- Corresponding Author:
- S. B. Wankhede
Department of Pharmaceutical Chemistry, Pad. Dr. D. Y. Patil Institute of Pharmaceutical Sciences and Research, Sant Tukaram Nagar, Pimpri, Pune-411 018, India. E- mail: sagar2277@rediffmail.com
| Date of Submission | 31 March 2009 |
| Date of Revision | 18 July 2009 |
| Date of Acceptance | 18 September 2009 |
| Indian J Pharm Sci, 2009, 71 (5): 563-567 |
Abstract
Two UV Spectrophotometric and one reverse phase high performance liquid chromatography methods have been developed for the simultaneous estimation of amlodipine besilate and olmesartan medoxomil in tablet dosage form. First UV spectrophotometric method was a determination using the simultaneous equation method at 237.5 nm and 255.5 nm over the concentration range 10-50 μg/ml and 10-50 μg/ml, for amlodipine besilate and olmesartan medoxomil with accuracy 100.09%, and 100.22% respectively. Second UV spectrophotometric method was a determination using the area under curve method at 242.5-232.5 nm and 260.5-250.5 nm over the concentration range of 10-50 μg/ml and 10-50 μg/ml, for amlodipine besilate and olmesartan medoxomil with accuracy 100.10%, and 100.48%, respectively. In reverse phase high performance liquid chromatography analysis carried out using 0.05M potassuim dihydrogen phosphate buffer:acetonitrile (50:50 v/v) as the mobile phase and Kromasil C18 (4.6 mm i.d.Χ250 mm) column as the stationery phase with detection wavelength of 238 nm. Flow rate was 1.0 ml/min. Retention time for amlodipine besilate and olmesartan medoxomil were 3.69 and 5.36 min, respectively. Linearity was obtained in the concentration range of 4-20 μg/ml and 10-50 μg/ml for amlodipine besilate and olmesartan medoxomil, respectively. Proposed methods can be used for the estimation of amlodipine besilate and olmesartan medoxomil in tablet dosage form provided all the validation parameters are met.
Keywords
Amlodipine besilate, area under curve method, olmesartan medoxomil, reverse phase high performance liquid chromatography, simultaneous equation method
Amlodipine besilate (AMLO), chemically, [3-ethyl- 5-methyl(4RS)-2-[(2-aminoethoxy) methyl]-4- (2-chlorophenyl)-methyl-1-dihydropyridine-3,5- dicarboxylate benzenesulfonate [1], is a long acting calcium channel blocker used which is used as an antihypertensive agent [2-4]. Olmesartan medoxomil (OLME), chemically, 2,3-dihydroxy-2-butenyl 4-(1-hydroxy-1-methylethyl)-2-propyl-1-[p-(o-1Htetrazol- 5-ylphenyl)benzyl]imidazole-5-carboxylate, cyclic-2,3-carbonate, is an angiotensin II receptor blockers used as an antihypertensive agent [5]. AMLO is official in BP [1], whereas OLME is not official in any pharmacopoeia. Both the drugs are marketed as combined dose tablet formulation in the ratio of AMLO:OLME 05:20 mg. Literature survey revealed that a number of methods have been reported for estimation of AMLO [6-15] and OLME [16,17] individually or in combination with other drugs. However, there is no analytical method reported for the simultaneous estimation of AMLO and OLME in a combined dosage formulation. Present work describes three simple, accurate, reproducible, rapid and economical methods for simultaneous estimation of AMLO and OLME in tablet formulation. A double-beam Shimadzu UV/Vis spectrophotometer, 1700 Pharmaspec, with spectral bandwidth of 2 nm, wavelength accuracy of ±0.5 nm and a pair of 1-cm matched quartz cells, was used to measure absorbance of the resulting solution. A gradient reverse phase high performance liquid chromatography (Merck Hitachi) with L-7100 double reciprocating pump, L-7400 UV detector, and Kromasil C18 (4.6 mm i.d.×250 mm) column as the stationery phase was used. The RP-HPLC system was equipped with Winchrom software for data processing. Standard gift sample of AMLO was provided by Glennmark Pharmaceuticals Ltd, Nashik, India and OLME by Macleods Pharmaceuticals Ltd, Mumbai, India. AMLO and OLME combination tablets (Olmezest- AM, 05 mg amlodipine besilate and 20 mg olmesrtan medoxomil; manufacture by SUN Pharmaceutical Industries, Dadra, India), were purchased from the local pharmacy. For UV-Spectrophotometry, methanol of analytical grade was used as solvent, standard stock solutions of AMLO (100 µg/ml) and OLME (100 µg/ml) were prepared in methanol and used for the analysis. For RP-HPLC, acetonitrile of HPLC grade was used, a buffer solution of 50 mM was prepared. A mixture of phosphate buffer and acetonitrile in the ratio of 50:50 v/v was used as mobile phase and was Þ ltered before use through 0.45 μ membrane filter. Kromasil C18 column (4.6 mm i.d.×250 mm) was used as stationary phase. Constant flow of 1.0 ml/min was maintained throughout the analysis. Detection was carried out using UV detector at 238 nm. Standard stock solutions of AMLO (100 µg/ml) and OLME (100 µg/ml) were prepared in mobile phase and used for the analysis.
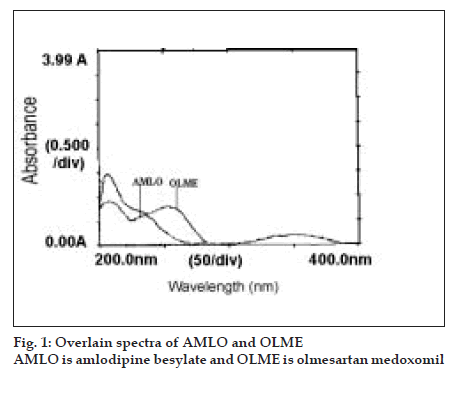
For the selection of analytical wavelength for the simultaneous equation method (method-A), solutions of AMLO and OLME (20 µg/ml, each), were prepared separately by appropriate dilution of standard stock solution and scanned in the spectrum mode from 200 nm to 400 nm. From the overlain spectra of both drugs (Þ g. 1), wavelengths 237.5 nm (λmax of AMLO) and 255.5 nm (λmax of OLME) were selected for the simultaneous equations. Calibration curves for AMLO and OLME were prepared in the concentration range of 10-50 µg/ml and 10- 50 µg/ml at both the wavelengths, respectively. The absorptivity values were determined for both the drugs at both the wavelengths and following Eqns were used, A1=41.86CAMLO+38.99COLME (1) and A2=28.25CAMLO+41.39COLME (2), where A1 and A2 are absorbances of the sample at 237.5 nm and 255.5 nm, respectively, 41.86 and 28.25 are absorptivities of AMLO at 237.5 nm and 255.5 nm, respectively, 38.99 and 41.39 are the absorptivities of OLME at 237.5 nm and 255.5 nm, respectively. CAMLO is the concentration of AMLO and COLME is the concentration of the OLME. The mixture concentration was determined by using the Eqns 1 and 2.
In the area under curve method (method-B), from the overlain spectra of both drugs (fig.1), wavelengths range 242.5-232.5 nm (for AMLO) and 260.5-250.5 nm (for OLME) were selected for the analysis. The calibration curves for AMLO and OLME were prepared in the concentration range of 10-50 µg/ml and 10-50 µg/ml at both the wavelength range, respectively. The absorptivity values were determined for both the drugs at both the wavelength range and following Eqns were used, A1 = 414.13CAMLO+389.32COLME (3) and A2 = 283.08CAMLO+408.97COLME (4), where A1 and A2 are area under curve of the sample at 242.5-232.5 nm and 260.5-250.5 nm, respectively, 414.13 and 283.08 are absorptivities of AMLO at 242.5-232.5 nm and 260.5- 250.5 nm, respectively, 389.32 and 408.97 are the absorptivities of OLME at 242.5-232.5 nm and 260.5- 250.5 nm, respectively. CAMLO is the concentration of AMLO and COLME is the concentration of the OLME. The mixture concentration was determined by using the Eqns 3 and 4.
In the reverse phase high performance liquid chromatography (method-C), standard stock solution of AMLO and OLME (1000 μg/ml) was prepared in mobile phase separately. The wavelength selected for analysis is 238 nm. The calibration curves for AMLO and OLME were prepared in the concentration range of 04-20 µg/ml and 10-50 µg/ml, respectively at 238 nm. Calibration curve was constructed by plotting concentration against peak area.
In the UV spectrophotometric method, for estimating AMLO and OLME in commercial formulations, twenty tablets were weighed and average weight was calculated. The tablets were crushed to obtain a fine powder. Tablet powder equivalent to 5 mg of AMLO was transferred to 25.0 ml volumetric flask containing 20.0 ml methanol and exposed to ultrasonic radiations for 20 min and then final volume was made up to the mark with methanol. The solution was then Þ ltered through a Whatmann filter paper No. 41. The filtrate was appropriately diluted with the same solvent to obtain final concentrations of 8 µg/ml for AMLO and 32 µg/ ml for OLME. Concentrations of both AMLO and OLME were determined by measuring the absorbance of the sample at 237.5 and 255.5 nm (method-A) and at 242.5-232.5 nm and 260.5-250.5 nm (method B) in the spectrum mode and values were substituted in the respective formulae to obtain concentrations. Results of the tablet analysis were analysed against the calibration curve in quantitation mode.
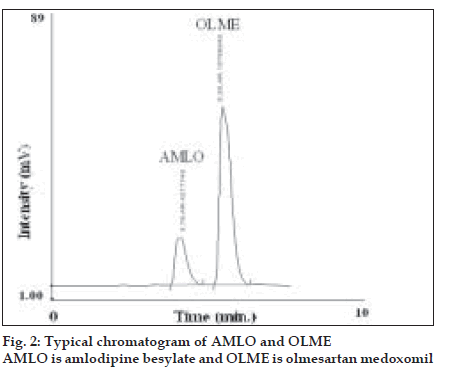
In the RP-HPLC method, for estimating AMLO and OLME in commercial formulation, tablet sample solution containing 8 µg/ml of AMLO and 32 µg/ ml of OLME was prepared in similar manner as described under UV spectrophotometric method using mobile phase as diluent instead of methanol. The diluted solutions were filtered through 0.20 μ Þ lter. Twenty microlitres of solutions were injected and chromatographed under above mentioned chromatographic conditions. A typical chromatogram of AMLO and OLME is shown in (fig. 2). The concentration of both AMLO and OLME was determined by comparing peak area of sample with that of standard at 238 nm. The results of tablet analysis are shown in Table 1.
| Method | Component | Label Claim (mg/tab) | Amount Found | Estimated Label Claim* | SD | CV |
|---|---|---|---|---|---|---|
| 100.27 | 0.1752 | 0.1747 | ||||
| A | AMLO | 05 | 5.012 | |||
| OLME | 20 | 20.091 | 100.46 | 0.2921 | 0.2920 | |
| B | AMLO | 05 | 5.01 | 100.10 | 0.1732 | 0.1730 |
| OLME | 20 | 20.07 | 100.36 | 0.2134 | 0.2126 | |
| C | AMLO | 05 | 5.012 | 100.25 | 0.6136 | 0.6120 |
| OLME | 20 | 20.065 | 100.32 | 0.2785 | 0.2776 |
*Average of six determinations, SD denotes standard deviation and CV denotes coefÞ cient of variation.
Table 1: Analysis of tablet formulation
The proposed chromatographic system was found suitable for effective separation and quantitation of AMLO (RT-3.79 min) and OLME (5.39 min). The system suitability parameters were found to be, resolution-2.22, tailing factor- 1.30 for AMLO and 1.17 for OLME. Recovery studies were carried out by standard addition method at three different levels 80, 100 and 120%. The % recovery of AMLO and OLME in the sample mixture was determined. The results of recovery studies obtained by proposed method were validated by statistical evaluation and are recorded in Table 2.
| Level of Recovery (%) | Amt. of PureDrug Added (mg) | Method-A % Recovery | Method-B % Recovery | Method-C % Recovery | ||||
|---|---|---|---|---|---|---|---|---|
| AMLO | OLME | AMLO | OLME | AMLO | OLME | AMLO | OLME | |
| 80 | 04 | 16 | 100.71 | 100.08 | 100.07 | 100.57 | 100.01 | 100.10 |
| 100 | 05 | 20 | 100.54 | 100.42 | 100.18 | 100.32 | 100.08 | 100.17 |
| 120 | 06 | 24 | 99.04 | 100.15 | 100.07 | 100.54 | 99.85 | 99.92 |
| Mean % Recovery | 100.10 | 100.22 | 100.11 | 100.48 | 99.98 | 100.06 | ||
| SD | 0.8422 | 0.1743 | 0.2575 | 0.1414 | 0.1685 | 0.1202 | ||
| CV | 0.8414 | 0.1739 | 0.2572 | 0.1407 | 0.1682 | 0.1201 | ||
| SE | 0.3439 | 0.0712 | 0.1051 | 0.0577 | 0.0688 | 0.0491 | ||
*Average of six determinations, SD denotes standard deviation and CV denotes coefÞ cient of variation.
Table 1: Analysis of tablet formulation
The methods discussed in the present work provide a convenient and accurate way for simultaneous analysis of AMLO and OLME. Percent label claim for AMLO and OLME in tablet, by all the methods, was found in the range of 98.57 to 101.56 %. Standard deviation and coefÞ cient of variance for six determinations of tablet sample, by these methods, was found to be less than ±2.0 indicating the precision of the methods. Accuracy of proposed methods was ascertained by recovery studies and the results are expressed as % recovery. Percent recovery for AMLO and OLME, by all three methods, was found in the range of 99.01 to 100.97%, values of standard deviation and coefÞ cient of variation were in the range of ±0.1202 to ±0.8422 and 0.1201 to 0.8414, respectively indicating the accuracy of both the methods. Based on the results obtained, it is found that the proposed methods are accurate, precise, reproducible and economical and can be employed for routine quality control of AMLO and OLME in combined dose tablet formulation.
Acknowledgements
The authors thank Dr. Avinash D. Deshpande, Director of Pharmacy, Pad. Dr. D. Y. Patil Institute of Pharmaceutical Sciences and Research, Pimpri, Pune for providing necessary facilities. The authors also thank Glennmark Pharmaceuticals Ltd, Nashik and Macleods Pharmaceuticals Ltd, for providing gift samples of drugs AMLO and OLME.
References
- British Pharmacopoeia, Vol. 1, London: Her Majesty's Stationary OfÞce; 2008. p. 137.
- Budavari S, editor. The Merck Index: An encyclopedia of chemicals, drugs and biologicals, 13th ed. Whitehouse Station, NJ: Merck and Co. Inc; 2001. p. 86.
- Hoffman BB. Therapy of Hypertension. In: Brunton LL, Lazo JS, Parker KL, editors. Pharmacological Basis of Therapeutics. 11th ed. New York: McGraw-Hill Medical; 2006. p. 845-68.
- Sweetman SC, editor. Martindale The complete drug reference, 33rd ed. London: Pharmaceutical Press; 2002. p. 862.
- Sellin L, Stegbauer J, Laeis P, Rump LC. Adding hydrochlorothiazide to olmesartan dose dependently improves 24-h blood pressure and response rates in mild-to-moderate hypertension. J Hypertens 2005;23:2083-92.
- Vora DN, Kadav AA. Development and validation of a simultaneous HPLC method for estimation of bisoprololfumarate and amlodipine besylate from tablets. Indian J Pharm Sci 2008;70:542-6.
- Chitlange SS, Imran M, Sakarkar DM. RP-HPLC method for simultaneous estimation of amlodipine and metoprolol in tablet formulation. Asian J Pharm Sci 2008;2:232-4.
- Mishra P, Gupta A, Shah K. Simultaneous estimation of atorvastatin calcium and amlodipine besylate from tablets, Indian J Pharm Sci 2007;69:831-3.
- Sahu R, Patel VB. Simultaneous spectrophotometric determination of amlodipine besylate and atorvastatin calcium in binary mixture. Indian J Pharm Sci 2007;69:110-1.
- Naidu KR, Kale UN, Shingare MS. Stability indicating RP-HPLC method for simultaneous determination of amlodipine and benzapril hydrochloride from their combination drug product. J Pharm Biomed Anal 2005;39:147-55.
- Gohil K, Trivedi P, Molvi KI, Spectrophotometric analysis of amlodipine besylate in bulk and in tablet dosage forms. Indian J PharmaSci 2005;67:376-8.
- Topale PR, Gaikwad NJ, Tajane MR. Simultaneous UV-Spectrophotometric estimation of losartan potassium and amlodipine in tablet. Indian Drugs 2003;40:119-21.
- Gawri N, Vaidhyalingam V, Santha A. HPTLC method for the simultaneous estimation of amlodipine besylate and benazepril HCl tablets. Indian Drugs 2003;40:645-8.
- Rao JR, Kadam SS, Mahadik KR. Reverse phase HPLC determination of amlodipine and benazepril HCl in tablets. Indian Drugs 2002;39:378-81.
- Dhake AS, Kasture VS, Syed MR. Spectrophotometric methods for estimation of Amlodipine besylate and Benidipine hydrochloride from tablets. Indian Drugs 2002;39:14-7.
- Patel CV, Khandhar AP, Captain AD, Patel KT. Validated Absorption Factor Spectrophotometric and Reverse-Phase High Performance Liquid Chromatography Methods for the Determination of Ramipril and OlmesartanMedoxomil in Pharmaceutical Formulations. Eurasian J Anal Chem 2007;2:159-71.
- Shah NJ, Suhagia BN, Shah RR, Patel NM. Development and validation of a simultaneous HPTLC method for the estimation of olmesartanmedoxomil and hydrochlorothiazide in tablet dosage form. Indian J Pharm Sci 2007;69:834-36.