- *Corresponding Author:
- Rongqi He
Department of Thoracic Surgery, Quanzhou First Hospital Affiliated to Fujian Medical University, Quanzhou, Fujian Province 362000, China
E-mail: Hrqfj@126.com
| Date of Received | 17 November 2022 |
| Date of Revision | 25 July 2023 |
| Date of Acceptance | 04 April 2024 |
| Indian J Pharm Sci 2024;86(2):484-493 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
MicroRNA-923 and microRNA-523 have been shown to be involved in various malignancies in addition to other biological processes. There is growing evidence that oxidative stress plays a crucial role in cancer. At present their role in esophageal squamous cell carcinoma is unclear. Reverse transcription polymerase chain reaction was used to detect the expressions of microRNA-923 and microRNA-523, enzyme-linked immunosorbent assay was used to detect oxidative stress-related factors, and Western blotting was used to detect the expressions of autophagy related proteins, endoplasmic reticulum stress-related proteins and apoptosis-related proteins. The effects of microRNA-923 and microRNA-523 on cell proliferation were detected by cell counting kit 8, and the formation of intracellular autophagosomes was analyzed by immunofluorescence. The results showed that the expression of microRNA-923 and microRNA-523 was significantly enhanced in esophageal squamous cell carcinoma serum exosomes. Light-chain 3 II, autophagy-related 5, autophagy related 12, nitric oxide and hydrogen peroxide expression was increased and total antioxidant capacity expression was decreased in esophageal squamous cell carcinoma tissues. Hydrogen peroxide induced oxidative stress promoted the expression of microRNA-923 and microRNA-523 as well as cell proliferation, apoptosis, autophagy and endoplasmic reticulum stress. MicroRNA-923 and microRNA-523 could also promote cellular autophagy and endoplasmic reticulum stress. Unexpectedly, microRNA-923 and microRNA-523 inhibited apoptosis, indicating that apoptosis was not involved in hydrogen peroxide induced microRNA-923 and microRNA-523 regulated cell proliferation. It was confirmed that hydrogen peroxide induced microRNA-923 and microRNA-523 mediated cell proliferation, autophagy and endoplasmic reticulum stress. Collectively, our study showed that oxidative stress induced microRNA-923 and microRNA-523 expression regulates cellular autophagy in esophageal squamous cell carcinoma. This will provide some clues to gain insight into the molecular and biological mechanisms of esophageal squamous cell carcinogenesis.
Keywords
Oxidative stress, autophagy, esophageal squamous cell carcinoma, microRNA-923, microRNA-523, exosomes
Esophageal cancer ranks as the 7th most common malignancy and the 6th leading cause of cancer-related death worldwide, with 604 000 new cases and 544 000 deaths in 2020[1]. Esophageal cancer is one of the most common malignancies of the digestive tract, with Esophageal Squamous Cell Carcinoma (ESCC) being the predominant pathological type of esophageal cancer worldwide, accounting for 90 % of all cases in China[2-4]. Despite advances in early diagnosis, surgery and radiotherapy, the prognosis of ESCC remains poor and remains a challenge[4-6]. Thereby, there is an urgent need to understand the occurrence of ESCC, which will support the development of diagnostic markers and new treatment strategies.
Oxidative stress as a key site of disease transformation can contribute to the progression of diseases and cancers by promoting phenomena such as oxidation of nucleic acids, proteins, lipids and other components, inflammatory responses and apoptosis through the increase of peroxides and free radicals[7-10]. In turn, Hydrogen peroxide (H2O2) can absorb large amounts of oxygen free radicals thereby inducing oxidative stress in cells, thus it is widely used as an apoptosis inducer[11]. Low concentrations of H2O2 function as signaling molecules to regulate signaling pathways at different levels thereby affecting cell division and growth[12,13]. Nevertheless, when H2O2 levels were excessive, the cell cycle would be blocked, which would further lead to apoptosis and autophagy[14,15]. The recent studies have shown that tumor oxidative stress can induce the Endoplasmic Reticulum Stress (ERS)[16]. In addition, the ERS can be inter-regulated with autophagy in several ways[17,18]. Therefore, research on the mechanisms of oxidative stress and related signaling pathways in cancer could help provide new strategies for cancer prevention and treatment.
It is well known that exosomes mediate communication across cells by transporting cell-derived proteins and nucleic acids, including various microRNA (miRNA)[19,20]. miRNAs are a class of small Ribonucleic Acid (RNA) of 18-25 nucleotides in length[21,22]. In part, miRNAs are dysregulated in response to oxidative stress[23,24]. These miRNAs respond to oxidative stress by regulating the expression of their target genes at the transcriptional and translational levels. The target genes can shift cells from proliferation to cellular arrest to prevent further cellular damage[25-27]. Studies have shown that abnormally high expression of miR-923 and miR-523 in breast cancer, liver cancer and melanoma is associated with poorer tumor prognosis[28-30]. Endoplasmic reticulum-based stress inducers induce miR-923 and miR-523 expression in human embryonic kidney HEK293 cells[31]. Currently, there are no relevant studies on the regulation of miR-923 and miR-523 expression by oxidative stress.
This study was to investigate the mechanism of oxidative stress on cellular autophagy in ESCC. In this study, we examined the levels of oxidative stress in the serum exosomes of ESCC patients and the expression of miR-923 and miR-523 in ESCC tissues. This analyzes the correlation between miR-923 and miR-523 expression, and oxidative stress in esophageal squamous carcinoma, and to elucidate the mechanism of miR-923 and miR-523 regulation of autophagy in esophageal squamous carcinoma cells.
Materials and Methods
Cell lines and culture:
ESCC cell line TE-13 was obtained from Zolgene Biotechnology Co., Ltd. (Fuzhou, China). TE-13 cells were cultured in Dulbecco's Modified Eagle Medium (DMEM)-high glucose medium (Gibco) containing 10 % Fetal Bovine Serum (FBS) (PAN biotech) at 37° in an incubator containing 5 % Carbon dioxide (CO2).
Transfection:
TE-13 cells were inoculated at 1×105 cells/well for 24 h in 24-well plates followed by transfection of miR-923 and miR-523 mimic inhibitors and their corresponding negative controls (NC-mimics, NC inhibitors) for 48 h with riboFECT CP Reagent (Ribo) according to the manufacturer’s instructions.
Treatment of ESCC cell with H2O2:
The TE-13 cells were inoculated in 96-well plates at 1×105 cells/well and incubated at 37° for 24 h in a 5 % CO2 incubator, followed by replacing the medium with fresh medium containing H2O2 so that the final concentrations of H2O2 were 0, 10, 50, 100, 200 and 400 µM, respectively, and assayed after 24 h of incubation. In order to establish a model of oxidative stress cells were treated with the appropriate concentration of H2O2 that had the best effect on cell viability. The experiments were performed in triplicate at least three times.
Reverse Transcription Polymerase Chain Reaction (RT-PCR):
We performed quantitative RT-PCR (qRT-PCR) to validate miR-923 and miR-523 expression in 80 serum exosome samples each from healthy individuals and ESCC patients. Serum exosomes were extracted by ultrafast centrifugation. To determine miR-923, miR-523, Light Chain 3 (LC3-II), Autophagy Related 5 (ATG5) and Autophagy Related 12 (ATG5) expressions in H2O2-induced TE-13 cells. Reverse transcription reactions were performed using Reverse Transcription System (Promega). Quantitative PCR amplification was performed using SYBR Green Real-time PCR Master Mix (Promega) and the reactions were incubated in an ABI 7500 PCR System (ABI, United States of America (USA)). Primers were as follows, miR-923 5’-CCAGGATTCCCTCAGTAATGG-3’ (forward) and 5’-AGTGCAGGGTCCGAGGTATT-3’ (reverse); miR-523 5’-CGCTCTAGAGGGAAGCGC-3’ (forward) and 5’-AGTGCAGGGTCCGAGGTATT-3’ (reverse); U6 5’-CTCGCTTCGGCAGCACA-3’ (forward) and 5’-AACGCTTCACGAATTTGCGT-3’ (reverse); Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH) 5’-AGAAGGCTGGGGCTCATTTG-3’ (forward) and 5’-AGGGGCCATCCACAGTCTTC-3’ (reverse); LC3-II 5’-GCGAGTTACCTCCCGCAG-3’ (forward) and 5’-TCATGTTGACATGGTCCGGG-3’ (reverse); ATG5 5’-GAGTAGTTGCCTGGAGGAGC-3’ (forward) and 5’-CCACTGCAGAGGTGTTTCCA-3’ (reverse); ATG12 5’-TGCTGGAGGGGAAGGACTTA-3’ (forward) and 5’-CACGCCTGAGACTTGCAGTA-3’ (reverse). The data were quantitatively analyzed by 2-ΔΔCt method.
Western blot:
Total cellular proteins were extracted by adding protein lysate containing Phenylmethylsulfonyl Fluoride (PMSF) (P8340, Solarbio) after cell collection precipitation, and proteins were separated on 4 % Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis (SDS/PAGE) gels and transferred to 8.5×6.5 cm nitrocellulose membranes (BioTrace, Pall, USA). The transferred NC membranes were closed with 5 % Bovine Serum Albumin (BSA) for 2 h and then incubated overnight at 4° with a specific primary antibody shaker to further incubate the blots with Horseradish Peroxidase (HRP)-conjugated secondary antibodies and visualize them using a chemiluminescence instrument (JS-2012, Shanghai Peiqing Technology Co., Ltd.). Antibody information is as follows; anti-beclin 1 antibody (ab210498, Abcam), anti-LC3B antibody (ab192890, Abcam), anti-ATG5 antibody (ab2108327, Abcam), anti-ATG12 antibody (abab109491, Abcam), Inositol-Requiring Enzyme 1 (IRE1); Endoplasmic Reticulum to Nucleus Signalling 1 (ERN1) polyclonal antibody (27528-1-AP, Proteintech), anti-ATF6 (ab227830, Abcam) and antibody anti-Protein Kinase-Like Endoplasmic Reticulum Kinase (PERK) antibody (ab229912, Abcam).
Enzyme-Linked Immunosorbent Assay (ELISA):
The caspase-3, caspase-8 and caspase-9 ELISA kits (Solarbio, USA, Cat: KHC4021 and Cat: BC3830, BC3850 and BC3890) were used according to the manufacturer’s guidelines to measure the TE-13 cells produced caspase-3, caspase-8 and caspase-9 concentrations.
Immunohistochemical analysis:
To begin with, paraffin sections were dewaxed and hydrated then rinsed with Phosphate Buffer Solution (PBS) followed by a peroxidase blocking agent to block endogenous peroxidase activity. Anti-LC3B antibody was added and incubated overnight at 4° followed by the addition of HRP enzyme-labeled secondary antibody for 30 min at 37°. After 3 washes in PBS, SABC HRP-streptavidin, 1:200-400 PBS dilution) was added and incubated for 30 min at 37°. After incubation, 3,3' Diaminobenzidine (DAB) was added dropwise for color development, hematoxylin was re-stained, and the slices were routinely dehydrated and sealed before microscopic examination and photography.
Total Antioxidant Capacity (TAC), H2O2 and Nitric Oxide (NO) detection:
TAC, H2O2 and NO in sera of healthy individuals and ESCC were assayed according to the TAC assay kit (Solarbio), H2O2 content assay kit (Solarbio) and H2O2 content assay kit (Solarbio). The ratio of 0.9 ml of reagent I was added to each 100 μl of serum and mixed thoroughly; the supernatant was centrifuged at 8000 g for 10 min at 4°, and the supernatant was extracted and assayed using an enzyme marker (DNM-9602, Beijing Plantronix Technology Co., Ltd.,).
Statistical analysis:
All experimental data were analyzed using Statistical Package for the Social Sciences (SPSS) 20.0 statistical software. For normally distributed data, t-test was used for two-by-two comparisons and paired t-test for paired data, one-way Analysis of Variance (ANOVA) was used for comparison of multiple groups of data. Data did not obey normal distribution, and non-parametric tests were used Spearman's analysis of correlation. p<0.05 indicates a statistically significant difference.
Results and Discussion
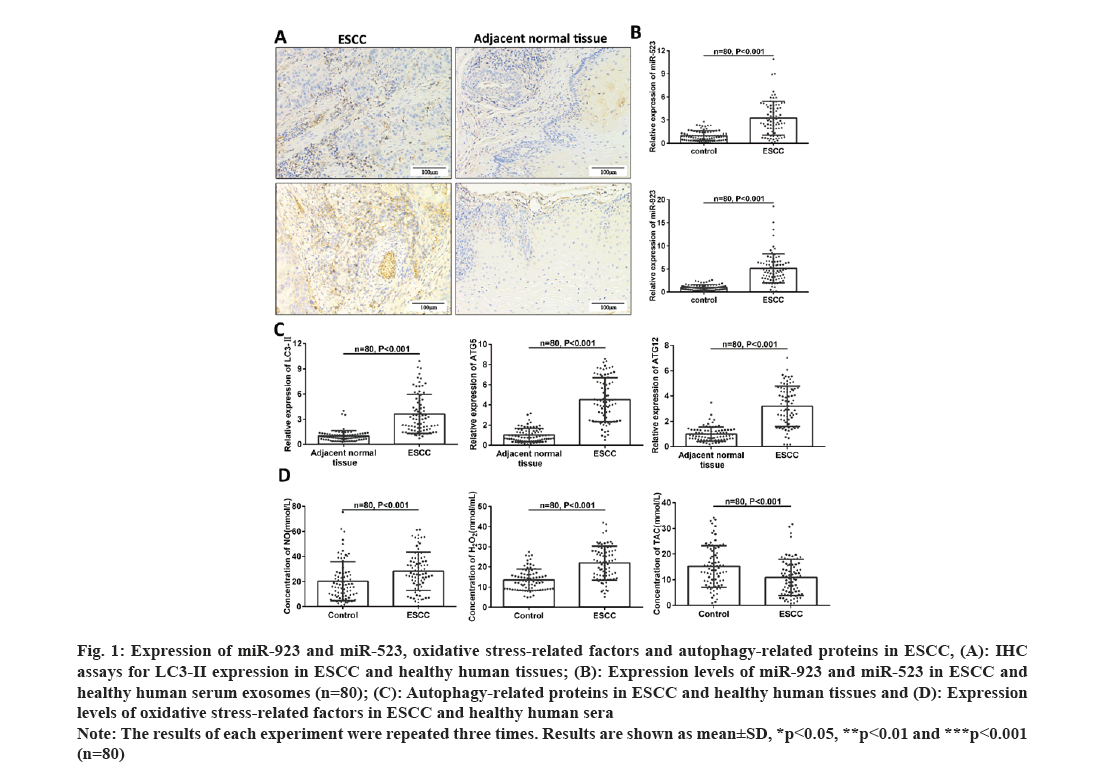
To investigate the expression of miR-923 and miR-523, oxidative stress and cellular autophagy levels in clinical samples into ESCC, we verified the expression of miR-523 and miR-932 in exosomes which were found to be significantly increased in the serum of patients with ESCC (fig. 1A and fig. 1B). The levels of oxidative stress (NO, H2O2 and TAC) in serum were examined in 80 pairs of healthy individuals and ESCC patients. As shown in fig. 1C, NO and H2O2 levels were significantly higher and TAC expression was significantly lower in serum of ESCC patients (fig. 1D). Since H2O2 could elicit cell damage thereby inducing cell death, to investigate whether oxidative stress induced cellular autophagy in esophageal cancer. We examined the expression levels of cellular autophagy-associated proteins LC3-II, ATG5 and ATG12 in tissue samples by IHC and RT-PCR. The results showed that the expression of LC3-II, ATG5 and ATG12 showed a significant increase in cancer patients (fig. 1A-fig. 1C). Above results suggest that miR-923 and miR-523 are abnormally highly expressed, oxidative stress and cellular autophagy levels increased in ESCC, yet whether there is an interrelationship between the three needs to be further investigated.
Fig. 1: Expression of miR-923 and miR-523, oxidative stress-related factors and autophagy-related proteins in ESCC, (A): IHC
assays for LC3-II expression in ESCC and healthy human tissues; (B): Expression levels of miR-923 and miR-523 in ESCC and
healthy human serum exosomes (n=80); (C): Autophagy-related proteins in ESCC and healthy human tissues and (D): Expression
levels of oxidative stress-related factors in ESCC and healthy human sera.
Note: The results of each experiment were repeated three times. Results are shown as mean±SD, *p<0.05, **p<0.01 and ***p<0.001
(n=80)
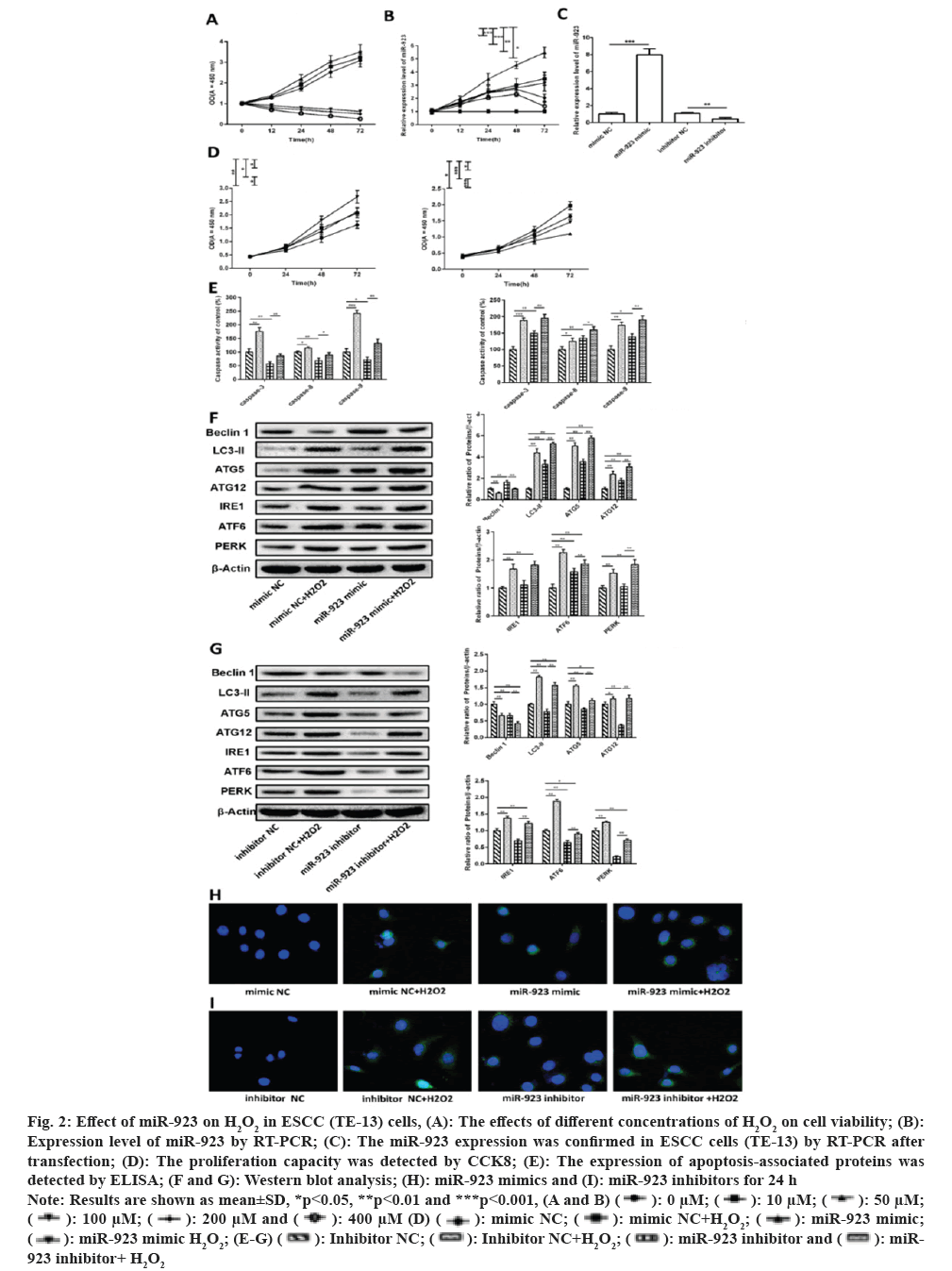
To investigate the further mechanism of action of oxidative stress on ESCC cells, we tested the effect of increased oxidative stress on ESCC cell viability by incubating the cells in different concentrations of H2O2 (0, 10, 50, 100, 200 and 400 µM). It was shown that cell viability was altered in a dose and time dependent manner in all groups (fig. 2A). As in the control group, cell viability increased with time in the 10 and 50 µM H2O2 groups. In contrast, in the group with higher H2O2 concentrations (100, 200 and 400 µM), cell viability was significantly reduced compared to cells treated with control and low concentrations of H2O2 (10 and 50 µM; p<0.05). In the high concentration H2O2-treated group, cell viability was consistently reduced for 72 h after H2O2 treatment, indicating that cell growth or apoptosis was affected by high oxidative stress. This indicates that oxidative stress induces cell damage thereby affecting cell viability.
Fig. 2: Effect of miR-923 on H2O2 in ESCC (TE-13) cells, (A): The effects of different concentrations of H2O2 on cell viability; (B):
Expression level of miR-923 by RT-PCR; (C): The miR-923 expression was confirmed in ESCC cells (TE-13) by RT-PCR after
transfection; (D): The proliferation capacity was detected by CCK8; (E): The expression of apoptosis-associated proteins was
detected by ELISA; (F and G): Western blot analysis; (H): miR-923 mimics and (I): miR-923 inhibitors for 24 h.
Note: Results are shown as mean±SD, *p<0.05, **p<0.01 and ***p<0.001, (A and B) 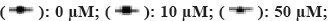
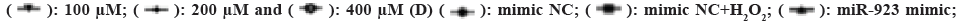
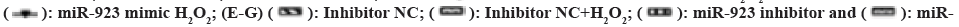 923 inhibitor+ H2O2.
923 inhibitor+ H2O2.
To explore whether oxidative stress regulates miR-923 expression, miR-923 expression levels in cells were examined after treatment with different concentrations of H2O2. In fig. 2B, miR-923 expression was significant elevated after H2O2 treatment of cells, where 50 µM H2O2-treated miR-923 was consistently elevated for 72 h after treatment, while miR-923 expression decreased to normal level after 72 h after high concentration of H2O2 treatment.
As above indicated that oxidative stress induces miR-923 expression, and then we further explore the impact of miR-923 on H2O2-induced proliferation, apoptosis, and autophagy in TE-13 cells. miR-923 mimic, miR-932 inhibitor, negative control mimic NC and inhibitor NC were transfected into H2O2-treated TE-13 cells (fig. 2C). As shown in fig. 2D, the cell proliferation after miR-923 overexpression and H2O2 treatment was higher than that of the control mimic NC, and the cell proliferation after miR-932 mimic and H2O2 co-treatment was higher than that of the other groups. The cell proliferation after miR-932 downregulation was significantly lower than that of control inhibitor NC, while the inhibition of cell proliferation by miR-923 inhibitor could be suppressed after H2O2 treatment. Results suggest that H2O2 induces miR-923 to promote TE-13 proliferation.
To determine whether apoptosis is involved in miR-932 mediated proliferation of TE-13 after activation of H2O2, we found that H2O2 promoted the expression of apoptosis-related proteins (caspase-3, caspase-8 and caspase-9) compared with control mimic NC, whereas miR-923 overexpression inhibited apoptosis and then co-treatment with H2O2 treatment counteracted the effect on apoptosis. Apoptosis was promoted when miR-923 was down-regulated and further increased by co-treatment with H2O2 (fig. 2E). Those results indicated that miR-932 could inhibit H2O2 mediated apoptosis of TE-13 cells and that apoptosis was not involved in H2O2 activation after miR-932 mediated proliferation of TE-13.
Western blotting was used to detect the expression of autophagy-related factors (Beclin 1, LC3-II, ATG5 and ATG12) and ERS related proteins (IRE, ATF6 and PERK). In fig. 2F, Beclin 1 expression was significantly decreased and LC3-II, ATG5, ATG12, IRE, ATF6 and PERK expression were significantly increased upon H2O2 treatment compared to control mimic NC, indicated that H2O2 induced oxidative stress induced ERS and further promoted cellular autophagy. In miR-932 overexpression, LC3-II, ATG5, ATG12 and ATF6 expression was significantly increased and Beclin 1 expression was significantly decreased, while IRE and PERK protein expression levels were not significantly different (fig. 2F). In contrast, the expression levels of Beclin 1, LC3-II, ATG5, ATG12, IRE, ATF6 and PERK showed a significant decrease after miR-932 downregulation (fig. 2G), suggested that miR-932 was involved in regulating cellular autophagy and ERS. Whereas either miR-932 overexpression or miR-932 downregulation was accompanied by H2O2 treatment, Beclin 1 expression was significantly reduced and LC3-II, ATG5, ATG12, IRE, ATF6 and PERK expression were significantly increased compared to miR-932 mimic or inhibitor groups. Since ERS can induce LC3-II expression and promote the formation of autophagosomes, we detected the formation of LC3-II autophagic spots by cellular immunofluorescence. As shown in fig. 2H and fig. 2I, H2O2 and miR-932 promote LC3-II (green) accumulation in cells. The results indicate that H2O2 promotes ERS as well as autophagy of miR-932 in TE-13 cells. Above results suggest that H2O2 induces miR-923 mediated proliferation and autophagy in ESCC cells.
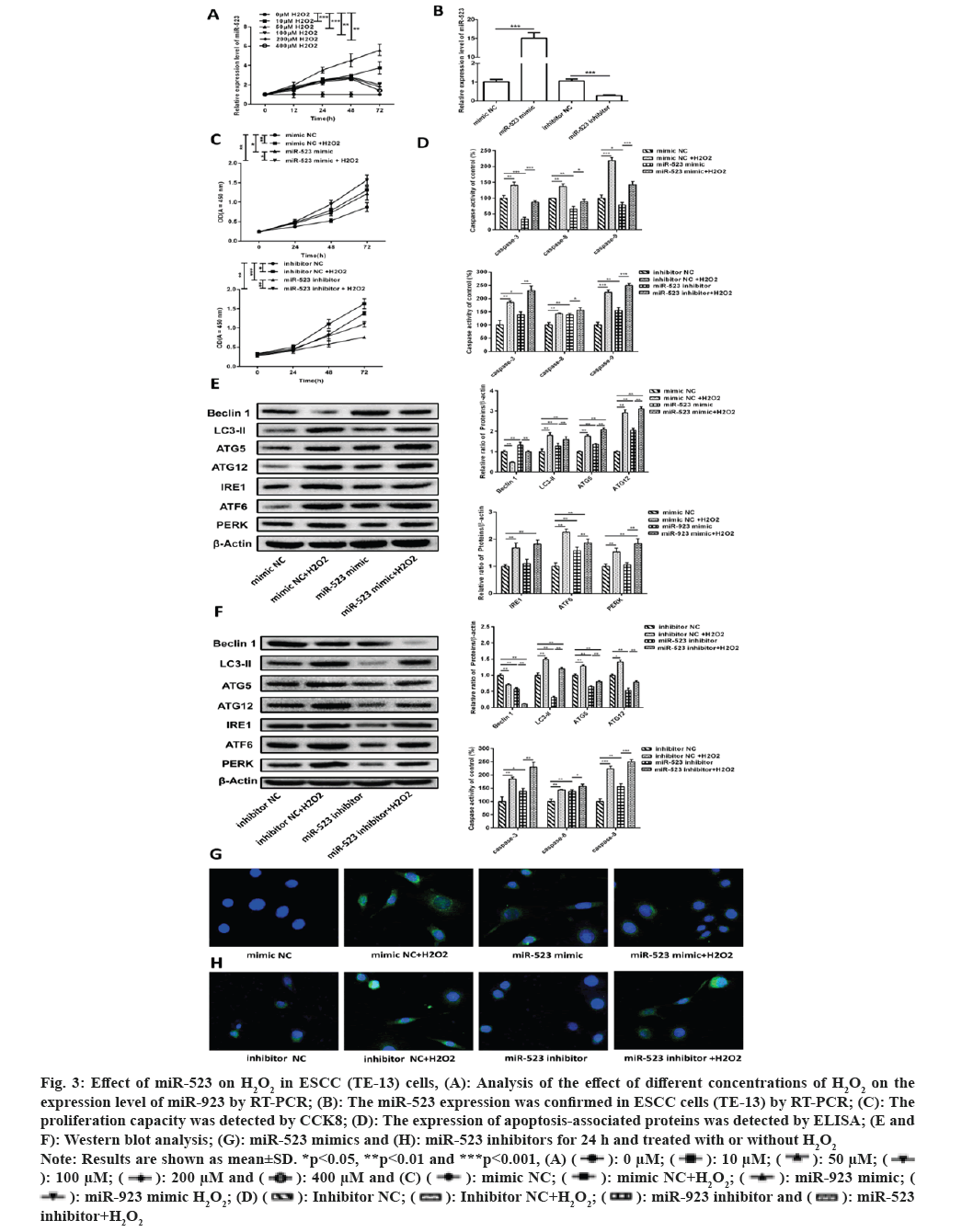
Since ERS inducers can induce miR-923 and miR-523 expression[6]. And we have previously verified that oxidative stress can induce ERS and regulate miR-923 expression. Therefore we further explored whether oxidative stress also has the same regulatory effect on miR-523. As shown in fig. 3A, miR-523 expression showed a significant increase after H2O2 treatment of cells, while miR-923 expression decreased to normal level after 72 h after high concentration of H2O2 treatment. And miR-523 in 50 µM H2O2 treated continued to be elevated at 72 h post-treatment (fig. 3B). The results confirm that oxidative stress promotes miR-523 expression.
Fig. 3: Effect of miR-523 on H2O2 in ESCC (TE-13) cells, (A): Analysis of the effect of different concentrations of H2O2 on the
expression level of miR-923 by RT-PCR; (B): The miR-523 expression was confirmed in ESCC cells (TE-13) by RT-PCR; (C): The
proliferation capacity was detected by CCK8; (D): The expression of apoptosis-associated proteins was detected by ELISA; (E and
F): Western blot analysis; (G): miR-523 mimics and (H): miR-523 inhibitors for 24 h and treated with or without H2O2.
Note: Results are shown as mean±SD. *p<0.05, **p<0.01 and ***p<0.001, (A) 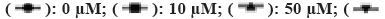
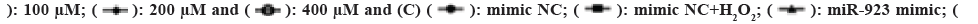
 inhibitor+H2O2.
inhibitor+H2O2.
Then, we further explored the effects of miR-523 on H2O2 induced proliferation, apoptosis, and autophagy in TE-13 cells. miR-523 mimic, miR-523 inhibitor and negative control mimic NC, inhibitor NC were transfected into H2O2 treated TE-13 cells (fig. 3B). In fig. 3C, cell proliferation was higher in the miR-523 overexpression and H2O2 group than in the control mimic NC, and cell proliferation was higher in the miR-532 overexpression and H2O2 co-treatment than in the other groups. Cell proliferation was significantly lower in the miR-523 downregulated group than in the control inhibitor NC, while when treated with H2O2 it inhibited the inhibition of cell proliferation by miR-523 inhibitor. The results suggest that H2O2 induces miR-523 to promote the proliferation of TE-13.
Also to determine whether apoptosis is involved in H2O2 activation after miR-523 mediated proliferation of TE-13, we performed apoptosis related protein assays (caspase-3, caspase-8 and caspase-9) after H2O2 and miR-523 treatment. In fig. 3D, H2O2 promotes apoptosis compared to control mimic NC, while miR-523 overexpression inhibits apoptosis. Caspase-3, caspase-8 and caspase-9 expression were significantly increased after miR-523 overexpression followed by H2O2 treatment in contrast to miR-523 mimic group. When miR-523 were promoted apoptosis after downregulation and then further promoted apoptosis after H2O2 treatment. This suggests that apoptosis is not involved in miR-523 mediated proliferation of TE-13 after activation of H2O2.
When treated with H2O2, Beclin 1 expression were significantly decreased and LC3-II, ATG5, ATG12, IRE, ATF6 and PERK expression were significantly increased compared to the mimic NC group, indicating that H2O2 induced oxidative stress induces ERS and further promotes the formation of cellular autophagosomes. In miR-523 overexpression, Beclin 1, LC3-II, ATG5, ATG12 and ATF6 expression was significantly increased, while IRE and PERK protein expression levels were not significantly different (fig. 3E). When miR-532 was down-regulated, the expression levels of Beclin 1, LC3-II, ATG5, ATG12IRE, ATF6 and PERK showed a significant decrease (fig. 3F), indicating that miR-523 regulates cellular autophagy and ERS. Whereas either miR-523 overexpression or miR-523 downregulation were accompanied by H2O2 treatment, Beclin 1 expression was significantly reduced and LC3-II, ATG5, ATG12, IRE, ATF6 and PERK expression were significantly elevated compared with miR-523 mimic or inhibitor groups. As shown in fig. 3G and fig. 3H, H2O2 and miR-523 promote LC3-II accumulation in cells. The results indicate the role of H2O2 induced miR-523 on autophagy in TE-13 cells and the regulation of ERS. Taken together, H2O2 induced miR-523 mediated proliferation and autophagy in ESCC cells.
This study investigated the relationship between oxidative stress, miR-923 and miR-523 and cellular autophagy to determine the effects of miR-923 and miR-523 on H2O2 induced oxidative damage in the ESCC cell line TE-13 with its mechanism. In this study, it was demonstrated that H2O2 induced oxidative damage could promote miR-923 and miR-523 expression, and increased cell proliferation, apoptosis, autophagy and ERS. Besides, miR-923 and miR-523 could also promote cell proliferation, autophagy and ERS. However, miR-923 and miR-523 inhibited the expression of apoptosis-related proteins (caspase-3, caspase-8 and caspase-9), which suggested that apoptosis was not involved in H2O2 induced miR-923 and miR-523 regulated cell proliferation. It was confirmed that H2O2 induced miR-923 and miR-523 mediated cell proliferation, autophagy and ERS.
ESCC is a fatal disease with a poor prognosis, which can be partly explained by the fact that many patients are diagnosed at an advanced stage of the disease[32,33]. Although ESCC has been extensively researched, the underlying mechanisms still remain unclear. Oxidative stress occurs in various cancers owing to increased oxidative and nitro radical cycling and disruption of cellular redox homeostasis leading to tumorigenesis. Previous studies have shown that oxidative stress affects ESCC occurrence and progression[34]. It was confirmed by our data that NO and H2O2 expressions were significantly higher and TAC levels were significantly lower in ESCC serum, suggesting that oxidative stress is a potential factor in esophageal carcinogenesis.
Oxidative stress has been reported to induce ERS pathways leading to cancer development. However, excessive activation of ERS could increase cellular damage and even lead to cell death[35-37]. Indeed, ERS can induce autophagy in multiple ways, and the interregulation of ERS and autophagy was a highly conserved process[18,19]. With the validation of the expression of LC3-II, ATG5 and ATG12 proteins in ESCC tissues, we found that they showed high expression in ESCC tissues, further illustrating the relevance of autophagy on ESCC progression. There is growing evidence that miRNAs have a critical role in oxidative stress response and carcinogenesis. It has been shown that miR-155 can protect endothelial cells from damage caused by intracellular oxidative stress[28]. The progression of ESCC is promoted by miR-126 through inhibition of apoptosis and autophagy[38]. Studies have shown that abnormally high expression of miR-923 and miR-523 in breast cancer, liver cancer and melanoma is associated with poorer tumor prognosis[29,30]. Yet, endoplasmic reticulum-based stress inducers induce miR-923 and miR-523 expression in human embryonic kidney HEK293 cells[32]. For our results, we also found abnormally high expression of miR-923 and miR-523 in ESCC serum exosomes. Hence, we further explored the mechanisms by which miR-923 and miR-523 regulate autophagy in ESCC cells in a cellular model.
We tested the cell viability of TE-13 cells treated with H2O2 with the finding that low concentrations of H2O2 did increase cell viability. However, when the concentration of H2O2 exceeded 100 µM, cell viability was inhibited. Also, miR-923 and miR-523 expression was examined and H2O2 was found to promote miR-923 and miR-523 expression. Previous studies have reported that H2O2 induced release of Reactive Oxygen Species (ROS) from cells can cause cellular damage. One of them, the LC3-I will be converted to LC3-II when conjugated with phosphatidylethanolamine, thus the LC3-II/I ratio was used to identify the activation of autophagy[39,40] . Hence, we examined the expression of apoptosis-related proteins, autophagy-related proteins, and ERS related proteins. The final results confirmed our hypothesis that H2O2 induced oxidative stress could induce miR-923 and miR-523 to mediate ERS and cellular autophagy, thereby promoting ESCC progression. This provides new insights into miRNA as a potential therapy for ESCC.
Acknowledgement
This study was awarded by the Department of Health (No: 2019-CX-46).
Conflict of interests:
The authors declared no conflict of interests.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70(1):7-30.
[Crossref] [Google Scholar] [PubMed]
- Kojima T, Doi T. Immunotherapy for esophageal squamous cell carcinoma. Curr Oncol Rep 2017;19(5):33.
[Crossref] [Google Scholar] [PubMed]
- Zhang BH, Cheng GY, Xue Q, Gao SG, Sun KL, Wang YG, et al. Clinical outcomes of basaloid squamous cell carcinoma of the esophagus: A retrospective analysis of 142 cases. Asian Pac J Cancer Prev 2013;14(3):1889-94.
[Crossref] [Google Scholar] [PubMed]
- Cools-Lartigue J, Spicer J, Ferri LE. Current status of management of malignant disease: Current management of esophageal cancer. J Gastrointest Surg 2015;19(5):964-72.
[Crossref] [Google Scholar] [PubMed]
- Wang GQ, Jiao GG, Chang FB, Fang WH, Song JX, Lu N, et al. Long-term results of operation for 420 patients with early squamous cell esophageal carcinoma discovered by screening. 2004;77(5):1740-4.
[Crossref] [Google Scholar] [PubMed]
- Lao-Sirieix P, Fitzgerald RC. Screening for oesophageal cancer. Nat Rev Clin Oncol 2012;9(5):278-87.
[Crossref] [Google Scholar] [PubMed]
- Ramundo V, Giribaldi G, Aldieri E. Transforming growth factor-beta and oxidative stress in cancer: A crosstalk in driving tumor transformation. Cancers 2021;13(12):3093.
[Crossref] [Google Scholar] [PubMed]
- Gupta S, Kaur P, Katoch N. Role of oxidative stress in cardiovascular diseases. Res J Pharm Biol Chem Sci 2014;4(3):655-8.
[Crossref] [Google Scholar] [PubMed]
- Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: The role of oxidant stress. Circ Res 2000;87(10):840-4.
[Crossref] [Google Scholar] [PubMed]
- Higashi Y, Noma K, Yoshizumi M, Kihara Y. Endothelial function and oxidative stress in cardiovascular diseases. Circ J 2009;73(3):411-8.
[Crossref] [Google Scholar] [PubMed]
- Gupta K, Sengupta A, Chakraborty M, Gupta B. Hydrogen peroxide and polyamines act as double edged swords in plant abiotic stress responses. Front Plant Sci 2016;7:1343.
[Crossref] [Google Scholar] [PubMed]
- Jiang S, Zhang D, Huang H, Lei Y, Han Y, Han W. Extracellular signal-regulated kinase 5 is required for low-concentration H2O2-induced angiogenesis of human umbilical vein endothelial cells. Biomed Res Int 2017;2017:6895730.
[Crossref] [Google Scholar] [PubMed]
- Medrao-Fernandez I, Sorrentino I, Galli M. Regulation of redox signaling mediated by H2O2 transporters. Free Radical Biology 2021;177(S1):S54.
- Panera N, Gnani D, Piermarini E, Petrini S, Bertini E, Nobili V, et al. High concentrations of H2O2 trigger hypertrophic cascade and phosphatase and tensin homologue (PTEN) glutathionylation in H9c2 cardiomyocytes. Exp Mol Pathol 2016;100(1):199-206.
[Crossref] [Google Scholar] [PubMed]
- Kwon SH, Pimentel DR, Remondino A, Sawyer DB, Colucci WS. H2O2 regulates cardiac myocyte phenotype via concentration-dependent activation of distinct kinase pathways. J Mol Cell Cardiol 2003;35(6):615-21.
[Crossref] [Google Scholar] [PubMed]
- Chong WC, Shastri MD, Eri R. Endoplasmic reticulum stress and oxidative stress: A vicious nexus implicated in bowel disease pathophysiology. Int J Mol Sci 2017;18(4):771.
[Crossref] [Google Scholar] [PubMed]
- Nakka VP, Prakash-Babu P, Vemuganti R. Crosstalk between endoplasmic reticulum stress, oxidative stress, and autophagy: Potential therapeutic targets for acute CNS injuries. Mol Neurobiol 2016;53(1):532-44.
[Crossref] [Google Scholar] [PubMed]
- Niso-Santano M, Bravo-San Pedro JM, Gómez-Sánchez R, Climent V, Soler G, Fuentes JM, et al. ASK1 overexpression accelerates paraquat-induced autophagy via endoplasmic reticulum stress. Toxicol Sci 2011;119(1):156-68.
[Crossref] [Google Scholar] [PubMed]
- Xiao J, Pan Y, Li XH. Cardiac progenitor cell-derived exosomes prevent cardiomyocytes apoptosis through exosomal miR-21 by targeting PDCD4. Cell Death Dis 2016;7(6):e2277.
[Crossref] [Google Scholar] [PubMed]
- El Andaloussi S, Mäger I, Breakefield XO, Wood MJ. Extracellular vesicles: Biology and emerging therapeutic opportunities. Nat Rev Drug Discov 2013;12(5):347-57.
[Crossref] [Google Scholar] [PubMed]
- Wang X, Kong D, Wang C, Ding X, Zhang L, Zhao M, et al. Circulating microRNAs as novel potential diagnostic biomarkers for ovarian cancer: A systematic review and updated meta-analysis. J Ovarian Res 2019;12(1):24.
[Crossref] [Google Scholar] [PubMed]
- Kan CW, Howell VM, Hahn MA, Marsh DJ. Genomic alterations as mediators of miRNA dysregulation in ovarian cancer. Genes Chromosomes Cancer 2015;54(1):1-19.
[Crossref] [Google Scholar] [PubMed]
- Ebrahimi SO, Reiisi S, Shareef S. miRNAs, oxidative stress, and cancer: A comprehensive and updated review. J Cell Physiol 2020;235(11):8812-25.
[Crossref] [Google Scholar] [PubMed]
- Engedal N, Zerovnik E, Rudov A. From oxidative stress damage to pathways, networks, and autophagy via microRNAs. Oxid Med Cell Longev 2018;2018:4968321.
[Crossref] [Google Scholar] [PubMed]
- Ma W, Ding F, Wang X, Huang Q, Zhang L, Bi C, et al. By targeting Atg7 microRNA-143 mediates oxidative stress-induced autophagy of c-Kit+ mouse cardiac progenitor cells. EBioMedicine 2018;32:182-91.
[Crossref] [Google Scholar] [PubMed]
- Ali Sheikh MS, Salma U, Zhang B, Chen J, Zhuang J, Ping Z. Diagnostic, prognostic, and therapeutic value of circulating miRNAs in heart failure patients associated with oxidative stress. Oxid Med Cell Longev 2016;2016:5893064.
[Crossref] [Google Scholar] [PubMed]
- Chen H, Liu Gao MY, Zhang L. MicroRNA-155 affects oxidative damage through regulating autophagy in endothelial cells. Oncol Lett 2019;17(2):2237-43.
[Crossref] [Google Scholar] [PubMed]
- Lasham A, Fitzgerald SJ, Knowlton N. A predictor of early disease recurrence in patients with breast cancer using a cell-free RNA and protein liquid biopsy. Clin Breast Cancer 2020;20(2):108-16.
[Crossref] [Google Scholar] [PubMed]
- Russo A, Caltabiano R, Longo A, Avitabile T, Franco LM, Bonfiglio V, et al. Increased levels of miRNA-146a in serum and histologic samples of patients with uveal melanoma. Front Pharmacol 2016;7:424.
[Crossref] [Google Scholar] [PubMed]
- Dai X, Huang R, Hu S, Zhou Y, Sun X, Gui P, et al. A novel miR-0308-3p revealed by miRNA-seq of HBV-positive hepatocellular carcinoma suppresses cell proliferation and promotes G1/S arrest by targeting double CDK6/cyclin D1 genes. Cell Biosci 2020;10:24.
[Crossref] [Google Scholar] [PubMed]
- Hiramatsu N, Chiang K, Aivati C. PERK-mediated induction of microRNA-483 disrupts cellular ATP homeostasis during the unfolded protein response. J Biol Chem 2020;295(1):237-49.
[Crossref] [Google Scholar] [PubMed]
- Lee NP, Chan CM, Tung LN, Wang HK, Law S. Tumor xenograft animal models for esophageal squamous cell carcinoma. J Biomed Sci 2018;25(1):66.
[Crossref] [Google Scholar] [PubMed]
- Tong DK, Law S, Kwong DL, Wei WI, Ng RW, Wong KH. Current management of cervical esophageal cancer. World J Surg 2011;35(3):600-7.
[Crossref] [Google Scholar] [PubMed]
- Zhang X, Lan L, Niu L. Oxidative stress regulates cellular bioenergetics in esophageal squamous cell carcinoma cell. Biosci Rep 2017;37(6):BSR20171006.
[Crossref] [Google Scholar] [PubMed]
- Moriya S, Miyazawa K, Kawaguchi T, Che XF, Tomoda A. Involvement of endoplasmic reticulum stress-mediated CHOP (GADD153) induction in the cytotoxicity of 2-aminophenoxazine-3-one in cancer cells. Int J Oncol 2011;39(4):981-8.
[Crossref] [Google Scholar] [PubMed]
- Tan Y, Dourdin N, Wu C, de Veyra T, Elce JS, Greer PA. Ubiquitous calpains promote caspase-12 and JNK activation during endoplasmic reticulum stress-induced apoptosis. J Biol Chem 2006;281(23):16016-24.
[Crossref] [Google Scholar] [PubMed]
- Li T, Su L, Zhong N, Hao X, Zhong D, Singhal S, et al. Salinomycin induces cell death with autophagy through activation of endoplasmic reticulum stress in human cancer cells. Autophagy 2013;9(7):1057-68.
[Crossref] [Google Scholar] [PubMed]
- Li M, Meng X, Li M. miR-126 promotes esophageal squamous cell carcinoma via inhibition of apoptosis and autophagy. Aging 2020;12(12):12107-18.
[Crossref] [Google Scholar] [PubMed]
- Weber M, Kim S, Patterson N, Rooney K, Searles CD. miRNA-155 targets myosin light chain kinase and modulates actin cytoskeleton organization in endothelial cells. Am J Physiol Heart Circ Physiol 2014;306(8):1192-203.
[Crossref] [Google Scholar] [PubMed]
- Chen G, Zhang W, Li YP, Ren JG, Xu N, Liu H, et al. Hypoxia-induced autophagy in endothelial cells: A double-edged sword in the progression of infantile haemangioma? Cardiovasc Res 2013;98(3):437-48.
[Crossref] [Google Scholar] [PubMed]