The time from the left hind paw touching the hot plate to the appearance of withdrawal, tiptoeing, struggling and squeaking licking foot response was recorded, each lasting 20 s[7].
Detection of adrenaline and noradrenaline: When the mice entered the deep anesthesia state, blood was collected from the tail artery, and serum was collected after centrifugation. The expression levels of adrenaline and noradrenaline in serum were analyzed according to the kit method[8].
qPCR detection of GABAA receptor transcription in mouse brain tissue: After the mice recovered, they were killed by decapitation and the brain tissue was removed and placed in 4° Phosphate Buffer Solution (PBS). Total Ribonucleic Acid (RNA) was extracted according to the RNA extraction kit procedure, and then reverse transcribed into complementary Deoxyribonucleic Acid (cDNA) according to the reverse transcription kit procedure. RT-PCR experiment was performed according to the procedure of Novozymes ChamQ Universal SYBR qPCR Master Mix kit. The primers for the internal reference gene GAPDH were; forward 5’-GACAACTTTGGCATCGTGGA-3’; reverse 5’-ATGCAGGGATGATGTTCTGG-3’, and the primers for the target gene GABAA were; forward 5’-AAAGTGCGACCATAGAACCGA AAG-3’ and reverse 5’ GCGGAAAGGCTATTCTTTTGACAGTG[9].
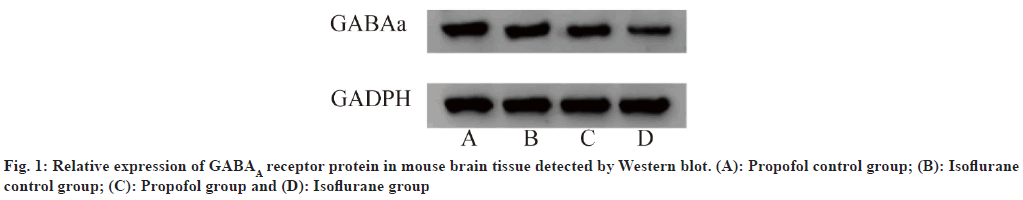
Western blot detection of GABAA receptor expression in mouse brain tissue: After the mice recovered, they were killed by decapitation and the brain tissue was removed and placed in a cryopreservation tube, then immediately placed in liquid nitrogen and transferred to a -80° freezer for storage. The brain tissue was taken out and placed at room temperature, and 100 mg of brain tissue was weighed with an analytical balance. The brain tissue was washed with sterile PBS and dried with filter paper. It was placed in 1 ml of lysis buffer on ice bath, and then homogenized to obtain a 10 % brain tissue homogenate. It was placed in a centrifuge (4°, 12 000 rpm, 10 min, centrifuged twice) to obtain the brain tissue supernatant. The protein concentration was determined by Bicinchoninic acid (BCA) protein quantification method, and 50 μg of protein per well was loaded. The concentration gel was run at 80 V, followed by 120 V for the separation gel. After electrophoresis, the membrane transfer (300 mA, 140 min) was performed. The membrane was blocked with 5 % skim milk at 4° overnight. The next day, the membrane was washed and incubated with GABAA receptor antibody (1:1000) and GADPH antibody (1:2000) at room temperature for 3 h. After washing with Tris-Buffered Saline with 0.1 % Tween® 20 Detergent (TBST) three times, the secondary antibody (1:20 000) was added and incubated at room temperature for 2 h. After washing with TBST three times, chemiluminescence liquid was added and photographed. The gray value of protein bands was analyzed by gel imaging system[10].
Immunofluorescence detection of GABAA receptor number in rat brain tissue: The extraction method of brain tissue supernatant is as described above. The brain tissue supernatant was added with 5 % goat serum and immunostaining permeabilization solution and left to stand for 1 h. Then GABAA antibody (1:1000) was added respectively, and incubated in a 4° refrigerator for 24 h. Then the secondary antibody was added and incubated at room temperature for 1 h. The nucleus was stained, washed, and sealed. The pictures were taken with a laser scanning confocal microscope, and the average number of GABAA receptors was calculated[11].
Statistical analysis:
The experimental data were expressed as mean±standard deviation (x͞ ±s), and analyzed by Statistical Package for the Social Sciences (SPSS) 19.0 software. One-way analysis of variance was used for multiple groups of data, and t-test was used for comparison between two groups.
Results and Discussion
The study found that Slack gene knockout mice had significantly reduced sensitivity to propofol. In the experiment, compared with the propofol control group, the induction period of the propofol group was significantly prolonged, while the duration of anesthesia was reduced, and the recovery period was shorter p<0.05 (Table 1).
| Group | n | Induction period/min | Anesthesia period/min | Awakening period/min |
|---|---|---|---|---|
| Isoflurane control | 8 | 5.16±2.12 | 28.54±6.24 | 5.31±1.86 |
| Propofol control | 8 | 3.54±1.06 | 36.25±8.47 | 7.26±2.18 |
| Isoflurane | 8 | 8.25±1.35* | 15.40±3.47* | 2.05±0.33* |
| Propofol | 8 | 6.26±0.95#& | 23.12±3.28#& | 4.32±1.48#& |
Note: Compared with propofol control, *p<0.05; compared with isoflurane control, #p<0.05 and compared with propofol group, &p<0.05
Table 1: Staging Time of Anesthesia in Four Groups of Mice
Compared with the propofol control group (0.23±0.19), the propofol group (0.42±0.49) had a significantly higher anesthesia depth score at the same dose, with a statistically significant difference, p<0.05; compared with the isoflurane control group (1.43±0.49), the isoflurane group (2.57±0.81) had a significantly higher anesthesia depth score at the same dose, with a statistically significant difference, p<0.05; in Slack gene knockout mice, the isoflurane group mice had a significantly higher anesthesia depth score than the propofol group, p<0.05 (Table 2).
| Group | n | Anesthesia depth |
|---|---|---|
| Isoflurane control | 8 | 1.43±0.49 |
| Propofol control | 8 | 0.23±0.19 |
| Isoflurane | 8 | 2.57±0.81* |
| Propofol | 8 | 0.42±0.49#& |
Note: Compared with propofol control, *p<0.05; compared with isoflurane control, #p<0.05 and compared with propofol group, &p<0.05
Table 2: Anesthesia Depth of Mice in Each Group
After the mice recovered, it was found that Slack gene knockout mice were more sensitive to the hot plate, and both propofol and isoflurane anesthesia mice showed shorter TWL response times, p<0.05; while after 2 h of recovery, Slack gene knockout mice showed more obvious thermal withdrawal responses, p<0.05 (Table 3).
| Group | n | T0/s | T2/s |
|---|---|---|---|
| Isoflurane control | 8 | 17.48±1.56 | 14.51±1.66 |
| Propofol control | 8 | 18.09±1.43 | 16.81±1.17 |
| Isoflurane | 8 | 13.57±2.11* | 5.21±0.63* |
| Propofol | 8 | 15.54±1.92#& | 8.89±1.13#& |
Note: Compared with propofol control, *p<0.05; compared with isoflurane control, #p<0.05 and compared with propofol group, &p<0.05
Table 3: Latency of Heat Shrinkage Foot in Each Group of Mice
ELISA analysis showed that compared with C57 BL/6J mice, the adrenaline and noradrenaline levels of Slack gene knockout mice increased significantly after anesthesia, p<0.05; in Slack gene knockout mice, the adrenaline and noradrenaline levels of the isoflurane group were significantly higher than those of the propofol group, p<0.05 (Table 4).
| Group | n | Adrenaline (pg/ml) | Norepinephrine (ng/ml) |
|---|---|---|---|
| Isoflurane control | 8 | 350.53±24.55 | 2.14±0.72 |
| Propofol control | 8 | 283.36±23.13 | 1.82±0.54 |
| Isoflurane | 8 | 560.66±48.51* | 5.77±1.62* |
| Propofol | 8 | 480.20±35.63#& | 4.26±1.18#& |
Note: Compared with propofol control, *p<0.05; compared with isoflurane control, #p<0.05 and compared with propofol group, &p<0.05
Table 4: Serum Epinephrine and Noradrenaline Levels in Mice
As shown in Table 5, the relative transcription levels (Δt) of GABAA protein in the propofol control group, isoflurane control group, propofol group, and isoflurane group were 17.89±1.34, 16.56±0.98, 7.36±1.28, and 5.37±0.87, respectively. Western blot experiments showed that compared with normal mice, the relative transcription level of GABAA receptor protein in Slack gene knockout mice decreased significantly, p<0.05.
| Group | n | Δt |
|---|---|---|
| Isoflurane control | 3 | 16.56±0.98 |
| Propofol control | 3 | 17.89±1.34 |
| Isoflurane | 3 | 5.37±0.87* |
| Propofol | 3 | 7.36±1.28#& |
Note: Compared with propofol control, *p<0.05; compared with isoflurane control, #p<0.05 and compared with propofol group, &p<0.05
Table 5: Amount of GABAA Receptor Transcription in Brain Tissue of Mice in Each Group
Protein in the propofol control group, isoflurane control group, propofol group, and isoflurane group were 0.90±0.12, 0.84±0.08, 0.51±0.14, and 0.45±0.10, respectively. As shown in fig. 1, Western blot experiments showed that compared with normal mice, the relative expression level of GABAA receptor protein in Slack gene knockout mice decreased significantly, p<0.05.
The number of GABAA receptors in the propofol control group, isoflurane control group, propofol group, and isoflurane group were 76.31±23.24, 67.86±15.45, 40.83±14.75, and 36.87±19.77, respectively. Immunofluorescence experiments showed that compared with normal mice, the number of GABAA receptors in Slack gene knockout mice decreased significantly, p<0.05 (Table 6).
| Group | n | Number |
|---|---|---|
| Isoflurane control | 3 | 67.86±15.45 |
| Propofol control | 3 | 76.31±23.24 |
| Isoflurane | 3 | 36.87±19.77* |
| Propofol | 3 | 40.83±14.75#& |
Note: Compared with propofol control, *p<0.05; compared with isoflurane control, #p<0.05 and compared with propofol group, &p<0.05
Table 6: Number of GABAA Receptors in Brain Tissue of Mice in Each Group
GABA is the main inhibitory neurotransmitter in the central nervous system. There are two major classes of proteins that mediate GABA responses; the ionotropic GABAA receptor family (a chloride ion channel) and the metabotropic GABAB receptor [12]. GABAA belongs to the ligand-gated chloride ion channel, which opens the channel when GABA binds to the GABAA receptor, allowing more chloride ions to enter the cell, making the neuronal membrane hyperpolarized, reducing the possibility of action potential generation, and thus inhibiting the activity of the next neuron[13]. Propofol is a GABAA receptor agonist, a common intravenous anesthetic, which is widely used for general anesthesia due to its rapid onset, complete recovery and no postoperative nausea and vomiting[14]. Isoflurane is also a GABAA receptor agonist, a common anesthetic inhalant, which is widely used in animal experiments due to its advantages of rapid anesthesia, rapid recovery, simple operation, continuous and controllable anesthesia depth[15].
This study found that propofol anesthesia induction period was significantly prolonged in Slack gene knockout mice compared with C57 BL/6J mice, and the duration of anesthesia was shortened, followed by a shortened recovery period. The results of isoflurane action on Slack gene knockout mice and C57 BL/6J mice were consistent with propofol, and the anesthetic effect of propofol was relatively improved compared with isoflurane. The evaluation of mouse anesthesia state during the anesthesia period found that after propofol or isoflurane anesthesia, C57 BL/6J mice showed eyelid reflex in one mouse in the isoflurane control group, and tail pinch reflex in one mouse, while only one mouse in the propofol control group showed eye response. In Slack gene knockout mice, all mice in the isoflurane group showed eyelid reflex and pedal reflex, and swallowing reflex and tail pinch reflex to varying degrees, while Slack mice in the propofol group also showed reflex state, and their anesthesia depth was significantly reduced compared with normal mice. This indicates that Slack gene knockout mice are easily stimulated by external stimuli during anesthesia, showing nervous excitement, which is consistent with the results reported by Zhang et al.[16]. Adrenaline is a hormone and neurotransmitter released by the adrenal glands[17]. In clinical studies, adrenaline has been used in combination with dexmedetomidine to enhance the anesthetic effect and duration[18,19]. The results showed that Slack knockout mice showed higher levels of adrenaline and noradrenaline during anesthesia, indicating sensitive sympathetic nerve activity, coping with respiratory depression or reduction caused by anesthesia, and promoting better oxygen supply for the body.
The absence of Slack channel in humans leads to the occurrence of early-onset epilepsy, which may be related to abnormal neuronal activity, but there are no reports on how Slack channel affects anesthesia effect and its underlying mechanism. In 2019, Kuchenbuch et al.[20] reported that Slack gene mutation reduced the firing rate of GABAergic interneurons and, to a lesser extent, pyramidal cells, resulting in increased cortical hyper excitability. Combined with the experimental results of this study, qPCR experiments showed that compared with normal mice, the relative transcription level of GABAA receptor protein in Slack gene knockout mice decreased significantly; Western blot experiments showed that compared with normal mice, the relative expression level of GABAA receptor protein in Slack gene knockout mice decreased significantly; immunofluorescence experiments showed that compared with normal mice, the number of GABAA receptors in Slack gene knockout mice decreased significantly; we speculate that Slack pathway deficiency leads to reduced GABAA receptor number, weakened neuronal inhibition, and thus when using propofol and isoflurane, which are GABAA receptor agonist type anesthetics, the anesthesia effect will be significantly reduced and the anesthesia depth will be relatively limited. This study provides reference for clinical treatment of patients with epilepsy or other abnormal neuronal activity who receive anesthesia treatment, and their anesthesia scheme applicability and dosage are different from normal people.
Funding:
This article was supported by the Suzhou Ninth Hospital Affiliated to Soochow University (No: 201923); and the Xuzhou Medical University (No: XZSYSKF2020043).
Conflict of interests:
The authors declared no conflict of interests.
References
- Hasan S, Balobaid A, Grottesi A, Dabbagh O, Cenciarini M, Rawashdeh R, et al. Lethal digenic mutations in the K+ channels Kir4. 1 (KCNJ10) and SLACK (KCNT1) associated with severe-disabling seizures and neurodevelopmental delay. J Neurophysiol 2017;118(4):2402-11.
[Crossref] [Google Scholar] [PubMed]
- Hu B. Mechanism of KCNE subunits regulating KCNQ1 channel and β2 subunit regulating drosophila BK channel. Thesis, Huazhong University of Science and Technology; Wuhan, China; 2019.
- Lin H, Jiang L. Research progress on epilepsy and its treatment related to KCNT1 gene mutation. Epilepsy J 2022;8:251-5.
- Zhang M, Chen Q, Tan C, Ma K, Li LI, Dai Z, et al. Expression and significance of PAR2 and TMEM16A on DRG rat modelin of neuropathic pain. J Pract Med 2017:3702-6.
- Guo M, Li W, Feng Z. Effect of ethanol gavage on propofol anesthesia effect and oxidative stress indicators in mice. J Acad Mil Med Sci 2016;37:641-3.
- Wang C, Chai Q, Gong H. Observation of the anesthetic effect of Shu Tai combined with chlorpromazine hydrochloride on the colonic dilatation model of rats. Exp Anim Comp Med 2022;42:31-35.
- Zhang L, Tao S. Effect of venlafaxine hydrochloride on neuropathic pain in rats with chronic constriction injury of sciatic nerve. J Gansu Coll Tradit Chin Med 2013;30:10-2.
- Wang W, Huang M. Effect of norepinephrine on left ventricular remodeling and cardiac function after acute myocardial infarction in mice. Chin J Clin Pharmacol Ther 2021;21:37.
- Yao C, Cai Z, Wang R. Sodium salicylate affects the expression of GABAA receptor subunits in cochlear spiral ganglion neurons. J Clin Otorhinolaryngol Head Neck Surg 2015;29(11):1024-9.
- Hu Y, Li L, Dai W. Effect of Huanglian Wendan decoction on sleep improvement and GABAergic system pathway neurotransmitter content and receptor expression in insomnia mice induced by chlorophenylalanine. Chin J New Drugs Clin Pharmacol 2022;33(8):1009-16.
- Yang P, He J. Effect of Zhi San needle electroacupuncture on working memory improvement and mechanism of vascular dementia mice. New Chin Med 2022;54(20):165-70.
- Li P, Stewart R, Butler A, Gonzalez-Cota AL, Harmon S, Salkoff L. GABAB controls persistent Na current and coupled Naactivated K current. eNeuro 2017.
[Crossref] [Google Scholar] [PubMed]
- Yuan D, Zhang Y, Luo M. Research progress of GABA receptor modulators in the treatment of Alzheimer's disease. Chin Pharm J 2022;57(21):1810-16.
- Li SQ. Propofol regulates AKT/P53 pathway to inhibit ferroptosis and reduce myocardial ischemia-reperfusion injury. Thesis Shandong Univ Jinan China 2022;52:42-6.
- Yang F. Effect of isoflurane anesthesia on neurovascular coupling mechanism of acute epilepsy attack in mice. Thesis Jilin Univ Changchun China 2021;9(4):13-9.
- Zhang D, Duan X, Hao C. Anesthetic effect and mechanism of isoflurane combined with propofol on rat colonic dilatation model. J Kunming Med Univ 2021;42(7):25-30.
- Wang X, Lu C, Shi T. Research progress on the mechanism of β-adrenergic receptor signaling pathway regulating cardiac fibrosis in heart failure. Chin J Contemp Med 2022;29(24):23-7.
- Li K, Zhao G, Li X, Zhu Z. Comparison of the effects of dexmedetomidine and adrenaline as adjuvants for local anesthesia on brachial plexus block. Int J Anesth Resusc 2016;8:692-5
- Wang S. Adrenaline combined with anesthetic for extraction of inflamed teeth in 480 cases. Natl Acad Confer Med 2010.
- Kuchenbuch M, Nabbout R, Yochum M, Sauleau P, Modolo J, Wendling F, et al. In silico model reveals the key role of GABA in KCNT1-epilepsy in infancy with migrating focal seizures. Epilepsia 2022;62(3):683-97.
[Crossref] [Google Scholar] [PubMed]