- *Corresponding Author:
- S. B. Bari
Department of Pharmaceutical Chemistry, R. C. Patel College of Pharmacy, Shirpur - 425 405, India
E-mail: sbbari@rediffmail.com
| Date of Submission | 31 March 2006 |
| Date of Revision | 27 April 2007 |
| Date of Acceptance | 8 December 2007 |
| Indian J. Pharm. Sci., 2007, 69 (6): 812-814 |
Abstract
Two new, simple, accurate and economical spectrophotometric methods have been developed for simultaneous estimation of drotaverine hydrochloride and mefenamic acid in two-component tablet formulation. The methods employed are, first derivative spectrophotometry, using zero crossing technique and multicomponent analysis. Both the drugs obey the Beer's law in the concentration range employed for these methods. The results of analysis are validated by statistical evaluation and recovery studies.
Keywords
Drotaverine hydrochloride is an analog of papaver and is used to reduce excessive labour pain [1]. Mefenamic acid is a benzoic acid derivative and is used as analgesic, antiinflammatory [2]. Chemically drotaverine hydrochloride (DH), is 1-[(3,4-diethox yphenyl)methylene]-6,7-diethoxy-1,2,3,4-tetrahydro isoquinoline [3] and mefenamic acid (MA), is 2-[(2,3- dimethylphenyl)amino] benzoic acid [3]. DH is official in Pharmacopoeia of Poland3; MA is official in IP [4], BP [5], and USP [6]. Literature survey revealed that HPLC method is reported for estimation of DH from human plasma [7] and spectrophotometric [4], HPLC [8] and HPTLC [9] methods have been reported for the estimation of MA. A combination of DH and MA is used to treat excessive labour pain [10].
Simultaneous analysis of DH and MA, using derivative spectrophotometric method and multicomponent method has been developed in the present investigation. Instrument involved is UV/Vis double beam spectrophotometer; model Shimadzu UV- 1601 with spectral bandwidth of 2 nm and wavelength accuracy of 0.5 nm with automatic wavelength correction and a pair of 10 mm matched quartz cells. Gift samples of DH and MA were obtained from Blue Cross Pharmaceuticals Ltd., Nashik. Standard stock solutions of 100 µg/ml were prepared by dissolving 10 mg of each in 100 ml of methanol (E. Merck).
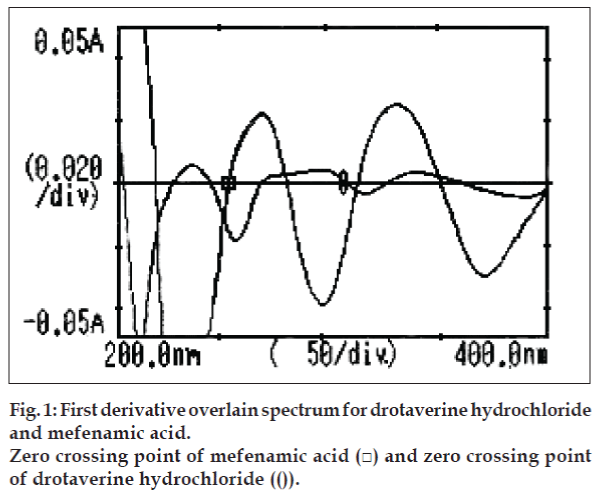
Derivative spectroscopy [11] offers, a useful approach for the analysis of drugs in multi-component mixtures. In the present work, derivative method employs zero-crossing wavelengths of MA and DH at 252.5 and 308 nm, respectively. Calibration curves were plotted between amplitudes observed at 1st order (key No. 2), for both the drugs at both the wavelengths against the concentration, in the range of 4-32 µg/ml. Estimation of these drugs was done by solving the regression equations, y= 0.0021x+(-0.0001)…. (1), y= 0.0006x+(- 0.0001)…. (2).
For multi-component method six mixed standards and two sampling wavelengths, as 279 and 308 nm were satisfactory to serve the purpose of experimentation. Six mixed standard solutions of DH and MA were prepared in the concentration ratio of 1:3.125. Concentrations were estimated by the multi-component mode.
Twenty tablets of Brand I (Detrim, DWD Pharmaceuticals Ltd, Mumbai, label claim 250 mg of MA and 80 mg of DH) and Brand II (Drota- M, Emcure Pharmaceuticals Ltd., Pune, label claim 250 mg of MA and 80 mg of DH) were weighed, average weight determined and finely powdered. An accurately weighed powdered sample, equivalent to average weight of one tablet was transferred to a beaker, dissolved in methanol, filtered through Whatmann filter paper No. 1 into 100 ml volumetric flask and the volume was made up to the mark with same solvent. Necessary dilutions were made with methanol to give final concentration of 25 µg/ml of MA (plus, 08 µg/ ml of DH). The absorbances were recorded at 252.5, 279 and 308 nm and concentrations were obtained by solving equation for calibration curves.
The 1st order overlain spectra of both drugs showed the wavelengths of zero crossing as 252.5 and 308 nm for MA and DH, respectively (fig. 1). Absorbances were determined at both the wavelengths. Calibration curves were plotted and regression analysis was carried out. Both these drugs obeyed linearity individually and in mixture within the concentration range of 4-32 µg/ml with correlation coefficient (r2< 1). Concentrations were calculated by solving Eqns. 1 and 2.
Analysis of both the brands was performed under multi-component mode of the instrument. For quantitative estimation, absorbances were measured at λmax of both the drugs viz. 279 and 308 nm respectively for MA and DH. The assay values for tablets, by both the methods were in the range of 99.06-99.12 % and 99.15-99.30 % for DH and MA, respectively. The results obtained, were comparable with the corresponding labeled amounts (Table 1). By observing the validation parameters [12] accuracy, precision, ruggedness, specificity, linearity (correlation coefficient, r2 <1) and range, both the methods were found to be specific, accurate, precise and reproducible.
| Brand | Parameter | % Label claim | % Recovery | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Method 1 | Method 2 | Method 1 | Method 2 | |||||||
| DH | MA | DH | MA | DH | MA | DH | MA | |||
| I | Mean | 99.06 | 99.15 | 99.08 | 99.21 | 99.27 | 99.72 | 99.19 | 99.57 | |
| SD | 0.18 | 0.08 | 0.14 | 0.09 | 0.30 | 0.26 | 0.23 | 0.28 | ||
| RSD | 0.002 | 0.001 | 0.001 | 0.001 | 0.003 | 0.003 | 0.002 | 0.003 | ||
| II | Mean | 99.12 | 99.30 | 99.12 | 99.18 | 98.45 | 99.24 | 98.81 | 99.20 | |
| SD | 0.13 | 0.11 | 0.15 | 0.12 | 0.45 | 0.30 | 0.17 | 0.21 | ||
| RSD | 0.001 | 0.001 | 0.002 | 0.001 | 0.005 | 0.003 | 0.002 | 0.002 | ||
Method 1 is the derivative spectrophotometric method while method 2 is multi-component mode method. Values for recovery are mean of nine estimations at three concentration levels, SD is standard deviation and RSD is relative standard deviation.
Table 1: Assay results of drotaverine hydrochloride and mefenamic acid in commercial formulations by method 1 and 2.
Acknowledgements
Authors are thankful to Blue Cross Pharmaceuticals Ltd., Nashik for the generous gift samples of drotaverine hydrochloride and mefenamic acid and also thankful to R.C. Patel College of Pharmacy, Shirpur for providing the instrumental facilities to carry out research work.
References
- Singh KC, Jain P, Goel N, Saxena A. Drotaverine hydrochloride for augmentation of labor. Inter J GynecoObst 2004;84:17-22.
- Budavari S, editor. The Merck Index. Whitehouse Station, NJ: Merck and Co. Inc; 2004.
- Sweetman SC, editor. Martindale: The complete drug reference. London: Pharmaceutical Press; 2002.
- The Indian Pharmacopoeia. New Delhi: The Controller of Publication; 1996. p. 459.
- The British Pharmacopoeia. London: HMSO Publication Center; 2002. p.1105.
- The United State Pharmacopoeia, XXIV, National Formulary, XX, Rockville MD: The US Pharmaceutical Convention, Inc; 2002. p. 1064.
- Bolaji OO, Onyeji CO, Ogungbamila FO, Ogunbona FA. High-performance liquid chromatographic method for the determination of drotaverine in human plasma and urine. J Chromatogr 1993;622:93-7.
- Lunn G. HPLC methods for pharmaceutical analysis. New York: John Wiley and Sons, Inc; 2000.
- Sethi PD. Quantitative analysis of drugs in pharmaceutical preparations. 3rd ed. New Delhi: CBS Publishers; 1997.
- Drug Index. New Delhi: Passi Publications; 2005. p. 286.
- Davidson AG. The basis of spectrophotometry. In: Beckett AH, Stenlake JB, editors. Practical pharmaceutical chemistry, 4th ed. New Delhi: CBS Publisher; 1997. p. 296-300.
- ICH guidelines, validation of analytical procedure: Methodology Q2B, Geneva: ICH Harmonized Tripartite Convention; 1996. p. 1-12.