- *Corresponding Author:
- I. Singhvi
Department of pharmaceutical sciences, M. L. sukhadia university, Udaipur - 313 001, India
E-mail: INDRAJEET_S@yahoo.com
| Date of Submission | 19 August 2006 |
| Date of Revision | 4 September 2007 |
| Date of Acceptance | 28 November 2007 |
| Indian J Pharm Sci, 2007, 69 (6): 780-783 |
Abstract
Three simple, accurate, economical and reproducible spectrophotometric methods for simultaneous estimation of two-component drug mixture of rosiglitazone maleate and glimepiride in combined tablet dosage form have been developed. Developed methods are based on direct estimation of rosiglitazone maleate at 318.0 nm, as at this wavelength glimepiride has zero absorbance and hence does not interfere. For estimation of glimepiride first developed method involves formation and solving of simultaneous equation at 238.0 nm. Second developed method makes use two wavelength spectroscopy using 244.8 nm and 257.2 nm as two wavelengths. Third developed method is based on first derivative spectroscopy using 252.0 nm as zero crossing point for estimation of glimepiride. All the developed methods obey Beer's law in the concentration ranges employed for the respective methods. The results of analysis were validated statistically and by recovery studies.
Keywords
Simultaneous spectrophotometric analysis, Rosiglitazone maleate, Glimepiride
Rosiglitazone maleate (RSGN) is a new oral antidiabetic drug and chemically, it is (±)-5-{p- [2- (methyl-2-pyridylamino)ethoxy]benzyl}-2,4- thiazolidinedione maleate [1]. The drug is not yet official in any of the pharmacopoeia. For analysis of RSGN two LC [2,3], one MEKC (Micellar Electrokinetic Chromatographic) [4] and three HPLC [5-7] methods have been reported in literature. Glimepiride (GLIM), is a sulphonylurea antidiabetic drug and chemically it is 3-ethyl-2,5-dihydro-4-methyl-N-[2-[4-[[[[(trans-4- methylcyclohexyl)amino]carbonyl]amino]sulphonyl] phenyl]ethyl] 2-oxo-1H-pyrrole-1-carboxamide [8]. The drug is not yet official in any of the pharmacopoeia. Literature survey revealed one spectrophotometric [9] and three HPLC [10-12] methods have been reported for the estimation of GLIM in pharmaceutical formulations. Although, some methods have been reported for the estimation of RSGN and GLIM from single component formulation, there is no single method reported for simultaneous estimation of these two drugs from combined tablet dosage form. An attempt in the present study has been made to develop simple, accurate and economical method for simultaneous estimation of RSGN and GLIM from combined tablet dosage form. The result of analysis using the developed spectrophotometric methods for simultaneous estimation was found to be satisfactory such that the developed methods can be used for routine analysis of drugs from combined pharmaceutical dosage form.
Materials and Methods
A PC based Systronic, UV/Vis double beam spectrophotometer (model No. 2101) with spectral bandwidth of 2 nm, wavelength accuracy ±0.5 nm (with automatic wavelength correction) and wavelength readability 0.1 nm increment was employed for all measurements using a matched pair of 10 mm quartz cells.
Standard bulk drug samples of RSGN and GLIM were provided by Dr. Reddy’s Laboratories, Hyderabad. Sodium hydroxide solution (0.1N) was used as solvent for the preparation of stock solution and for further dilutions. Double distilled water was used for the preparation of 0.1 N sodium hydroxide solution. The tablet samples of combined dosage form of RSGN and GLIM (Rosicon G, Glenmark Pharmaceuticals, Mumbai and Enseline-2G, Torrent Pharmaceuticals Ltd., Ahmedabad) were procured from local pharmacy.
Method I, simultaneous equation method
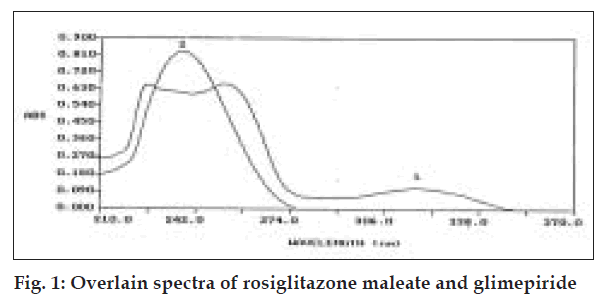
From the overlain spectra of RSGN and GLIM in 0.1 N sodium hydroxide solution (fig. 1), it was observed that GLIM has zero absorbance at 318.0 nm, where RSGN has substantial absorbance so RSGN was estimated directly at this wavelength. For estimation of GLIM simultaneous equation was framed. Pure drug sample of RSGN and GLIM were dissolved separately in 0.1 N sodium hydroxide solution so as to give several dilutions of standard in concentration range of 0-30 µg/ml of each drug. All dilutions were scanned in wavelength range of 210.0 nm-370.0 nm. Absorbance was measured at 318.0 nm against reagent blank and plotted a calibration curve. For estimation of GLIM simultaneous equation was framed on the basis of absorptivity coefficient of two drugs at 238.0 nm. Absorptivity coefficient for GLIM at 238.0 nm was calculated as 418.63 cm-1g-1l and for RSGN 394.72 cm-1g-1l. These calculated values are the means of four independent determinations. Equation framed for GLIM estimation was, A= 418.63 C1+394.72 C2, where A is absorbance of sample solution at 238.0 nm, C1 and C2 are concentration of GLIM and RSGN respectively in g/l. Validity of above framed equation was checked by preparing five mixed standards using pure drug sample of two drugs, measuring their absorbance at respective wavelengths and calculating concentration of two components. Results of which are reported in Table 1.
| Sample no. | Conc. Present | %Conc. Found | ||||
|---|---|---|---|---|---|---|
| µg/ml | Method I | Method III | ||||
| RSGN | GLIM | RSGN | GLIM | RSGN | GLIM | |
| 1 | 5 | 30 | 100. | 99. | 100. | 102. |
| 2 | 10 | 25 | 99. | 100. | 99. | 103. |
| 3 | 15 | 20 | 98. | 99.10 | 98. | 103. |
| 4 | 20 | 15 | 101. | 100. | 101. | 102. |
| 5 | 25 | 10 | 100. | 101. | 100. | 100.00 |
RSGN is rosiglitazone maleate, GLIM is glimepiride
Table 1: Results of validation studies for method I and Iii using mixed standards
Method II, two wavelength calculation method
Estimation of RSGN was carried out in a manner similar to method-I. In this developed method for estimation of GLIM two wavelength calculation method was used. Two wavelengths selected for estimation of GLIM are 244.8 nm and 257.2 nm based on the principle that absorbance difference between two points on a mixture spectra is directly proportional to concentration of component of interest and independent of interfering component. Five mixed standards of pure drugs containing 0-30 µg/ml of each drug were prepared in 0.1N sodium hydroxide solution. Absorbance of all standards were recorded at two wavelengths 244.8 nm and 257.2 nm, determined absorbance difference (A1-A2) values and plotted calibration curve between absorbance difference values and concentration of drug for estimation of GLIM.
Method III, first order derivative spectroscopy method
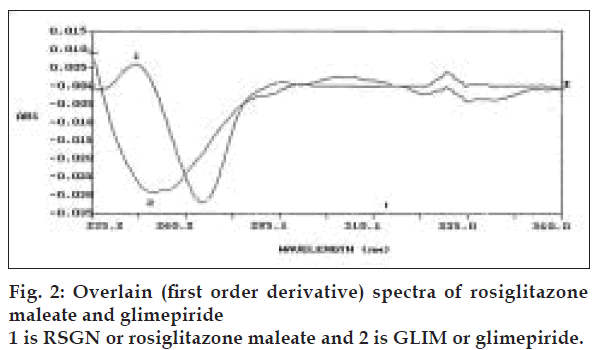
Estimation of RSGN was carried out in a manner similar to method-I. For estimation of GLIM first order derivative spectroscopy method was used. From first order derivative spectra of RSGN and GLIM in 0.1 N sodium hydroxide (fig. 2) zero crossing point 252.0 nm was selected for estimation of GLIM. Accurately weighed pure drug sample of RSGN and GLIM were dissolved in 0.1 N sodium hydroxide solution so as to give several dilutions in range of 0-30 µg/ml of each drug. The absorbance of these dilutions was recorded in first order derivative mode at 252.0 nm for estimation of GLIM and calibration curve was prepared. Validity of proposed method was checked by preparing five mixed standards using pure drug sample of two drugs and absorbance was measured at selected zero crossing point and determined concentration of GLIM using calibration curve. Results of validation studies are reported in Table 1.
Analysis of commercial formulation
For method I, twenty tablets of each brand were weighed accurately and finely powdered. From each triturate of the twenty tablets an amount equivalent to 2 mg rosiglitazone maleate was accurately weighed and extracted four times with 20 ml portions of 0.1 N sodium hydroxide solution and filtered through Whatman filter paper no. 41 into a 100 ml volumetric flask. Washed residue with 0.1N sodium hydroxide solution and added washings to filtrate, volume of filtrate was made to 100 ml mark with 0.1N sodium hydroxide solution. Absorbance of this dilution was measured at 238.0 nm and 318.0 nm. Concentration of RSGN was calculated directly from absorbance value at 318.0 nm from calibration curve prepared using standard drug solution. Concentration of GLIM was calculated using above framed equation. Results of analysis of tablet formulation are reported in Table 2.
| Method | Batch | Label claim mg/Tab | % Label claim estimated* | Standard deviation | % Conc. Recovered** | ||||
|---|---|---|---|---|---|---|---|---|---|
| RSGN | GLIM | RSGN | GLIM | RSGN | GLIM | RSGN | GLIM | ||
| Method I | A | 2 | 1 | 100. | 101. | 0.4171 | 0.3040 | 100. | 100. |
| B | 2 | 1 | 100. | 101. | 0.8343 | 0.0848 | 100. | 99. | |
| Method II | A | 2 | 1 | 100. | 101. | 0.4171 | 0.3889 | 100. | 100.30 |
| B | 2 | 1 | 100. | 101.10 | 0.8343 | 0.7778 | 101. | 101. | |
| Method III | A | 2 | 1 | 100. | 99. | 0.4171 | 0.7495 | 100. | 101. |
| B | 2 | 1 | 100. | 101. | 0.8343 | 0.8697 | 100. | 98. | |
*Averageofthreeestimations, **Averageofrecoverystudiesatthreedifferentconcentrationlevels.AisRosiconG(GlenmarkPharmaceuticals, Mumbai)andBisEnseline-2G (TorrentPharmaceuticalsLtd.,Ahmedabad). RSGNisrosiglitazone maleate,GLIMisglimepiride. MethodIisSimultaneousEquation method,MethodII isTwoWavelength Calculationmethod, MethodIII is Firstorder derivative spectroscopymethod
Table 2: Results of analysis of commercial formulation
For method II, preparation of tablet sample solution and estimation of RSGN was carried out in a manner similar to method I. For estimation of GLIM final dilution was analyzed by recording absorbance at 244.8 nm and 257.2 nm and absorbance difference values were noted and concentration was calculated from the respective calibration curve. Results of analysis are reported in Table 2.
For method III, preparation of tablet sample solution and estimation of RSGN was carried out in a manner similar to method I. For estimation of GLIM absorbance of sample was recorded at 252.0 nm from first order derivative spectra of sample and amount of GLIM was calculated using respective calibration curve. Results of analysis are reported in Table 2.
Recovery studies
To study the accuracy, reproducibility and precision for all the three developed methods, recovery studies were carried out by the addition of standard drug solution to pre-analyzed tablet sample with proper dilutions at three different concentration levels with in the range of linearity for both the drugs. Results of recovery studies were found to be satisfactory and are reported in Table 2.
Results and Discussion
The proposed methods described for the simultaneous analysis of RSGN and GLIM in combined tablet dosage form has been found to be simple, accurate, rapid, economical and sensitive to be applied in routine analysis of tablets. In the described methods there are no additional extraction or separation procedures to extract the drug from the formulation excipient matrix thereby decreasing the error in quantitation.
The developed methods are based on direct estimation of RSGN at wavelength maxima of RSGN, where GLIM has no absorbance, however for estimation of GLIM special techniques were used. First developed method involving formation and solving of simultaneous equation is based on absorptivity coefficient of two drugs at wavelength maxima of GLIM i.e. once the equation is framed then it is just required to measure the absorbance of sample solution at selected wavelengths and few calculations that can be manually done. Framed equation was validated using laboratory prepared mixed standards of two drugs which gave satisfactory results.
Second developed method for estimation of GLIM makes use of two wavelength calculation method so as to remove interference between two components. Proper selection of two wavelengths for estimation of a component is critical.
Third developed method for simultaneous analysis of RSGN and GLIM from combined dosage form makes use of first order derivative ultraviolet spectrophotometry based on principle that at zero crossing point of one component, the other component has substantial absorbance.
The results of analysis of two drugs from tablet formulation using all the three developed methods were found close to 100% for both RSGN and GLIM, standard deviation was satisfactorily low indicating accuracy and reproducibility of the methods. Recovery studies were satisfactory which shows that there is no interference of excipients. The developed methods were found to be simple, rapid, accurate and can be used for routine estimation of two drugs from tablet formulations.
Acknowledgements
Authors would like to acknowledge Dr. Reddy’s Laboratories, Hyderabad, India for providing pure drug samples of RSGN and GLIM.
References
- Budavari S, editor. The Merck Index. 13th ed. Whitehouse Station (NJ): Merck and Co Inc; 2001. p. 1484.
- Kolte BL, Raut BB, Deo AA, Bagool MA, Shinde DB. Liquid chromatographic method for the determination of rosiglitazone in human plasma. J Chromatogr B AnalytTechnol Biomed Life Sci 2003;88:37-44.
- Radhakrishna T, Satyanarayana J, Satyanarayana A. LC Determination of rosiglitazone in bulk and pharmaceutical formulation. J Pharm Biomed Anal 2002;29:873-80.
- Gomes P, Sippel J, Jablonski A, Steppe M. Determination of rosiglitazone in coated tablets by MEKC and HPLC methods. J Pharm Biomed Anal 2004;36:909-13.
- Mamidi RN, Benjamin B, Ramesh M, Srinivas NR. Simple method for the determination of rosiglitazone in human plasma using a commercially available internal standard. Biomed Chromatogr 2003;17:417-20.
- Muxlow AM, Fowles S, Russell P. Automated HPLC method for the determination of rosiglitazone in human plasma. J Chromatogr B Biomed SciAppl 2001;752:77-84.
- Kim KA, Park JY. Simple and extractionless HPLC determination of rosiglitazone in human plasma and application to pharmacokinetics in human plasma. Biomed Chromatogr 2004;18:613-5.
- Budavari S, editor. The Merck Index. 13th ed. Whitehouse Station (NJ): Merck and Co Inc; 2001. p. 790.
- Altinoz S, Tekeli D. Analysis of glimepiride by using derivative UV spectrophotometric method. J Pharm Biomed Anal 2001;24:507-15.
- Pistos C, Astraka C, Kalovidouris M, Vassilopoulos E, Koutsopoulou M. Bioequivalence evaluation of two brands of glimepiride 4 mg tablets in healthy subjects. Int J ClinPharmacolTher 2005;43:203-8.
- Pistos C, Koutsopoulou MI. Improved liquid chromatographic tandem mass spectrometric determination and pharmacokinetic study of glimepiride in human plasma. Biomed Chromatogr 2005;19:394-401.
- Wanjari DB, Gaikwad NJ. Reversed phase HPLC method for determination of glimepiride in tablet dosage forms. Indian J Pharm Sci 2005;67:253-5.