- *Corresponding Author:
- D. J. Singhavi
Institute of Pharmaceutical Education and Research, Borgaon (Meghe), Wardha-442 001, India
E-mail: dileshsinghavi@rediffmail.com
| Date of Submission | 16 April 2019 |
| Date of Revision | 22 July 2019 |
| Date of Acceptance | 20 October 2019 |
| Indian J Pharm Sci 2019;81(6):1099-1106 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The present investigation was aimed at enhancing the dissolution properties of carvedilol, a poorly water-soluble drug using a combination of solid dispersion and semisolid-filled capsule. The use of lauroyl polyoxyl-6 glycerides as a carrier in a semisolid base to improve the dissolution behaviour of carvedilol was investigated. Solid dispersions containing carvedilol were prepared and percent drug content was assessed. In vitro dissolution studies, Fourier-transform infrared spectroscopy, differential scanning calorimetry and scanning electron microscopy were used to characterize solid dispersions. Semisolid-filled capsules of carvedilol were prepared using different bases, and their percent drug content and in vitro dissolution behaviour were studied. The optimized solid dispersion containing 3 parts of poloxamer and 0.2 part of Plasdone K90 with respect to 1 part of carvedilol was incorporated into an optimized semisolid base containing 20 parts of lauroyl polyoxyl-6 glycerides with respect to 1 part of carvedilol). The combination of solid dispersions and semisolid-filled capsule produced a significant increase in the rate of release of the drug. The differential scanning calorimetry thermogram of the optimized semisolid formulation did not show carvedilol peak, which suggested that carvedilol had dissolved in the base in the presence of lauroyl polyoxyl-6 glycerides. The dissolution of carvedilol was improved and the lag time of drug release was reduced in the semisolid-filled capsules in which lauroyl polyoxyl-6 glycerides was used as a carrier.
Keywords
Dissolution, carvedilol, lauroyl polyoxyl-6 glycerides, semisolid-filled capsules
Most of the active pharmaceutical ingredients discovered recently through screening and combinatorial chemistry are poorly water-soluble drugs[1,2]. The therapeutic efficacy of an orally administered drug mainly depends on its solubility, dissolution and absorption[3,4]. One of the major challenges in drug development lies in enhancing the solubility, dissolution and bioavailability of poorly water-soluble drugs[5]. Various approaches that have been used to improve the dissolution behaviour of such drugs involve the use of salt formation[6], self-emulsifying drug delivery systems[7], inclusion complexes[8], co-crystallization[9], solid dispersions (SD)[10] and semisolid matrix filled capsules (SSCs)[11]. SD techniques, of all these approaches, have been used most to enhance the solubility of water-insoluble drugs. SSCs are being used more recently because of several benefits such as reduced weight variations, content uniformity, enhanced stability and improved dissolution of poorly water-soluble drugs[12].
The major disadvantage of the use of a single approach to enhance dissolution is that in emergencies such as heart attacks, myocardial infarctions and asthma, the requirement of dissolution enhancement of a poorly water-soluble drug to achieve therapeutic concentration within a short period might not be achieved[11]. Any delay in achieving therapeutic concentration due to poor dissolution would result in a delayed onset of action and might worsen the emergency situation. Thus increasing the aqueous solubility and dissolution behaviour of the drug is of therapeutic importance. A combination of approaches and material attributes might provide results in terms of dissolution. Selecting a suitable material with the desirable attributes in relation to solubility enhancement is more vital in shaping a drug delivery device. Lauroyl polyoxyl-6 glycerides (LP6G) is a transesterified product of a C12-18 glyceride and polyethylene glycol, with a melting point of about 35-40°[13]. It is an inert, waxy, semisolid material with unique emulsifying properties[14,15]. LP6G is preferred for faster drug release due to its high hydrophilic lipophilic balance (HLB)[16].
Carvedilol (CV) is a non-cardioselective β-blocker used in the treatment of hypertension, congestive cardiac failure and myocardial infarction[17]. If the therapeutic concentration of CV is to be achieved immediately, as in an emergency, the dissolution at the absorption site should be increased within a short period of time. Several approaches have been used to improve the dissolution of CV, such as the use of SD[18], inclusion complexes[19] and nanotubes[20]. Yuvaraja and Khanam developed a SD containing CV using different carriers such as β-cyclodextrin (βCD), hydroxypropyl-β-cyclodextrin (HPβCD), tartaric acid (TA), polyvinyl pyrrolidone K-30 (PVP K-30) and poloxamer-407 (PLX-407). They found that the solubility of CV was increased because of increased wettability and transformation of the crystalline form of the drug to an amorphous form[18]. Zheng et al. successfully improved the dissolution of CV by synthesizing nanotubes[20]. No reports have yet been reported the incorporation of LP6G as a carrier of CV in a semisolid base formulation.
Considering the benefits of a combination of approaches and materials with the required attributes, the present study was carried out to formulate a combination of SD and SSC of CV that contained LP6G. The combined effect of adding LP6G and using SD to improve the solubility and reduce the lag time of dissolution of CV at the absorption site is reported for the first time in the present study.
Materials and Methods
CV was obtained as a gift sample from Cipla Pharmaceutical Pvt. Ltd., Pune, India. Poloxamer 188 (PO) and Plasdone K90 (PD) were gifted by Signet Chemical Corporation Pvt. Ltd., Mumbai, India. PEG 400 and PEG 6000 were obtained from Loba Chemie Pvt. Ltd, Mumbai, India, and LP6G was obtained from Gattefossé SAS, Mumbai. All other materials used were of analytical grade. They were procured from commercial sources.
Determination of saturation solubility of CV: The solubility of CV in distilled water and phosphate buffer (pH 7.4) at room temperature (25°) was determined. An excess amount of CV (20 mg) was added to 10 ml of these media in screw-capped glass vials. Next, the vials were sonicated in an ultrasonic water bath for 1 h, stirred on a magnetic stirrer at a speed of 50 rpm for 7 d at 25°±0.5°[18]. After a period of 24 h (for the 7 d stirring), the suspension in each vial was withdrawn and filtered through Whatman filter paper no. 42 (pore size 2.5 μM), and the amount of drug dissolved in the media was determined using a UV double-beam spectrophotometer (UV 2401 PC, Shimadzu Corporation, Singapore). Each experiment was carried out in triplicate.
Phase solubility analysis:
A phase solubility study was performed according to the method described by Higuchi and Connors[21]. An excess amount of CV (10 mg) was added to distilled water containing various concentrations of PO and PD (2-12 mM) in 10-ml screw capped bottles. The contents were stirred for 72 h on a rotary flash shaker at 25°. After equilibrium was attained, the samples were filtered through Whatman filter paper no. 42, and the absorbance was recorded at 241 nm. The apparent complexation constant (K, 1:1) of CV-PO and CV-PD was calculated from the slopes and intercepts of the straight lines of the phase solubility diagrams, according to the following Eqn., K1:1 = slope/(S0 (1–slope), where, K 1:1 = apparent complexation constant and S0 (intercept) = the solubility of the compound in the absence of a complexing agent.
Preparation of SD:
PO and PD were weighed in different ratios as shown in Table 1 and heated on a water bath to a temperature above their melting points. Weighed amount of CV was gradually incorporated into the molten mass. The mixture was cooled with constant stirring to disperse CV throughout the matrix homogeneously, without melting CV into it. The cooled mass was crushed in a glass mortar, passed through a 355 μm sieve and stored in a desiccator with anhydrous calcium chloride until use[11,22,23].
| Formulations | Formulation ingredients ratio | Drug content (% w/w)* | ||
|---|---|---|---|---|
| Carvedilol phosphate | Poloxamer | Plasdone | ||
| SD1 | 1 | 1 | 75.02±0.15 | |
| SD2 | 1 | 1 | 0.2 | 80.42±0.12 |
| SD3 | 1 | 1 | 0.4 | 85.89±0.21 |
| SD4 | 1 | 2 | 0.2 | 88.12±0.13 |
| SD5 | 1 | 2 | 0.4 | 89.15±0.28 |
| SD6 | 1 | 3 | 0.2 | 91.16±0.15 |
| SD7 | 1 | 3 | 0.4 | 95.05±0.19 |
*Mean±SD (n=3)
Table 1: Composition of Solid Dispersion Batches
Preparation of SSCs:
The semisolid matrix was prepared by the melting method. Different amounts of PEG 400, LP6G and PEG 6000 were weighed (Table 2) and mixed together in a beaker. The mixture was heated on a water bath at 80° with continuous stirring to obtain a homogeneous melt, into which CV was gradually incorporated. The heated mixture was poured into a preheated (by immersing in the hot water at 80°) plastic injector and transferred into hard gelatin capsules, which were stored in a desiccator until use[11,12,24].
| Code | Formulation ingredients ratio | Average weight** | Drug content (% w/w)** | Dispersal homogeneity **(% drug content) | ||||
|---|---|---|---|---|---|---|---|---|
| CV | PEG 400 | Labrafil M 2130 CS | PEG 6000 | Top layer | Bottom layer | |||
| SSC 1 | 1 | 15 | 5 | 1.5 | 239.53±0.42 | 92.16±0.24 | 92.78±0.65 | 92.09±0.19 |
| SSC 2 | 1 | 15 | 10 | 1.5 | 285.92±0.31 | 94.42±0.15 | 94.55±0.72 | 94.92±0.46 |
| SSC 3 | 1 | 15 | 15 | 1.5 | 235.63±0.68 | 95.08±0.35 | 95.84±0.18 | 95.61±0.78 |
| SSC 4 | 1 | 15 | 20 | 1.5 | 381.90±0.81 | 98.21±0.14 | 98.63±0.64 | 98.08±0.35 |
| SSC 5 | 1 | 20 | 15 | 1.5 | 382.20±0.73 | 93.78±0.29 | 93.98±0.69 | 94.01±0.88 |
| SSC 6 | 1 | 25 | 15 | 1.5 | 431.49±0.56 | 91.25±0.17 | 91.65±0.17 | 91.99±0.87 |
| SSC 7 | 1 | 30 | 15 | 1.5 | 481.12±0.46 | 97.41±0.32 | 97.81±0.72 | 97.44±0.66 |
| SSC 8 | 1* | 15 | 20 | 1.5 | 521.36±0.46 | 93. 25±0.62 | 93.72±0.65 | 94.15±0.84 |
*SD equivalent to amount of CV was taken, **mean±SD (n=3)
Table 2: Composition of Semi-Solid Matrix Filled Capsules
Preparation of SSCs containing optimized SD incorporated in optimized semisolid base:
SSCs (SSC8) were prepared by the melting method. PEG 400, LP6G, and PEG 6000 were weighed in the amount mentioned in optimized semisolid base formula (SSC4) as shown in Table 2 and mixed together in beaker. The mixture was heated on water bath at 80° with continuous stirring to obtain a homogeneous melt to which optimized SD (SD6) was gradually incorporated. The heated mixture was poured into a preheated plastic injector and transferred in to hard gelatin capsules, and stored in desiccator until use[11].
Weight variation test:
Ten capsules from each formulation batch were weighed. The percent weight variation was calculated using the following formula[25], weight variation = (weight of capsule–average weight of capsule)/average weight of capsule.
Drug content:
SD equivalent to 10 mg was dispersed in a 10-ml volumetric flask using phosphate buffer (pH 7.4). Then it was stirred for 24 h at 25±0.5° at a speed of 50 rpm using a thermostatically controlled magnetic stirrer. After a period of 24 h, the volume was made up to 100 ml with pH 7.4 phosphate buffer. The dispersion was then filtered through a Whatmann filter paper no. 42. The required dilutions were made and the absorbance was taken at 241.0 nm. In case of SSCs, the formulation was dissolved in a 10-ml volumetric flask with pH 7.4 phosphate buffer. Further tests were similar to those carried out on the SD. Percent drug content of formulations was calculated using the following Eqn., % drug content = actual amount of drug present in formulations (mg)/theoretical amount of drug in formulations (mg)×100.
Dispersion homogeneity test of semisolid mix: This test was performed to ensure the homogeneity of a semisolid mix and, in turn, the homogeneity of CV within the system before filling it into the capsules. A weighed quantity of the SSC formulation was transferred to polypropylene centrifuge tubes (15 ml, PW 1231, Himedia), mixed with 10 ml of distilled water and centrifuged at 5000 rpm for 15 min. The material was examined visually for homogeneity, any sediment at the bottom of the tube and any floating residue on the top layer. Additionally, 1 ml volume was sampled from the top and bottom of the tube each and CV content was determined spectrophotometrically[11].
In vitro dissolution study:
Dissolution studies were carried out on the pure CV (10 mg) and the SD equivalent to 10 mg CV using USP type II (paddle method) apparatus with 500 ml of phosphate buffer (pH 7.4) as a dissolution medium. The rotation speed was maintained at 100 rpm and the temperature at 37±0.5°. Five milliliter aliquots were withdrawn at 5, 10, 20, 30, 40, 50 and 60 min and the volume was made up with fresh dissolution medium after each sampling. The drawn samples were filtered and analysed using a double-beam UV spectrophotometer at 241.0 nm. In vitro release test similar to that carried out on the SSC formulation except for that the sampling time points were 0, 5, 15 and 20 min[26].
FTIR spectroscopy:
Potassium bromide discs containing CV, the polymer, a physical mixture of the two and the optimized SD were prepared to record the spectrum in the range of 4000-400 cm-1 using FTIR spectrophotometer (model no: 84005 Shimadzu Asia Pacific Pvt. Ltd., Singapore).
Differential scanning calorimetry (DSC):
The samples were sealed in aluminum pans and analysed using a DSC 60 (Shimadzu, Japan). Five to ten milligrams of the sample was sealed in an aluminum pan and analysed at a scanning rate of 10°/min between 30 and 300°. A nitrogen purge (20 ml/min) was maintained throughout the runs, using an empty sealed pan as the reference. Temperature and heat flow calibrations were performed using indium as the standard.
Scanning electron microscopy (SEM):
SEM was utilized to study the external morphology (size, shape and surface) of the prepared SD. The optimized SD was placed on a double-sided copper conductive tape (NEM Nisshin EM Co. Ltd.) fixed on aluminum stubs and sputter coated with a thin layer of gold in vacuum for 45 s at 20 Ma using a coating unit (Cresssington 108 auto Sputter Coater, UK) to make it electrically conductive. Formulation SD6 was analysed using the SEM instrument (Supra 5, Carl Zeiss, Germany) which was operated at 10 kV.
Stability studies:
A stability study of the optimized formulation was carried out by storing the optimized formulations wrapped in aluminum foil at 40±2° and 75±5 % relative humidity for 3 mo. The physical appearance, drug content and in vitro release were determined at the end of 1, 2 and 3 mo.
Results and Discussion
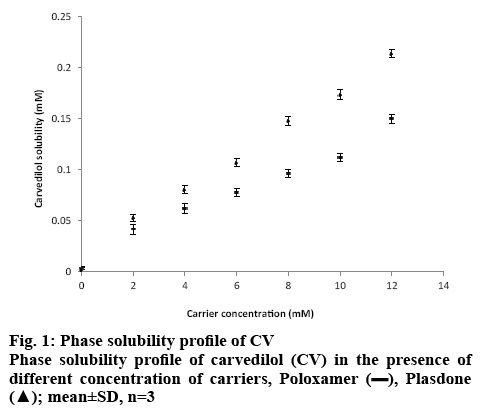
Several potent drug candidates are promising candidates for treatment of emergency conditions. Due to their poor solubility, achieving the therapeutic concentration promptly is difficult. CV is one of these drugs. The saturation solubility of CV in distilled water and in phosphate buffer (pH 7.4) was found to be 0.00022±0.0015 and 0.0058±0.0013 mg/ml, respectively. In basic pH, the solubility of CV is poor owing to its basic nature[27]. As it remains in the unionized form in distilled water and in pH 7.4 phosphate buffer, it dissolves at a lower rate in these media. Figure 1 shows the solubility of CV in the presence of carriers (PO and PD alone) of different concentration at room temperature. It is evident from the results that as the concentration of these carriers was increased, the solubility of CV in distilled water and pH 7.4 phosphate buffer increased. The rise in solubility of CV due to the carrier might be because of the formation of soluble complexes between hydrophilic carriers and the poorly soluble drug[28]. Therefore, PO and PD were selected as carriers for the formation of SDs. On the basis of the phase solubility observations, SDs containing CV were prepared using PO and PD in different ratios to analyse the effects of PD in the binary SDs. Table 1 shows that as the proportion of carrier with respect to the drug was increased, the percent drug content increased. The high proportion of carriers led to a greater association of the drug with the carriers, which resulted in high percent drug content. As shown in figure 2A, a higher dissolution rate than that of the pure drug was found in all the SD formulations. The SD of CV with PO in the weight ratio 1:1 showed 41.52±0.84 % drug release at 60 min. However, the formulations SD2, SD3, SD4, SD5, SD6 and SD7 showed drug releases of 56.15±0.85, 49.45±0.84, 66.23±0.97, 58.63±0.98, 78.12±0.97 and 73.53± 0.84 %, respectively. The improved dissolution rates of CV in the SDs might be due to the hydrophilic attribute of the carriers, which were in intimate contact with the drug particles as a result of mixing. Due to contact with aqueous dissolution medium, the hydrophilic carrier particles present in dispersion undergo fast hydration, which leads to increased wettability and dissolution of the drug particles. On increasing the concentration of PO with respect to the drug, the dissolution rate of the drug also increased. This might be due to the association of monomolecular micelles of PO to form aggregates of varying size, which have the ability to increase the dissolution rate of the drug, and is consistent with the findings of phase solubility studies[11]. When a second carrier PD was included at 20 % of the drug amount, again the drug dissolution rate of CV is enhanced. However, a slight reduction in the dissolution rate of CV was observed when PD was used at 40 % of the drug amount. This might be due to the formation of a thick layer of PD around the CV molecules that hindered the release medium to reach CV molecules. Thus, it was observed that PD inhibited dissolution above a certain concentration. Batch SD6, which showed maximum drug release of all batches at 60 min, was selected as an optimal batch for further formulations. LP6G is an amphiphilic base, it possesses solubility and dissolution enhancement properties via emulsification properties. The dispersions of PEG 400 and PEG 6000 with LP6G have unique semisolid characteristic. All the SSC formulations complied with the specified requirements in terms of quality control tests, namely, percent drug content and weight uniformity.
After various semisolid formulations were prepared, these were centrifuged at 4000 rpm for 10 min. No precipitation was observed at the bottom of the tube. It was also observed that there was no significant difference in the drug content between the samples collected from the top and bottom of the tube. The results indicated that stable, homogeneous semisolid dispersions were formed.
The in vitro drug release profiles of the SSCs showed a biphasic release characterised by an initial burst phase within 5 min that resulted in CDR values in the range from 50.51±0.99 to 64.83±0.81 %, which was followed by a slower release up to 20 min in the range from 75.02±0.82 to 93.56±0.95 %. This release pattern is similar to that displayed by SD6, but SD6 showed a biphasic release of lower magnitude (52.75±0.82 % at 5 min and 62.03±0.79 % at 20 min) compared with all the SSC formulations. This clearly emphasized the superiority of the SSCs over SDs and which is desirable for antihypertensive drugs such as CV, which required a faster onset of action.
Among all the SSC formulations, SSC4, which had the lowest level of PEG 400 and the highest level of LP6G, showed the maximum drug release, 93.56±0.95 %, up to 20 min. In contrast, formulation SSC7, which had the lowest LP6G content and the highest PEG 400 content, showed the least release up to 20 min, 75.02±0.82 %. Clearly, the levels of both LP6G and PEG 400 played a key role in enhancing the drug release. Batch SSC4, with the maximum drug release at the end of 20 min was selected as the optimized batch. The optimized solid dispersion SD6 was incorporated in the semisolid base formula of the formulation SSC4 to prepare semisolid matrix capsules (SSC8) to improve the dissolution behaviour.
LP6G is able to self-emulsify on contact with aqueous media, forming a coarse dispersion, i.e. emulsion or fine dispersion or microemulsion[29]. This leads to improvement of the solubility of the drug, which facilitates absorption. Another hydrophilic component, PEG 400, is a low molecular weight molecule that improves the solubility of the drug by creating a less polar environment[30]. A comparison was made between SD6, SSC4 and SSC8 (figure 2B). The drug release values at 5 min in these formulations were 52.75±0.82, 64.83±0.81 and 79.62±0.88 %, respectively. When SD6 was added to the SSC4 formulation base containing LP6G, there was a further increase in the dissolution rate. A comparison was made between a commercial formulation and the optimized formulation SSC8. Formulation SSC8 released more than 80 % of the CV in 5 min and its lag time was less than that of the commercial formulation to release the CV up to the same level. This improvement in drug release in formulation SSC8 is due to the combined benefits of an SD and a semisolid base.
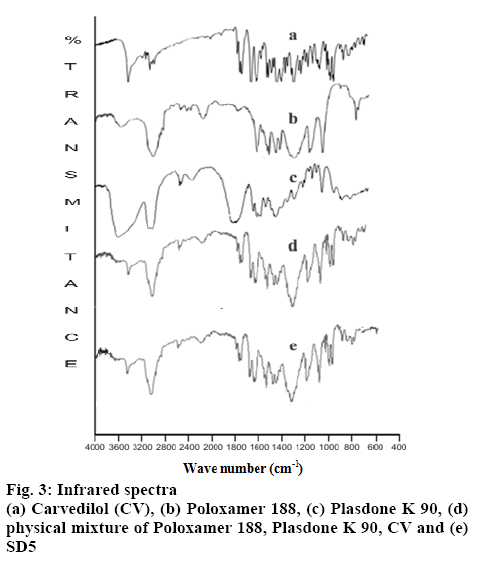
The FTIR spectra of CV, the excipients and the optimized SD were obtained using the KBr disk method (figure 3). The FTIR spectra of the optimized solid dispersion and the semisolid matrix filled capsule were compared with the spectrum of pure CV. The FTIR spectrum of CV exhibited characteristic peaks at 3342.41, 3242.12, 3058.89 and 2900.11 cm-1, corresponding to stretching vibrations of OH, NH, the aromatic ring and CH, respectively. PO showed a broad band of OH stretching vibrations at 3600- 3300 cm-1 in addition to CH stretching vibrations at 2900 cm-1. The spectrum of PD displayed the characteristic stretching vibrations of CO at 1700.56 cm-1. The FTIR spectra of both PEG 400 and PEG 6000 showed the characteristic stretching vibrations of OH in a broad band at 3600-3200 cm-1. The spectrum of LP6G reveals the presence of COOH by demonstrating OH and CO stretching vibrations at 3600-3200 cm-1 and 1750 cm-1, respectively. The presence of a long linear-chain hydrocarbon was confirmed by coupled vibrations at 2900 cm-1 in addition to scissoring vibrations at 1464 cm-1.
The fingerprints of the FTIR spectra of the physical mixture and CV were superimposable. Characteristic drug peaks at 3242.41, 3058.89 and 2900.11 cm-1 were detected in the spectrum of the physical mixture. There was no evidence of any additional peak or any significant peak shifts, indicating no chemical interaction between the drug and excipients and hence the mixture was found to be stable. Both stretching and fingerprint region in the FTIR spectrum of optimized SD and CV were almost superimposable. As with the physical mixture, no evidence of additional peak was observed. These results suggest that the formulations did not undergo any change during their preparation and were stable.
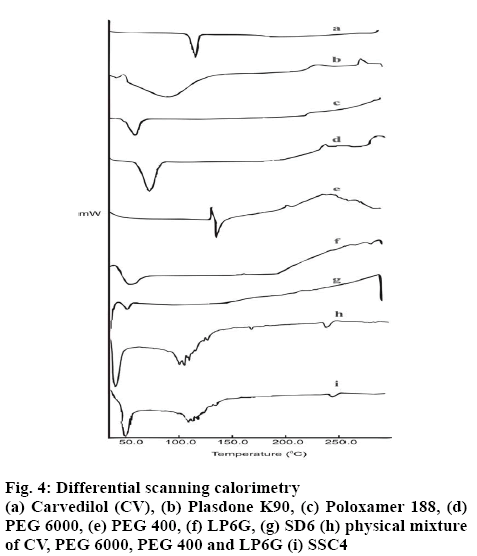
The DSC thermogram of CV exhibited a single sharp melting point in the form of an endothermic peak at 119.0° (figure 4). No sign of a phase transition or degradation of CV was observed before and after the melting point, respectively. The DSC scan of PO showed that the melting point is at 60.7°. No sharp melting point was observed in the DSC scan of PD; instead, a rapid loss of moisture up to 89.6° was found. The DSC thermogram of PEG 400 exhibited initial recrystallization, with an exothermic peak, immediately followed by melting at 137.4°. The DSC thermogram of LP6G showed a rapid loss of moisture up to 45.2°, followed by a gradual rise in temperature up to 200°, followed by oxidation. The DSC scan of PEG 6000 showed a sharp melting point with a single endothermic band at 65.3°.
In the DSC thermogram of the optimized SD, the endothermic melting peak of CV was not to be found, and a less intense endotherm was observed at 41°. These findings from DSC clearly indicate a strong possibility of the transformation of the crystalline form of CV to its amorphous form in the matrix, which might be responsible for the improved dissolution. DSC analysis of the optimized SSC did not show the endothermic peak corresponding to the melting of CV at 119.0°. The thermograms of the other excipients used in the preparation were not present. The disappearance of the melting peak of CV can be attributed to the complete dissolution of the drug in the carrier and a possibility of a transition from the crystalline state to an amorphous state as shown by the glass transition peak that was recorded for CV at 109.5°.
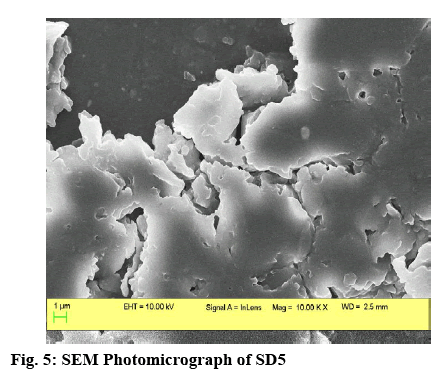
From the SEM photography (figure 5), it was found that the optimized SD6 had a non-uniform, porous, amorphous surface texture comprising numerous depressions and striations. The optimized batch SSC8 was used in a stability study. It was observed that the batch was stable for a period of 3 mo at 40±2° and 75±5 % RH. SSCs of a poorly water-soluble drug CV were successfully designed using LP6G as a carrier. CV was dispersed uniformly throughout the carrier in an SD, and hence fast dissolution could be achieved. However, for an antihypertensive drug, an almost zero lag time is desirable. Therefore, SSCs with reduced lag time were formulated. A combination of an SD and an SSC containing LP6G improved the dissolution of CV the most. LP6G acted as an excellent carrier for enhancing the dissolution behaviour of CV using a combination of SD and SSC.
References
- Bikiaris DN. Solid dispersions. Part I: Recent evolutions and future opportunities in manufacturing methods for dissolution rate enhancement of poorly water-soluble drugs. Expert Opin Drug Deliv 2011;8:1501-19.
- Cooper ER. Nanoparticles: A personal experience for formulating poorly water soluble drugs. J Control Release 2010;141:300-2.
- Khadka P, Ro J, Kim H, Kim I, Kim JT, Kim H, et al. Pharmaceutical particle technologies: An approach to improve drug solubility, dissolution and bioavailability. Asian J Pharm Sci 2014;9(6):304-16.
- Venkatesh DN, Sangeetha S, Samanta MK, Suresh B, Ramesh N, Faisal MM, et al. Dissolution enhancement of domperidone using water soluble carrier by solid dispersion technology. Int J Pharm Sci Nanotechnol 2008;1(3):221-6.
- Tang B, Cheng G, Gu JC, Xu CH. Development of solid self-emulsifying drug delivery systems: Preparation techniques and dosage forms. Drug Discov Today 2008;13:606-12.
- Parikh RK, Mansuri NS, Gohel MC, Sonlwala MM. Dissolution enhancement of nimesulide using complexation and salt formation techniques. Indian Drugs 2005;42(3):149-54.
- Yoo JH, Shanmugam S, Thapa P, Lee ES, Balakrishnan P, Baskaran R, et al. Novel self-nanoemulsifying drug delivery system for enhanced solubility and dissolution of lutein. Arch Pharm Res 2010;33:417-26.
- Loh GO, Tan YT, Peh KK. Enhancement of norfloxacin solubility via inclusion complexation with β-cyclodextrin and its derivative hydroxypropyl-β-cyclodextrin. Asian J Pharm Sci 2016;11:536-46.
- Sugandha K, Kaity S, Mukherjee S, Isaac J, Ghosh A. Solubility enhancement of ezetimibe by a cocrystal engineering technique. Cryst Growth Des 2014;14:4475-86.
- Sareen S, Mathew G, Joseph L. Improvement in solubility of poor water-soluble drugs by solid dispersion. Int J Pharm Investig 2012;2:12.
- Tyagi VK, Singh D, Pathak K. Semisolid matrix-filled hard gelatin capsules for rapid dissolution of amlodipine besilate: Development and assessment. J Adv Pharm Technol Res 2013;4:42.
- Alladi S, Shastri NR. Semi solid matrix formulations of meloxicam and tenoxicam: An in vitro and in vivo evaluation. Arch Pharm Res 2015;38:801-12.
- Ambuhl M, Haeberlin B, Luckel B, Meinzer A, Lambert O, Marchal L. Pharmaceutical compositions. United States patent US20060134203A1. 2006 June 22.
- Langlois C. Pharmaceutical microspheres containing valproic acid for oral administration. Indian Patent 209019. 2007 Sep 21.
- Avachat AM, Patel VG. Self-nanoemulsifying drug delivery system of stabilized ellagic acid–phospholipid complex with improved dissolution and permeability. Saudi Pharm J 2015;23:276-89.
- Borhade V, Pathak S, Sharma S, Patravale V. Clotrimazole nanoemulsion for malaria chemotherapy. Part I: Preformulation studies, formulation design and physicochemical evaluation. Int J Pharm 2012;431:138-48.
- Stafylas PC, Sarafidis PA. Carvedilol in hypertension treatment. Vasc Health Risk Manag 2008;4:23.
- Yuvaraja K, Khanam J. Enhancement of carvedilol solubility by solid dispersion technique using cyclodextrins, water soluble polymers and hydroxyl acids. J Pharm Biomed Anal 2014;96:10-20.
- Zoghbi A. Wang B. Carvedilol solubility enhancement by inclusion complexation and solid dispersion. J Drug Deliv Ther 2015;13:1-8.
- Zheng X, Wang T, Jiang H, Li Y, Jiang T, Zhang J, et al. Incorporation of carvedilol into PAMAM-functionalized MWNTs as a sustained drug delivery system for enhanced dissolution and drug-loading capacity. Asian J Pharm Sci 2013;8:278-86.
- Higuchi T, Conners KA. Phase solubility techniques. Adv Anal Chem Instrum 1965;4:117–212.
- Dhirendra K, Lewis S, Udupa N, Atin K. Solid dispersion: A review. Pak J Pharm Sci 2009;22:234-46.
- Jagadeesan R, Radhakrishnan M. Novel approaches in the preparation of solid dispersion: A review. Int J Pharm Sci 2013;5(3):1000-4.
- Cole ET, Cade D, Benameur H. Challenges and opportunities in the encapsulation of liquid and semi-solid formulations into capsules for oral administration. Adv Drug Deliv Rev 2008;60:747-56.
- Kanabar VB, Doshi SM, Patel VP. Duocap: A capsule technology. Int Res J Pharm 2015;6(2):86-9.
- Galal SA, El-Massik MA, Abdallah OS, Daabis NA. Formulation of fast release glibenclamide liquid and semi-solid matrix filled capsules. Acta Pharm 2003;53:57-64.
- Timmins P, Pygall SR. Microenvironment pH control and mixed polymer approaches to optimize drug delivery with hydrophilic matrix tablets. In: Timmins P, Pygall SR, Melia CD, editors. Hydrophilic matrix tablets for oral controlled release. New York: AAPS Press; 2009. p. 257-80.
- Medarevic DP, Kachrimanis K, Mitric M, Djuris J, Djuric Z, Ibric S. Dissolution rate enhancement and physicochemical characterization of carbamazepine– poloxamer solid dispersions. Pharm Dev Tech 2016;21:268-76.
- Nakarani M, Patel J, Patel P, Vaghani S. Self-microemulsifying drug delivery systems of efavirenz: Formulation design, in vitro and in vivo assessment. Acta Pharma Sci 2011;53:517-34.
- Nayak AK, Panigrahi PP. Solubility enhancement of etoricoxib by cosolvency approach. ISRN Phys Chem 2012;18: 2012.
 , Plasdone
, Plasdone  ; mean±SD, n=3
; mean±SD, n=3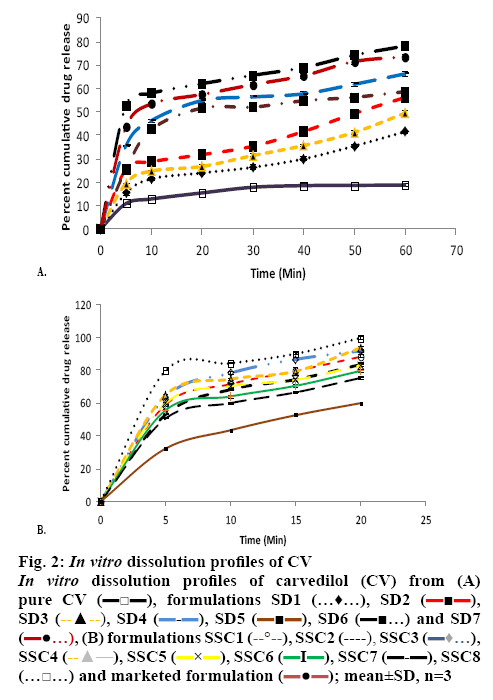
 , formulations SD1
, formulations SD1  , SD2
, SD2  , SD3
, SD3  , SD4
, SD4  , SD5
, SD5  , SD6
, SD6  and SD7
and SD7  , (B) formulations SSC1
, (B) formulations SSC1  , SSC2
, SSC2  , SSC3
, SSC3  , SSC4
, SSC4  , SSC5
, SSC5  , SSC6
, SSC6  , SSC7
, SSC7  , SSC8
, SSC8  and marketed formulation
and marketed formulation  ; mean±SD, n=3
; mean±SD, n=3