- Corresponding Author:
- H. A. RajDepartment of Quality Assurance, Shri Sarvajanik Pharmacy College, Near Arvind Baug, Mehsana - 384 001, India
E-mail: contacthasu@yahoo.co.in
| Date of Submission | 20 June 2006 |
| Date of Revision | 16 August 2007 |
| Date of Acceptance | 15 November 2007 |
| Indian J Pharm Sci, 2007, 69 (6): 759-762 |
Abstract
A simple, accurate and precise spectroscopic method was developed for simultaneous estimation of ezetimibe and simvastatin in tablets using first order derivative zero-crossing method. Ezetimibe showed zero crossing point at 245.4 nm while simvastatin showed zero crossing point at 265.2 nm. The dA/dl was measured at 265.2 nm for ezetimibe and 245.4nm for simvastatin and calibration curves were plotted as dA/dl versus concentration, respectively. The method was found to be linear (r 2 >0.9994) in the range of 5-40 µg/ml for ezetimibe at 265.20 nm. The linear correlation was obtained (r 2 >0.9935) in the range of 5-80 µg/ml for simvastatin at 245.4 nm. The limit of determination was 0.39 and 0.12 µg/ml for ezetimibe and simvastatin, respectively. The limit of quantification was 1.10 and 0.4 µg/ml for ezetimibe and simvastatin, respectively. The method was successfully applied for simultaneous determination of ezetimibe and simvastatin in binary mixture.
Keywords
Ezetimibe, simvastatin, simultaneous estimation, zero crossing method, antihyperlipidemic agents
Ezetimibe is a new antihyperlipidemic agent and chemically is, 1-(4-fluorophenyl)-3(R)- [3-(4-fluorophenyl)-3(S)-hydroxypropyl]-4(S)-(4- hydroxyphenyl)-2-azetidinone [1]. It is a selective cholesterol absorption inhibitor that effectively blocks intestinal absorption of dietary and biliary cholestrol. [2-6] Simvastatin inhibits the enzyme 3- hydroxy-3-methyl glutaryl coenzyme A (HMG CoA) reductase [7]. Simvastatin when combined in low doses i.e. 10-20 mg/day; with ezetimibe can be a potent and safe combination for reduction of LDL-cholestrol [8]. A combination formulation Simvotin™EZ 10, Ranbaxy, containing 10 mg of simvastatin and 10 mg of ezetimibe is available in the market. Simvastatin is official in BP and the official method is a LC method [9]. Other methods reported in literature are TLC [10], HPLC [10,11]. One HPLC [12] method and GC10 method are available for simultaneous estimation of simvastatin and Lovastatin. Similarly for ezetimibe, RP-HPLC [13], HPLC [14], LCMSMS [15] and TLC [16] methods are reported. But no official or reported procedure is present for simultaneous determination of ezetimibe and Simvastatin in pharmaceutical preparations. The reported procedures are time consuming expensive and relatively complicated. Derivative spectroscopy provides a greater selectivity than common spectroscopy and offers a powerful approach for resolution of band overlapping quantitative analysis of multicomponent mixture [17,18]. The aim of this study was to develop a simple, fast and sensitive derivative spectroscopic method for simultaneous determination of ezetimibe and simvastatin in pharmaceutical preparations on the basis of zerocrossing measurement.
Materials and Methods
Ezetimibe and simvastatin were obtained as a gift samples from Sun Pharmaceutical Ltd. Baroda. Methanol used was of analytical grade and obtained form S. D. Fine Chemicals. A commercial tablet formulation (Simvotin™ EZ 10, Ranbaxy) each containing 10 mg of simvastatin and 10 mg of ezetimibe were procured from the local pharmacy. A Shimadzu UV-1700 double beam UV/Vis spectrophotometer with software of UVprobe was used for all measurements. The zero order absorption spectra were recorded over the wavelength range of 200-380 nm, against a solvent blank, in quartz cuvettes with 1 cm diameter. For all solutions, the derivative spectra were obtained over 200-380 nm range at 2 nm slit width (Δλ).
Standard and calibration solutions
Standard stock solution of ezetimibe and simvastatin were prepared by separately dissolving 10 mg of ezetimibe and simvastatin, respectively in 100 ml methanol. Accurate volumes were transferred into two sets of 10 ml calibrated flask. The first series contained varying concentrations of ezetimibe (1- 40 μg/ml). The second series contained varying concentration of simvastatin (1-40 μg/ml). The calibration curves for derivative spectroscopy were constructed by plotting drug concentration versus the absorbance values of the first derivate spectrum (D1) at 265.20 nm for ezetimibe and at 245.4 nm for simvastatin and regression equations were computed.
Spectroscopic measurements
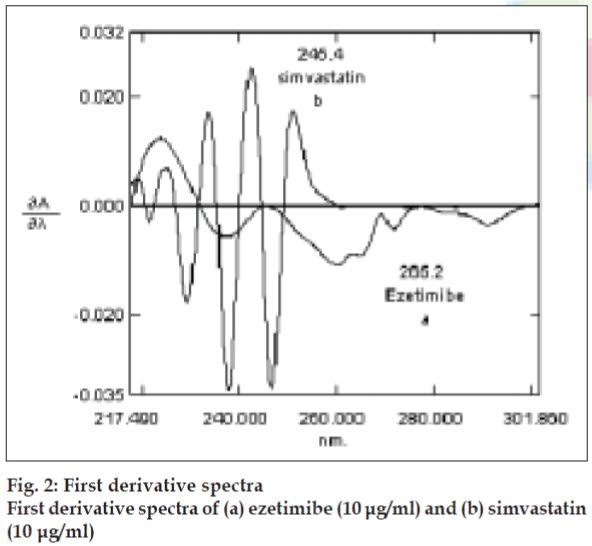
The difference between spectra of standard solutions of ezetimibe and simvastatin versus their solvent blanks were recorded in the range of 200-380 nm. The first order derivative spectra of the standard solutions of each drug and those containing mixtures of both drugs were obtained in the same range of wavelength (200-380 nm) against blanks. The values of D1 amplitudes for ezetimibe in the presence of simvastatin and vice versa measured at 265.20 nm (zero-crossing of simvastatin) and 245.4 nm (zero crossing of ezetimibe), respectively.
Accuracy and precision
To establish the reliability of the proposed method, two series of solutions containing 10, 20, 30 and 40 μg/ml of ezetimibe plus in each 10 μg/ml of simvastatin and 10, 20, 30 and 40 μg/ml of simvastatin plus in each 10 μg/ml ezetimibe were prepared, respectively, and analyzed as discussed above. Precision of the procedure was calculated by within - day and between-day variations. Accuracy of the method was measured as percentage of deviation between added and measured concentrations (recovery study).
Analysis of tablets
Twenty tablets of Simvotin™ EZ 10, Ranbaxy, were powdered. The powder equivalent to 10 mg of simvastatin and 10 mg of ezetimibe was weighed accurately and transferred to 100 ml volumetric flask. Twenty milliliters methanol was added to the flash and sonicated for 20 min. The solution was filtered through Whatman filter paper No. 41 and the volume was adjusted up to the mark with menthol. This solution is expected to contain 100 µg/ml simvastatin and 100 µg/ml ezetimibe. From the stock solution 1 ml was taken in to a 10 ml volumetric flask and the volume make up to the mark with methanol to get a final concentration of simvastatin (10 µg/ml) and ezetimibe (10 µg/ml). The concentration of ezetimibe and simvastatin in tablets were calculated using the corresponding calibrated curve.
Results and Discussion
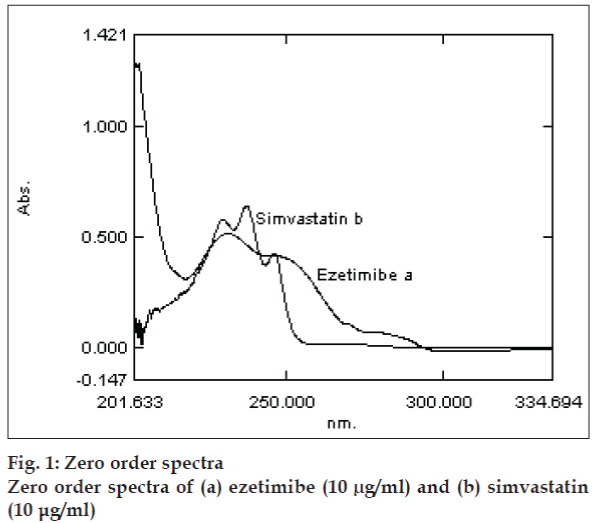
Zero-order absorption spectra of ezetimibe and simvastatin showed overlapping peaks that interfere with the simultaneous determination of this formulation (fig. 1). Derivative spectroscopy, based on a mathematical transformation of the spectra zeroorder curve into the derivative spectra, allows a fast, sensitive and precise resolution of a multicomponent mixture and overcomes the problem of overlapping of a multi-component system. Derivative spectroscopy on the basis of zero-crossing measurements involves measurement of the absolute value of the total derivative spectrum at an abscissa value corresponding to the zero-crossing wavelength of the derivative spectra of individual components, which should be only a function of the concentration of other componet [19]. The spectroscopic parameters including derivative order, wavelength and Δλ values should be optimized to obtain maximum resolution, sensitivity and reproducibility [19-21]. In this study first-derivative technique (D1) traced with Δλ= 2 nm was used to resolve the spectral overlapping. Zero-crossing points of 200-380 nm is presented in fig. 2. The optimum D1 values without interference for ezetimibe and simvastatin were 265.20 and 245.4 nm, respectively (fig. 2).
The linearity of the method was established form first-derivative spectra by measurement of the absorbance of standard solutions containing varying concentrations of each compound in the presence of constant concentration of the other one. The calibration curves were constructed by plotting the D1 value against ezetimibe or simvastatin concentration at the zero-crossing wavelength of simvastatin (265.20 nm) or ezetimibe (245.4 nm), respectively. The results obtained are summarized in Table 1. The linearity of the calibration curves and the adherence of the method to Beer’s law are validated by the high value of the correlation coefficient and the value of intercept on ordinate which is close to zero.
| Parameters | Ezetimibe | Simvastatin |
|---|---|---|
| Wavelength (nm) | 265.20 | 245.4 |
| Linearity (μg/ml) | 1 - 40 | 1- 40 |
| Regression equation * | Y= 0.0018x ± 0.00001 | Y= 0.0014x ± 0.0003 |
| Correlation coefficient | 0.9993 | 0.9923 |
| Limit of detection (μg/ml) | 0.39 | 0.12 |
| Limit of quantification (μg/ml) | 1.10 | 0.4 |
*Y=bx + a, where x is the concentration of drug in μg/ml Y is the amplitude at the specified wavelength, b is slope and a is intercept.
Table 1: Statistical data of calibration curves of ezetimibe and simvastatin using first-derivative spectra
The limit of detection that was found to be 0.39 μg/ml and 0.12 μg/ml for ezetimibe and simvastatin. The accuracy and precision were determined by using synthetic mixture of ezetimibe and simvastatin in the laboratory. The mean recoveries and SD are illustrated in Tables 2 and 3. Data of these tables showed a good accuracy and precision over the entire concentration range. The within-day and between-day variations showed co-efficient of variation (CV%) values less than 1% for both ezetimibe and simvastatin respectively in all four selected concentrations. The data indicate that the proposed derivative spectroscopic method is highly precise during one analysis and between different runs.
| Added amount of ezetimibe (μg/ml) | Found (μg/ml) SD | |
|---|---|---|
| Within day* | Between day* | |
| 10 | 10.22 ± 0.15 | 10.01 ± 0.51 |
| 20 | 20.02 ± 0.21 | 19.89 ± 0.27 |
| 30 | 30.13 ± 0.10 | 29.91 ± 0.11 |
| 40 | 39.99 ± 0.17 | 40.11 ± 0.21 |
Accuracy and precision data of determination of ezetimibe in the presence of simvastatin (10 μg/ml) using first derivative spectroscopy. *Mean of six determinations
Table 2: Accuracy and precision of determination of ezetimibe in the presence of simvastatin
| Added amount of simvastatin (μg/ml) | Found (μg/ml) SD | |
|---|---|---|
| Within day* | Between day* | |
| 10 | 10.11 ± 0.17 | 10.19 ± 0.45 |
| 20 | 20.02 ± 0.19 | 20.19 ± 0.22 |
| 30 | 29.89 ± 0.14 | 30.00 ± 0.28 |
| 40 | 39.98 ± 0.10 | 39.99 ± 0.05 |
Accuracy and precision data for determination of simvastatin in the presence of ezetimibe (10 μg/ml) by first derivative spectroscopy. *Mean of six determinations
Table 3: Accuracy and precision of determination of simvastatin in the presence of ezetimibe
The percentage of recovery in each case was calculated. The results obtained from the recoveries of both drugs (Tables 2 and 3) showed excellent accuracy. The influence of excipients was studied by mixing two formulation containing 10 μg/ml of ezetimibe and 10 μg/ml of simvastatin. No interference was observed from the presence of excipient in the amounts, which are commonly present in tablet dosage forms. Study of stability of ezetimibe and simvastatin in the solutions during analysis showed that analytes were stable at least for 72 h in solutions.
The proposed method was successfully applied to analyze preparation containing ezetimibe and simvastatin. The results are summarized in Table 4. The results obtained are in good agreement with the labeled content.
| Formulation | Simvastatin % Found ± SD (n=4)* | Ezetimibe % Found ± SD (n=4)* |
|---|---|---|
| Sample 1 | 99.8 ± 0.09 | 98.9 ± 0.21 |
| Sample 2 | 99.5 ± 0.44 | 99.4 ± 0.37 |
*Mean of four determinations. Both the sample from simvotin™ EZ 10, Ranbaxy, contenting 10 mg of simvastatin and 10 mg of ezetimibe. SD is the standard deviation
Table 4: Results of the analysis of commercial product
Acknowledgements
The authors are thankful to Sun Pharmaceutical Pvt. Ltd. Baroda, for providing standards sample of drug and also the Shri Sarvajanik Pharmacy College Mehsana for providing facilities to carry out work.
References
- Rosenblum SB, Huynh T, Afonso A. Discovery of 1-(4-flurophenyl)-(3R)-[(4-flurophenyl)-(3S)-hydroxypropyl]-(4s)-(4-hydroxyphenyl)-2-azetididnone (SCH 58253): A designed, potent, orally active inhibitor of cholesterol absorption. J Med Chem 1998; 41: 973-980.
- Van HM, Framce CF, Compton DS, McLeod RL, Yamibe NP, Altone KB, et. al. In vivo metabolism based discovery of a potent cholesterol absorption inhibitor, SCH 58235, in the rat and monkey through the identification of the active metabolites of SCH 48461. J PharmacolExpTher 1997; 283: 157-163.
- Hanefled M. Clinical rationale for rosuvastatin, a potent new HMG-CoA reductase inhibitor. Int J ClinPract 2001; 55:6: 399-402.
- Vijiayvergia R, Sridhar G, Grover A. Ezetimibe: A novel cholesterol absorption inhibitor. Drug Bulletin 2000; 29:1: 35-43.
- British Pharmacopoeia, Vol. II, London: Published by Controller of Her Majesty’s StationalryOffice; 2000; 1535.
- Moffat Article for copyediting. Osselton MD. Widdop B. Clark’s Analysis of Drugs and Poisons, 3rd ed. Vol. 2. London: pharmaceutical press; 1995. p. 1561-63.
- Pasha MK, Muzee BS, Basha SJ. Mullangi R. Analysis of five HMG-CoA reductase inhibitors atorvastatin, lovastatin, pravastatin, rosuvastatin and simvastatin: pharmacological, pharmacokinetic and analytical overview and development of a new method for use in pharmaceutical formulations analysis and in vitro metabolism studies. Biomed Chromatogr 2006; 20(3):282-93.
- Srinivasu MK, Narasa RA, Rao DS. Determination of lovastatin and simvastatin in pharmaceutical dosage form by HPLC. Indian Drugs 2004; 41: 156-158.
- Sistla R, Tata VSSK, Kashyap YV, Chandrasekar D, Diwan PV. Development and validation of reversed phase HPLC method for the determination of ezetimibe in pharmaceutical dosage forms. J Pharm Bio Anal 2005; 39: 3-4:517.
- Ghosal A, Hapangama N, Yuan Y, Iunnucci R, Alton K, Patrick J E, et al. Identification of human UDP-Glucuronosyltransferase enzyme(s) responsible for the glucuronidation of ezetimibe. D M D 2002; 30: 4: 314-320.
- James E, Kosoglou J, Stauber KL, Alton KB. Disposition of the selective cholesterol absorption inhibitor ezetimibe in healthy male subject. D M D 2002; 30: 4: 430-437.
- Van HM, Farket C, Compton DS, Hoos LM, Torhan S, Davis HR. Ezetimibe potently inhibit cholesterol absorption but dose not affect acute hepatic or intestinal cholesterol synthesis in rats. J Brit Pharmacology 2003; 138: 1459-1464.
- Karpinska J, Mulikowsha M. Simultaneious determination of zinc(II) manganese (II) and iron(II) in pharmaceutical preparation. J Pharm Bio Anal 2002; 29: 153-158.
- Morelli B. Determination of a quanternary mixture of vitamins B6, B1 and B12 and uridin 5’-triphosphate, by derivative spectrophotometry. J Pharm Sci 1995; 84: 1: 34-37.
- Ragno G, Garofalo A, Vetuschi C. Photodegradation monitoring of amlodipine by derivative spectrophotometry. J Pharm Bio Anal 2002; 27:1-.2: 19-24.
- Bebawy LI, Moustafa AA, Abo-Talib NF. Stability-indicating methods for the determination of doxazosinmezylate and celecoxib. J Pharm Bio Anal 2002; 27:5: 779-793.
- El-Gindy A, El-Zeany B, Awad T, Shabana MM. Derivative spectrophotometric, thin layer chromatographic-densitometric and high performance liquid chromatographic determination of trifluoperazine hydrochloride in presence of its hydrogen peroxide induced-degradation product. J Pharm Bio Anal 2002; 27:1-2: 9-18.