- *Corresponding Author:
- Le Zhou
Department of Cardiology, Shibei Hospital of Jingan District, Jingan, Shanghai 200435, China
E-mail: 18018501521@163.com
| Date of Received | 22 December 2022 |
| Date of Revision | 10 July 2023 |
| Date of Acceptance | 15 November 2023 |
| Indian J Pharm Sci 2023;85(6):1725-1730 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To probe the action of shikonin on cardiomyocyte damage triggered by high glucose and its possible mechanism. High glucose was used to induce rat cardiomyocyte H9C2 to establish a cell injury model, and different doses of shikonin were used to treat H9C2 cells. Flow cytometry tested cell apoptosis. The enzyme-labeled method and water-soluble tetrazolium salt method were used to detect superoxide dismutase and malondialdehyde content, respectively. Quantitative reverse transcription polymerase chain reaction assayed circ_0000491 levels. After shikonin treatment, the apoptosis rate of H9C2 cells, levels of malondialdehyde in cells, and circ_0000491 contents were declined, and superoxide dismutase content was increased in a dose-dependent manner. After transfection with si-circ_0000491, the apoptosis rate and malondialdehyde levels were suppressed, but superoxide dismutase content was increased in H9C2 cells. Transfection of plasmid cloning deoxyribonucleic acid-circ_0000491 could attenuate these inhibitory effects of shikonin on high glucose-induced H9C2 cell apoptosis and oxidative stress. Shikonin could inhibit cell apoptosis and oxidative stress by down-regulating circ_0000491, thereby improving high glucose-induced cardiomyocyte damage.
Keywords
Rat cardiomyocyte H9C2, shikonin, circ_0000491, apoptosis, oxidative stress
Diabetes is a metabolic disease and can lead to hyperglycemia, which is able to damage heart tissue and then because diabetes cardiomyopathy, moreover, sustained High Glucose (HG) levels in the circulatory system can promote reactive oxygen species generation, proinflammatory factors release, then evoke myocardial damage[1,2]. At present, there is no effective treatment for diabetes cardiomyopathy.
Currently, it has been revealed that active ingredients of Traditional Chinese Medicine (TCM) have antioxidant and anti-inflammatory activities, and can slow down the development process of diabetes cardiomyopathy[3,4]. Shikonin is a Chinese herbal medicine, and can exert antioxidant action by clearing oxygen free radicals[5]. Shikonin could suppress acetaminophen-evoked acute liver injury via reducing inflammatory reaction and oxidative damages[6]. However, the therapeutic effect of shikonin on diabetic cardiomyopathy and its possible mechanism are unknown. Circular Ribonucleic Acids (circRNA) are one of non- coding RNA molecules formed by covalent bonding that lacks the 5' and 3' ends. Research has shown that circ_0000491 was elevated in diabetes nephropathy and could promote glomerular mesangial cell damage[7]. Whereas, the effects of circ_0000491 diabetes cardiomyopathy and whether circ_0000491 can be used as a potential target of shikonin in the treatment of diabetes cardiomyopathy have not been clarified.
Therefore, this study established a cell injury model in rat H9C2 cardiomyocytes that was induced by HG to investigate whether shikonin could regulate circ_0000491 expression to control HG-induced myocardial cell damage.
Materials and Methods
Materials and reagents:
Shikonin (Yili Naique Biotechnology Co., Ltd., purity ≥98 %); H9C2 cells (Shanghai Tongpai Biotechnology Co., Ltd); Dulbecco's Modified Eagle Medium (DMEM), Fetal Bovine Serum (FBS), trypsin, and cell apoptosis detection kit (Beyotime, Beijing, China); Lipofectamine 2000 and Trizol reagent (Invitrogen, Camarillo, California, United States of America (USA)); Superoxide Dismutase (SOD) and Malondialdehyde (MDA) detection kits (Nanjing Jiancheng Biotechnology Research Institute); the reverse transcription kit and SYBR Green kit (Beijing Tiangen Biochemical Technology Co., Ltd); si-NC, si-circ_0000491, plasmid cloning Deoxyribonucleic Acid (pcDNA), and pcDNA-circ_0000491 (Geenseed, Guangzhou, China); caspase-3, caspase-9, B-Cell Lymphoma 2 (Bcl-2), Bcl-2 Associated X (BAX), Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH), and Horseradish Peroxidase (HRP)- labeled Immunoglobulin G (IgG) antibodies (Abcam, Cambridge, Massachusetts, USA).
Experimental grouping:
H9C2 cells were inoculated onto a 6-well plate (1×105 cells/well) and incubated for 24 h in DMEM with 5.5 mmol/l glucose, namely NG group. Cell incubated for 24 h with 33 mmol/l glucose in DMEM were named as HG group. Cells in HG group were treated with 2.5 μmol/l, 5 μmol/l or 10 μmol/l for 24 h, namely HG+low shikonin, HG+medium shikonin, and HG+high shikonin groups. The si- NC or si-circ_0000491 were transfected into H9C2 cells using Lipofectamine 2000, and then incubated in DMEM medium with HG for 24 h, named as HG+si-NC or HG+si-circ_0000491 group. In addition, pcDNA and pcDNA-circ_0000491 were also transfected into H9C2 cells, then treated with shikonin 10 μmol/l, followed by incubating with HG, named as HG+shikonin+pcDNA group or HG+shikonin+pcDNA-circ_0000491.
Flow cytometry:
H9C2 cells were added with 500 μl binding buffer containing Annexin V-Fluorescein Isothiocyanate (FITC) (5 μl) and Propidium Iodide (PI) (5 μl) and incubated avoiding light for 10 min. Then flow cytometry was adopted to detect cell apoptosis rate.
Detection of SOD activity and MDA level:
Digest H9C2 cells in each group with 0.25 % trypsin, then the levels of intracellular MDA and SOD activity were assayed using enzyme-linked assay and Water-Soluble Tetrazolium Salt (WST-1) assay strictly following the instructions of the reagent kits.
Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR):
The total RNA of H9C2 cells was extracted using Trizol, and the purity of RNA were detected using NanoDropTM 2000c spectrophotometer. The Optical Density (OD) 260/OD280 range of RNA was between 1.8 and 2.0. Then complementary DNA (cDNA) generation by reverse transcription and qRT-PCR reaction were performed. qRT-PCR reaction conditions; 95° 2 min, 95° denaturation 15 s, 60° 30 s, 72° 30 s, a total of 40 cycles. The normalization control we used was GAPDH, and levels of circ_0000491 were assayed by 2-ΔΔCt method.
Western blot:
Following measuring the concentration of isolated protein by Radioimmunoprecipitation Assay (RIPA) lysis buffer in H9C2 cells, the protein samples (about 40 μg per lane) were subjected to 10 % separating gel. After shifting onto the Polyvinylidene Difluoride (PVDF) membranes, BAX (1:800), caspase-3 (1:1000), caspase-9 (1:800), Bcl-2 (1:800) and GAPDH (1:3000) diluents were applied to incubate with PVDF membrane all night at 4°. After secondary incubation with second antibody diluent (1:5000), Enhanced Chemiluminescence (ECL) solution was employed to observe the combined signals.
Statistical analysis:
The data were presented by (x? ±s). The comparison of the two groups or multiple groups was conducted using t-test or Analysis of Variance (ANOVA). p<0.05 indicated significant difference.
Results and Discussion
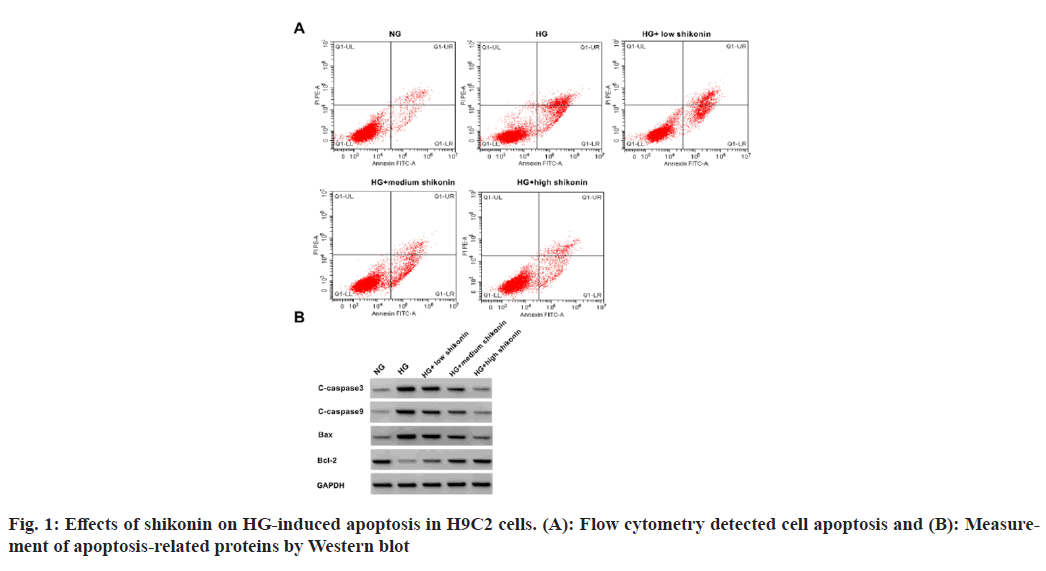
The HG group showed increase in cell apoptosis rate and proteins of BAX, caspase-3, and caspase-9, and a decrease in Bcl-2 protein level in H9C2 cells relative to NC group. In addition, compared with HG group, shikonin treatment dose-dependently suppressed H9C2 cell apoptosis, decreased BAX, caspase-3, and caspase-9 contents, and increased Bcl-2 content (fig. 1 and Table 1).
| Group | Apoptosis rate/% | Caspase-3 | Caspase-9 | BAX | Bcl-2 |
|---|---|---|---|---|---|
| NG | 6.80±0.31 | 0.22±0.02 | 0.13±0.02 | 0.22±0.03 | 0.80±0.07 |
| HG | 24.31±1.47* | 0.76±0.07* | 0.74±0.05* | 0.81±0.06* | 0.18±0.02* |
| HG+low shikonin | 21.16±1.07# | 0.56±0.05# | 0.52±0.05# | 0.66±0.04# | 0.36±0.06# |
| HG+medium shikonin | 17.71±0.97#& | 0.40±0.05#& | 0.38±0.04#& | 0.43±0.04#& | 0.60±0.05#& |
| HG+high shikonin | 12.42±0.83#&@ | 0.25±0.03#&@ | 0.21±0.02#&@ | 0.25±0.03#&@ | 0.75±0.06#&@ |
| F | 435.785 | 203.786 | 363.831 | 345.506 | 207.96 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Note: Relative to NG group, *p<0.05; relative to HG group, #p<0.05; relative to HG+low shikonin group, &p<0.05 and relative to HG+medium shikonin group, @p<0.05
Table 1: Effects of Shikonin on HG-Induced Apoptosis in H9C2 Cells
Compared with NG group, SOD activity in HG group was declined, while an elevated MDA level was observed in HG group; compared with HG group, SOD activity in HG+low shikonin group, HG+medium shikonin group and HG+high shikonin group was dose-dependently increased, while MDA level was dose-dependently decreased as shown in Table 2.
| Group | SOD/U·l-1 | MDA/nmol·ml-1 |
|---|---|---|
| NG | 341.04±29.01 | 116.84±8.94 |
| HG | 45.18±2.91* | 627.69±42.49* |
| HG+low shikonin | 98.46±7.49# | 529.39±25.74# |
| HG+medium shikonin | 165.21±13.00#& | 361.70±34.04#& |
| HG+high shikonin | 270.01±14.29#&@ | 193.74±17.89#&@ |
| F | 518.821 | 522.319 |
| p | 0.000 | 0.000 |
Note: Relative to NG group, *p<0.05; relative to HG group, #p<0.05; relative to HG+low shikonin group, &p<0.05 and relative to HG+medium shikonin group, @p<0.05
Table 2: Effects of Shikonin on SOD and MDA Levels in H9C2 Cells Treated by HG
Relative to NG group, levels of circ_0000491 in HG group was increased. Relative to HG group, levels of circ_0000491 in HG+low shikonin group, HG+medium shikonin group and HG+high shikonin group was dose-dependently decreased as shown in Table 3.
| Group | circ_0000491 |
|---|---|
| NG | 1.00±0.00 |
| HG | 3.48±0.13* |
| HG+low shikonin | 3.02±0.12# |
| HG+medium shikonin | 2.45±0.11#& |
| HG+high shikonin | 1.39±0.12#&@ |
| F | 865.394 |
| p | 0.000 |
Note: Relative to NG group, *p<0.05; relative to HG group, #p<0.05; relative to HG+low shikonin group, &p<0.05 and relative to HG+medium shikonin group, @p<0.05
Table 3: Effects of Shikonin on circ_0000491 Expression in HG-induced H9C2 Cells
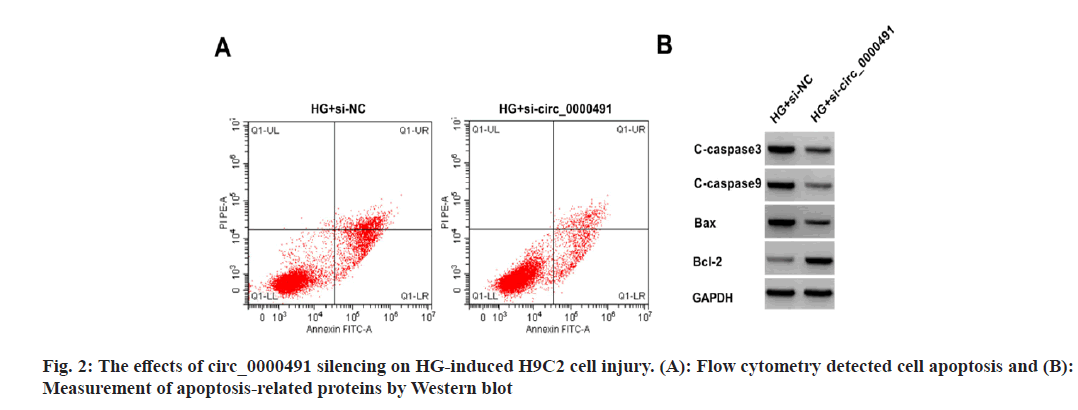
Relative to HG+si-NC group, the apoptosis rate and the levels of BAX, caspase-3 and caspase-9 protein in HG+si-circ_0000491 group were decreased, while Bcl-2 protein level was increased, moreover, SOD activity was increased and MDA content was decreased (fig. 2 and Table 4).
| Group | circ_0000491 | Apoptosis rate/% | Caspase-3 | Caspase-9 | BAX | Bcl-2 | SOD/U·L-1 | MDA/nmol·ml-1 |
|---|---|---|---|---|---|---|---|---|
| HG+si-NC | 1.00±0.00 | 24.19±1.34 | 0.75±0.07 | 0.76±0.06 | 0.82±0.06 | 0.19±0.03 | 47.36±5.04 | 607.26±42.03 |
| HG+si-circ_0000491 | 0.42±0.05* | 13.62±0.52* | 0.32±0.05* | 0.25±0.02* | 0.32±0.03* | 0.66±0.07* | 233.38±17.35* | 236.52±21.56* |
| t | 34.8 | 22.061 | 14.996 | 24.191 | 22.361 | 18.514 | 30.888 | 23.545 |
| P | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Note: Relative to HG+si-NC, *p<0.05
Table 4: The Effects of circ_0000491 Silencing on HG-induced H9C2 Cell Injury
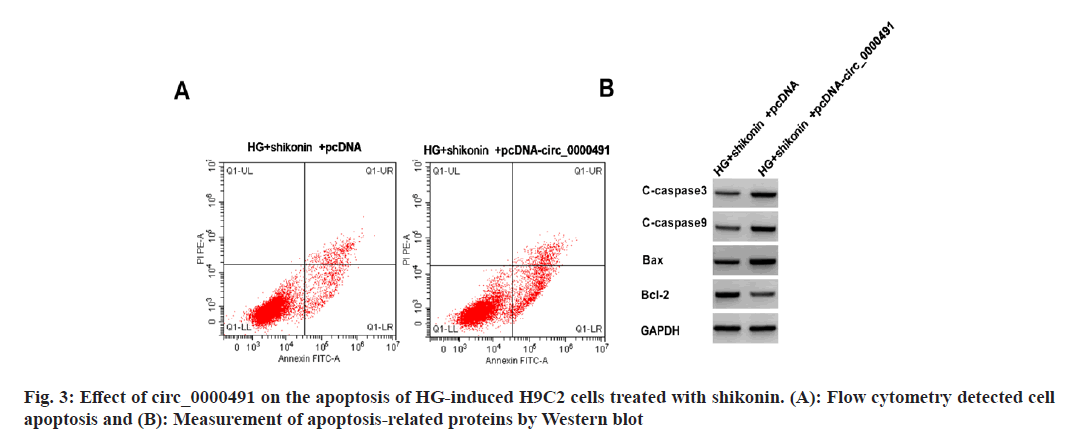
Compared with HG+shikonin+pcDNA group, cells in HG+shikonin+pcDNA-circ_0000491 group showed increased apoptosis rate and contents of BAX, caspase-3, and caspase-9 protein, as well as declined Bcl-2 content (fig. 3 and Table 5).
| Group | circ_0000491 | Apoptosis rate/% | Caspase-3 | Caspase-9 | BAX | Bcl-2 |
|---|---|---|---|---|---|---|
| HG+shikonin+pcDNA | 1.00±0.00 | 12.30±0.77 | 0.26±0.04 | 0.21±0.03 | 0.27±0.02 | 0.74±0.07 |
| HG+shikonin+pcDNA-circ_0000491 | 2.63±0.14* | 20.85±1.31* | 0.50±0.05* | 0.44±0.04* | 0.54±0.04* | 0.40±0.03* |
| t | 34.929 | 16.880 | 11.245 | 13.800 | 18.112 | 13.393 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Note: Relative to the HG+shikonin+pcDNA, *p<0.05
Table 5: Functions of circ_0000491 on the Apoptosis of HG-Induced H9C2 Cells Treated with Shikonin
Relative to HG+shikonin+pcDNA group, H9C2 cells in HG+shikonin+pcDNA-circ_0000491 group showed decreased SOD level and increased MDA level as shown in Table 6.
| Group | SOD/U·l-1 | MDA/nmol·ml-1 |
|---|---|---|
| HG+shikonin+pcDNA | 262.89±22.13 | 202.97±22.57 |
| HG+shikonin+pcDNA-circ_0000491 | 125.04±13.03* | 449.03±17.68* |
| t | 16.103 | 25.747 |
| p | 0.000 | 0.000 |
Note: Relative to HG+shikonin+pcDNA group, *p<0.05
Table 6: Effect of circ_0000491 on the Levels of MDA and SOD in HG-induced H9C2 Cells Treated with Shikonin
HG induced myocardial cell injury is a key process in the occurrence and progression of diabetic cardiomyopathy, sustained HG environment results in oxidative injury in myocardial cells, importantly, TCM has been revealed that can impair HG-evoked cardiomyocyte damage[8-10]. Some circRNAs have been discovered that can regulate the proliferation, apoptosis and other biological behaviors of cardiomyocytes, which may also be a potential drug for diabetes cardiomyopathy therapy[9,11]. However, whether circRNA can be used as a target of TCM in diabetes cardiomyopathy therapy has not been clarified.
Shikonin is a type of TCM, it was found to reduce the death and oxidative injury of cardiomyocyte induced by hypoxia/reoxygenation, and promote cell viability[12]. Guo et al.[13] showed that shikonin treatment improved the cardiac function and survival rate, and repress inflammatory reaction in the mouse model with cardiac dysfunction. In addition, shikonin could recover cardiac function, reduce myocardial infarction, and inhibit autophagy in rats with myocardial ischemia/ reperfusion damage[14]. Here, we speculated shikonin might also have protective effects on HG-induced myocardial cell. In our work, we found HG evoked cardiomyocyte apoptosis and oxidative stress by increasing MDA level and reducing SOD activity, indicating the successful establishment of a cardiomyocyte injury model. Then we found shikonin could dose-dependently reduce HG-induced apoptosis in cardiomyocytes, moreover, shikonin also could reduce MDA levels and promote SOD activity in HG-treated cardiomyocytes in a concentration dependent manner, implying the anti-oxidative stress action of shikonin in cardiomyocytes.
Circ_0000491 was elevated in HG-induced mouse mesangial cells, and circ_000049 deletion could reversed HG-evoked apoptotic, inflammatory, and oxidative damages as well as fibrosis in cells[15]. Here, the results of this study showed that HG elevated circ_000049 levels in cardiomyocytes, which were reduced by shikonin treatment in cells, suggesting that shikonin might inhibit circ_0000491 expression to slow down the development of diabetes cardiomyopathy. Meanwhile, we discovered that circ_0000491 deficiency restrained HG-evoked cardiomyocyte apoptotic and oxidative damages. Moreover, circ_0000491 overexpression abolished the protective effects of shikonin on HG-treated cardiomyocytes, hinting that shikonin inhibited circ_0000491 expression to ameliorate HG-evoked cardiomyocyte damage.
In summary, shikonin could reduce HG-evoked cardiomyocyte apoptosis and oxidative stress, and its mechanism of action was related to the inhibition of circ_0000491 expression, which laid an experimental foundation for further research on the protective effect of shikonin on diabetes cardiomyopathy and for determination of the molecular mechanism of cardiomyocyte damage caused by hyperglycemia. Nevertheless, further experiments in animals are needed to investigate the molecular mechanism of shikonin on HG- induced myocardial injury.
Author’s contributions:
Le Zhou and Xiaohong Geng have contributed equally to this work.
Conflict of interests:
The authors declared no conflict of interests.
References
- Lv J, Ji M, Yang H, Wang C, Zhang L, Ni H. Rutaecarpine attenuates high glucose-induced damage in AC16 cardiomyocytes by suppressing the MAPK pathway. J Appl Toxicol 2023;43(9):1306-18.
[Crossref] [Google Scholar] [PubMed]
- Cheng G, Li L. High-glucose-induced apoptosis, ROS production and pro-inflammatory response in cardiomyocytes is attenuated by metformin treatment via PP2A activation. J Biosci 2020;45:126.
[Google Scholar] [PubMed]
- Wu D, Zhang Q, Yu Y, Zhang Y, Zhang M, Liu Q, et al. Oleanolic acid, a novel endothelin a receptor antagonist, alleviated high glucose-induced cardiomyocytes injury. Am J Chin Med 2018;46(6):1187-201.
[Crossref] [Google Scholar] [PubMed]
- Jiang Z, Fu L, Xu Y, Hu X, Yang H, Zhang Y, et al. Cyclovirobuxine D protects against diabetic cardiomyopathy by activating Nrf2-mediated antioxidant responses. Sci Rep 2020;10(1):6427.
- Sun Q, Gong T, Liu M, Ren S, Yang H, Zeng S, et al. Shikonin, a naphthalene ingredient: Therapeutic actions, pharmacokinetics, toxicology, clinical trials and pharmaceutical researches. Phytomedicine 2022;94:153805.
[Crossref] [Google Scholar] [PubMed]
- Guo H, Sun J, Li D, Hu Y, Yu X, Hua H, et al. Shikonin attenuates acetaminophen-induced acute liver injury via inhibition of oxidative stress and inflammation. Biomed Pharmacother 2019;112:108704.
[Crossref] [Google Scholar] [PubMed]
- Mou X, Chenv JW, Zhou DY, Liu K, Chen LJ, Zhou D, et al. A novel identified circular RNA, circ_0000491, aggravates the extracellular matrix of diabetic nephropathy glomerular mesangial cells through suppressing miR-101b by targeting TGFβRI. Mol Med Rep 2020;22(5):3785-94.
- Ren BC, Zhang YF, Liu SS, Cheng XJ, Yang X, Cui XG, et al. Curcumin alleviates oxidative stress and inhibits apoptosis in diabetic cardiomyopathy via Sirt1-Foxo1 and PI3K-Akt signalling pathways. J Cell Mol Med 2020;24(21):12355-67.
[Crossref] [Google Scholar] [PubMed]
- Wang J, Chen P, Cao Q, Wang W, Chang X. Traditional Chinese medicine ginseng Dingzhi decoction ameliorates myocardial fibrosis and high glucose-induced cardiomyocyte injury by regulating intestinal flora and mitochondrial dysfunction. Oxid Med Cell Longev 2022;2022:9205908.
[Crossref] [Google Scholar] [PubMed]
- Chang X, Zhang T, Wang J, Liu Y, Yan P, Meng Q, et al. SIRT5-related desuccinylation modification contributes to quercetin-induced protection against heart failure and high-glucose-prompted cardiomyocytes injured through regulation of mitochondrial quality surveillance. Oxid Med Cell Longev 2021;2021:5876841.
[Crossref] [Google Scholar] [PubMed]
- Yang F, Li A, Qin Y, Che H, Wang Y, Lv J, et al. A novel circular RNA mediates pyroptosis of diabetic cardiomyopathy by functioning as a competing endogenous RNA. Mol Ther Nucl Acids 2019;17:636-43.
[Crossref] [Google Scholar] [PubMed]
- Wang S, Zhu Y, Qiu R. Shikonin protects H9C2 cardiomyocytes against hypoxia/reoxygenation injury through activation of PI3K/Akt signaling pathway. Biomed Pharmacother 2018;104:712-7.
[Crossref] [Google Scholar] [PubMed]
- Guo T, Jiang ZB, Tong ZY, Zhou Y, Chai XP, Xiao XZ. Shikonin ameliorates LPS-induced cardiac dysfunction by SIRT1-dependent inhibition of NLRP3 inflammasome. Front Physiol 2020;11:570441.
[Crossref] [Google Scholar] [PubMed]
- Liu T, Ling C, Tian J, Ma F. Protective effect of shikonin in myocardial ischemia/reperfusion injury in rats by inhibition of autophagy through the Hippo pathway. Biochem Biophys Res Commun 2022;613:87-93.
[Crossref] [Google Scholar] [PubMed]
- Wang J, Yang S, Li W, Zhao M, Li K. circ_0000491 promotes apoptosis, inflammation, oxidative stress, and fibrosis in high glucose-induced mesangial cells by regulating miR-455-3p/Hmgb1 axis. Nephron 2022;146(1):72-83.
[Crossref] [Google Scholar] [PubMed]