- Corresponding Author:
- Sejal K. Patel
S. K. Patel College of Pharmaceutical Education and Research, Ganpat University, Kherva, Mehsana-382 711, India
E-mail: skpatel_2@rediffmail.com
| Date of Submission | 08 August 2008 |
| Date of Revision | 20 May 2009 |
| Date of Acceptance | 8 September 2009 |
| Indian J. Pharm. Sci., 2009, 71 (5): 545-547 |
Abstract
A binary mixture of trifluoperazine HCl and chlordiazepoxide was determined using reversed-phase liquid chromatography method using methanol:water (97:03, v/v) pumped at a flow rate of 1.0 ml/min. Quantification was achieved with ultraviolet detection at 262 nm over concentration ranges of 0.1-1 and 0.5-5 μg/ml; mean accuracies were 101.05±0.47 and 98.97±0.33 %, respectively. The method was successively applied to tablet dosage forms as no chromatographic interferences from the tablet excipients were observed. The method retained its accuracy and precision when the standard addition technique was applied.
Keywords
Trifl uoperazine HCl, chlordiazepoxide, RP-HPLC
Trifluoperazine HCl (TFP) is official in IP, BP and USP. Both IP[1] and USP[2] describe nonaqueous titration method whereas BP[3] describes UV spectroscopy method for estimation of TFP. A literature survey revealed spectrophotometric[4,5], HPLC[6], HPTLC[7] methods for simultaneous estimation of TFP in pharmaceutical formulation with other drugs. Chlordiazepoxide (CLR) is official in IP, BP and USP. The IP[8], BP[9] and USP[2] describe non-aqueous titration, potentiometry titration and HPLC methods, respectively for estimation of CLR. Literature survey indicated difference spectrophotometric[10], micellar liquid chromatography[11], derivative spectrophotometry[12] methods for CLR with other drugs in pharmaceutical formulations. Literature survey revealed spectrophotometry method[13] and RP-HPLC method[14] for the simultaneous determination of these two drugs. The present RP-HPLC method uses simple mobile phase ratio (methanol: water- 97:03), higher sensitivity and analysis will complete before 6 min. Therefore the present study was to determine both drugs concurrently by sensitive, accurate, rapid and precise RP-HPLC method for routine analysis.
The chromatography was performed on a Shimadzu (Columbia, MD) RP-HPLC instrument (LC-2010CHT) equipped with PDA detector, Phenomenex (Torrance, CA) C18 column (250×4.6 mm id, 5 µm particle size) was used as stationary phase. Standard samples of TFP and CLR and market samples of Serepose tablets (Unimarck Pharma, Chandigarh), each tablet contained 1 mg TFP and 10 mg CLR were used. Triple distilled water, methanol (S. D. Fine Chemical, Ahmedabad, India) used were of HPLC grade.
TFP and CLR stock solutions (40 µg/ml and 200 µg/ml, respectively) were prepared by weighing accurately 2 mg TFP and 10 mg CLR powder into 2 separate 50 ml volumetric ß asks; 25 ml methanol was added, shaken for a few minutes, and diluted to volume with methanol. From these solutions (2.5 ml) were transferred into 2 separate 10 ml volumetric ß asks and diluted to the mark with methanol to give final concentrations of 10 and 50 µg/ml, respectively. Accurate aliquots equivalent to 0.1-1 µg TFP from its working solution (10 µg/ml) and aliquots equivalent to 0.5-5 µg CLR from its working solution (50 µg/ml) were transferred into 2 separate sets of 5 ml volumetric flasks and diluted to volume with methanol. Powder from the mixed contents of 20 tablets, equivalent to 1 mg TFP and 10 mg CLR, was transferred accurately to a 50 ml volumetric flask and diluted to volume with methanol. The solution was diluted to the same concentrations of working standard solutions and treated according to the linearity for the RP-HPLC method. The separation was done on a C18 column using methanol:water (97:03, v/v) as the mobile phase. The chromatogram was recorded under the following instrumental parameters: 20 µl injection volume, flow rate, 1.0 ml/min at 400 temperature and the eluent monitored at 262 nm. Calibration curves for both TFP and CLR were plotted, and the corresponding regression equations were calculated.
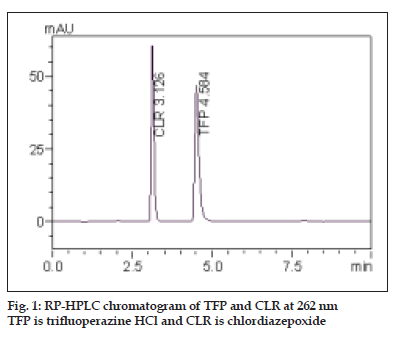
The aim of this work was to develop sensitive, accurate, precise and rapid analytical method for the simultaneous determination of TFP and CLR. This was achieved using RP-HPLC method. To optimize the proposed RP-HPLC method, all of the experimental conditions were investigated. For the choice of the stationary phase, reversed-phase separation was preferred due to the drawbacks of the normal phase, e.g., hydration of silica with water that can cause peak tailing. To optimize the mobile phase, different systems were tried for chromatographic separation of the two components by combining homogenous design and solvent polarity optimization. The best resolution was achieved using a mobile phase consisting of methanol:water (97:03, v/v), which gave good resolution and sensitivity of both drugs (fig. 1).
| Parameters | RP-HPLC method | |
|---|---|---|
| TFP ± % RSDa | CLR ± % RSDa | |
| Retention time, min | 4.58±0.19 | 3.12±0.20 |
| Tailing factor | 1.12±0.67 | 1.34±0.56 |
| Asymmetry factor | 1.12±0.93 | 1.30±0.39 |
| Theoretical plates | 5253.8±1.13 | 5283.98±1.33 |
| Repeatability of | 0.67 | 0.48 |
| measurement (nb = 6) |
Table 1: System Suitability Test Parameters For Tfp And Clr For Proposed Method.
| Formulation | Amount of drug taken (mg) | Amount of drug found (mg) | % Amount found (na=3) ± SDb | |||
|---|---|---|---|---|---|---|
| TFP | CLR | TFP | CLR | TFP | CLR | |
| Tablets | 1 | 10 | 1.00894 | 9.91332 | 100.89 ± 0.95 | 99.13 ± 0.87 |
| 1 | 10 | 1.0106 | 9.8964 | 101.06 ± 0.20 | 98.96 ± 1.00 | |
Table 2: Assay Results For Tablets Using The Proposed Method.
System suitability testing of the RP-HPLC method gave good relative retention time = 1.46; theoretical plates= 5253.8 and 5283.98; asymmetry factor (A)= 1.12 and 1.30; and tailing factor (T)= 1.12 and 1.34 for TFP and CLR, respectively (Table 1). A linear relation was obtained between peak area and the concentration of the two drugs in the range of 0.1-1 and 0.5-5 µg/ml for TFP and CLR, respectively. The linear regression equations were computed, Y=117926 X+10901, r= 0.9976 and Y=123994 X+35915, r= 0.9979, where Y is the area under the peak, X is the concentration in µg/ml, and r is the correlation coefficient. Results obtained by applying the RPHPLC procedure showed that TFP and CLR can be simultaneously analyzed in the prepared mixtures with mean recoveries of 101.05±0.47 and 98.97±0.33 %, respectively. The proposed method has been applied to assay TFP and CLR in tablets without any interference from the additives (Table 2). The limit of detection for TFP and CLR were found to be 0.05 µg/ml and 0.05 µg/ml, respectively; the limit of quantification for TFP and CLR were found to be 0.1 µg/ml and 0.5 µg/ml, respectively by visual method. The low % CV values of intra-day (0.35-1.56 for TFP and 0.48-1.31 for CLR) and inter-day (0.48-1.67 for TFP and 0.57-1.62 for CLR) precision reveal that the proposed method is precise. Thus, the proposed procedure can be used in routine analysis.
Acknowledgements
The authors are thankful to managements of Shree S. K. Patel College of Pharmaceutical Education and Research, Ganpat University, Kherva, Mehsana for providing needed facilities for this work.
References
- Indian Pharmacopoeia, Vol. 3. Government of India, Ghaziabad: The Indian Pharmacopoeia Commission: 2007. p. 1828.
- The United States Pharmacopoeia. 28th Revision. US Pharmacopoeial convention. Inc. Rockville, MD: 2005. p. 1974, 435.
- British Pharmacopoeia. Vol. I. Her Majesty’s Stationary Office. London: UK; 2005. p. 2101.
- El-Gindy A, El-Zeany B, Awad T, Marwan MS. Spectrophotometric determination of trißuoperazineHCl and isopropamide iodide in binary mixture using second derivative and second derivative of the ratio spectra methods. J Pharm Biomed Anal 2001;26:203-10.
- Ruedas RMJ, Ruiz MA, Diaz AM. Bead injection spectroscopy-flow injection analysis (BIS-FIA): an interesting tool applicable to pharmaceutical analysis Determination of promethazine and trißuoperazine. J Pharm Biomed Anal 2004;35:1027-34.
- Patela YP, Dhorda UJ, Sundaresan M. A comparative study using HPLC and packed column supercritical ßuid chromatography for the assay of three anti-psychotic dosage forms. Talanta 1998;47:625-30.
- Maslanka A, Krzek J. Densitometric High Performance thin-layer chromatography identification and quantitative analysis of psychotropic drugs. J AOAC Int 2005;88:70-9.
- Indian Pharmacopoeia, Vol. 2. Government of India Ghaziabad: The Indian Pharmacopoeia Commission; 2007. p. 905.
- British Pharmacopoeia. Vol. 1. London: Her Majesty’s Stationary Office; 2005. p. 463.
- Davidson AG. Assay of chlordiazepoxide and demoxepam in chlordiazepoxide formulations by difference spectrophotometry. J Pharm Sci 1984;73:55-8.
- Cholbi-Cholbi MF, Martinez-Pla JJ, Sagrado S, Villanueva-Camanas RM, Medina-Hernandez MJ. Determination of anticonvulsant drugs in pharmaceutical preparations by micellar liquid chromatography. J LiqChromatogr Related Tech 2004;27:153-70.
- Toral MI, Richter P, Lara N, Jaque P, Soto C, Saavedra M. Simultaneous determination of chlordiazepoxide and clidinium bromide in pharmaceutical formulations by derivative spectrophotometry. Int J Pharm 1999;189:67-74.
- Saudagar RB, Saraf S, Saraf S. Spectrophotometric determination of chlordiazepoxide and trifluoperazine hydrochloride from combined dosage form. Indian J Pharm Sci 2007;69:149-52.
- Jadhao VM, Gide PS, Kadam VJ. Reverse phase high performance liquid chromatographic determination of chlordiazepoxide and trißuoperazine hydrochloride in tablets. Indian Drugs 2006;43:99-101.