- *Corresponding Author:
- N. M. Patel
Shri. B. M. Shah College of Pharmaceutical Education and Research, Modasa - 383 315, India
E-mail: nmp_pharmacist@rediffmail.com
| Date of Submission | 3 January 2006 |
| Date of Revision | 25 August 2006 |
| Date of Acceptance | 23 March 2007 |
| Indian J Pharm Sci, 2007, 69 (2): 219-225 |
Abstract
The present investigation describes the influence of content of polyethylene oxide and ratio of lactose to starch 1500 on dipyridamole release from self correcting floating matrix tablets using 32 full factorial design. Tablets were evaluated for in vitro floating ability and drug release study using USP 24 type II apparatus using 0.1 N HCI at 100 rpm and temperature of 37±0.50. Multiple regression analysis and two way analysis of variance followed by Tukey test were performed for dependent variables. All formulations floated within 2 min regardless of factors studied and had total floating time of more than 12 h. It was observed that both the factors had significant influence on all dependent variable studied ( P< 0.05) except the ratio of lactose to starch 1500 did not significantly contribute for Q1 ( P > 0.05). As content of polymer increased the release rate declined with increase in value of diffusion exponent giving anomalous drug release to zero order drug release ( P < 0.05). It was observed that above a certain threshold level of polymer content further increase did not contribute significantly for percentage drug release. Lactose gave higher drug release with release mechanism towards zero order compared to starch 1500 which gave slower release with release mechanism towards diffusion based. Although both the factors significantly contribute for percentage drug release at different time point, the content of polymer dominated. It was observed that polymer content was a dominant controlling factor for drug release kinetics and it could be controlled by employing various blends of fillers.
Gastric emptying of dosage forms is an extremely variable process and ability to prolong and control the emptying time is a valuable asset for dosage forms which reside in the stomach for a longer period of time than conventional dosage forms [1]. The transit of drug or formulation through gastrointestinal (GI) tract will determine how long a compound will be in contact with its preferred absorptive site [2]. Prolonged gastric retention improves bioavailability, reduces drug waste and improves solubility for drugs that are less soluble in a high pH environment. It has applications also for local drug delivery to the stomach and proximal small intestines [3]. Controlling the residence time of a drug delivery system in a particular region of GI tract can be achieved by several approaches: intragastric floating system, high density system, mucoadhesive system, unfolding, extendable or expandable systems and superporous hydrogels [3,4]. From the technological point of view, a floating drug delivery system is more convenient and logical approach to prolong gastric residence time.
The attainment of high gel viscosity, maintenance of constant gel layer or synchronous erosion/dissolution in a monolithic sense for linear drug release over a prolong period of time is not easily achievable. The various dynamic phases in the rate process of polymer relaxation, disentanglement and/or erosion during dissolution rate manifest themselves in a nonconstant manner hence realization of zero order drug release from such monolithic devices is difficult. Pillay and Fassihi [5] reported that the limitation of hydrophilic polymers may be circumvented through modification of physical and chemical infrastructure of the polymeric gel system by inducing in situ reaction between electrolytes and hydrophilic polymer. This produces a heterogeneous domain within the swollen gel boundary referred to as a ‘metamorphic scaffold’ characterizes self correcting matrices.
Polyethylene oxide (PEO) is among various hydrophilic polymers that, in presence of water, control the release of the active moiety either by swelling or by swelling/ erosion by forming a hydrogel. PEOs have been proposed as alternatives to cellulose or other ethylene glycol derivatives in the production of controlled released drug delivery system6. In the present study, high molecular weight PEO (mw = 7 × 106) was used since it was reported that it swells to greater extent and forms a stronger gel that is less prone to erosion. Further it also has mucoadhesive properties which may assist in prolonging the gastric residence time [7]. The rate of drug release from hydrophilic matrix is dependent on various factors such as types of polymer, solubility of drug, polymer content, particle size of drug and polymer as well as types and amount of filler used in the formulation [8]. The adjustment of polymer concentration, viscosity grade and addition of different types and levels of excipients to the polymer matrix can modify the kinetics of drug release [9].
Dipyridamole, poorly-soluble weak base, dissolves rapidly in stomach but incompletely in intestine [10] and absorption is remarkably lower in elevated gastric pH [11] making it an ideal candidate for floating drug delivery system. The present investigation describes the influence of content of PEO WSR 303 and ratio of lactose to Starch® 1500 (PPS) on dipyridamole release from self correcting floating matrices using 32 full factorial statistical design.
Materials and Methods
Dipyridamole was received as a gift sample from Sun Pharmaceutical Ltd., Vadodara (India). Poly (ethylene oxide) WSR 303 (Polyox® WSR 303, mw= 7 × 106) was received as a gift sample from Dow Chemical Company, New Jersey (USA). Tablettose 80 (directly compressible lactose) was received as a gift sample from Meggle GMBH, Germany. Partially pregelatinized maize starch (Starch® 1500) was obtained as a gift sample from Colorcon Asia Pvt. Ltd., Goa (India). All other ingredients were procured from Lesar chemicals, Vadodara (India) and of analytical grade. All materials used through out the study conformed to USP 24 standards.
Full factorial design
Two factors were evaluated each at three levels and experimental trials were performed at all possible nine combinations. In the present investigation, content of PEO (X1) and ratio of lactose to PPS (X2) were selected as independent variables. The diffusion exponent (n), release rate constant (k), percentage drug release at 1 h (Q1), 4 h (Q4), 8 h (Q8) and 12 h (Q12) were selected as dependent variables. The experimental design with corresponding formulations are outlined in Table 1. Content of PEO was evaluated at 20%, 30% and 40% of total tablet weight while ratio of lactose to PPS was evaluated at 1:0, 0.5:0.5 and 0:1.
| Batch Code | Coded value | Diffusion exponent (n) | Release rate constant (k) | Percentage drug release | ||||
|---|---|---|---|---|---|---|---|---|
| X1 | X2 | Q1 | Q4 | Q8 | Q12 | |||
| P1 | -1 | 1 | 0.752 | 0.158 | 15.28 | 45.61 | 77.39 | 97.34 |
| P2 | 0 | 1 | 0.804 | 0.111 | 10.51 | 35.23 | 59.20 | 77.73 |
| P3 | 1 | 1 | 0.890 | 0.084 | 07.96 | 30.09 | 55.45 | 75.33 |
| P4 | -1 | 0 | 0.744 | 0.149 | 14.47 | 42.14 | 71.38 | 92.83 |
| P5 | 0 | 0 | 0.786 | 0.109 | 10.71 | 33.69 | 56.17 | 75.74 |
| P6 | 1 | 0 | 0.881 | 0.079 | 07.35 | 25.65 | 52.86 | 73.41 |
| P7 | -1 | -1 | 0.732 | 0.141 | 13.86 | 38.65 | 64.94 | 85.55 |
| P8 | 0 | -1 | 0.761 | 0.107 | 11.52 | 29.82 | 50.99 | 73.66 |
| P9 | 1 | -1 | 0.866 | 0.076 | 08.47 | 27.13 | 50.28 | 71.49 |
For coded values, -1, 0 and 1, the actual values are for X1- 20, 30 and 40 and for X2- 0:1, 0.5:0.5 and 1:0, respectively. X1 is content of PEO (%) and X2 is ratio of lactose to PPS. Q1, Q4, Q8 and Q12 are percentage drug release at 1 h, 4 h, 8 h and 12 h, respectively. Each batch contains 30% of dipyridamole, 10% of sodium bicarbonate, 1% of magnesium stearate and quantity sufficient of filler.
Table 1: Formulation and dissolution characteristics of batches in a 32 full factorial design.
A statistical model incorporating interactive and polynomial terms was utilized to evaluate the response, Y=b0+b1X1 +b2X2+b12X1X2+b11X12+b22X22, where Y is the dependent variable, b0 is the arithmetic mean response of the 9 runs and bi is the estimated coefficients for the factor Xi. The main effect (X1 and X2) represents the average result of changing one factor at a time from its low to high value. The interaction term (X1X2) show how the response changes when two factors are changes simultaneously. The polynomial terms (X12,X22) are included to investigate nonlinearity.
Preparation of dipyridamole floating tablets
Dipyridamole (30%) was mixed with require quantity of polymer, sodium bicarbonate (10%) and filler by mixing in laboratory cube blender for 15 min. The powder blend was then lubricated with magnesium stearate (1%) for additional 3 min and compressed on 10 station rotary tablet machine (Rimek, Ahmedabad, India) using 10 mm standard flat face punch. Compression force was adjusted to obtain tablets with hardness in range of 5-6 kg/cm2. The tablets weighed 500±2 mg, had a round flat face with average diameter of 10±0.1 mm and a thickness of 4.5±0.2 mm
In vitro buoyancy study
The in vitro buoyancy was characterized by floating lag time and total floating time. The test was performed using USP 24 type II paddle apparatus using 900 ml of 0.1 N HCl at paddle rotation of 100 rpm at 37±0.5°. The time required for tablet to rise to surface of dissolution medium and duration of time the tablet constantly float on dissolution medium were noted as floating lag time and total floating time, respectively (n=3).
In vitro drug release study
The in vitro drug release was performed using USP 24 type II paddle apparatus using 900 ml of 0.1 N HCl at paddle rotation of 100 rpm at 37±0.50. The samples were withdrawn at predetermined time intervals for period of 12 h and replaced with the fresh medium. The samples were filtered through 0.45 μm membrane filter, suitably diluted and analysed at 283 nm using double beam UV/ Vis spectrophotometer (Shimadzu Corporation, UV-1601, Japan). The content of drug was calculated using equation generated from standard calibration curve. The test was performed in triplicate. High reproducibility of data was obtained (SD< 3%), hence only the average values were considered.
Statistical analysis
The statistical analysis of the factorial design batches were performed by multiple regression analysis using Microsoft Excel®. To evaluate contribution of each factors with different levels on responses, two way analysis of variance (ANOVA) followed by Tukey test was performed using Sigma Stat software (Sigma Stat 2.03, SPSS, USA). To graphically demonstrate the influence of each factor on responses, the response surface plots were generated using Sigma Plot Software (Sigma Plot Software 8.0, SPSS, USA). The P<0.05 was considered to be significant.
Results and Discussion
In order to achieve linear, bimodal or zero-order release, various strategies that seek to manipulate tablet structure or geometry have been developed, though many such strategies often increase the number of manufacturing steps and complexity of production, involving specialized equipment and non-conventional excipients. An alternative to physical modifications of tablet structure (layered and reservoir coated tablets) is modification of the simple monolithic matrix formulations. The present work describes the application of a novel ‘self-correcting’ hydrophilic matrix employing the principles of the colloidal chemistry ‘salting-out’ phenomenon to modulate the swelling and erosion kinetics of the matrix containing the drug, excipients, and electrolytes. The presence of these electrolytic compounds in the form of ionizable salts allows for non-collapsible diffusion channels to form. The electrolytes also provide microenvironments within the tablet, whose pH is mediated by the nature of the electrolyte, thus, either enhancing or suppressing the solubility of the drug itself. As the matrix hydrates, the electrolytes and polymer compete for water of hydration with the drug, resulting in controlled solubilization and diffusion of drug [12,13]. In the present study sodium bicarbonate was used as an electrolyte because of its dual properties, Firstly releases carbon dioxide in presence of acidic medium thus enabling the dosage form to float by entrapment of gas inside the matrices and secondly, it provides an alkaline environment inside the matrices leading to controlled solubilization and dissolution of dipyridamole.
The floating lag time for tablets of all batches was found to be below 2 min regardless of the content of polymer and ratios of lactose to PPS used as the polymer level of 20% or more, the particles of polymer are close enough to permit faster establishment of gel layer leads to minimization of influence of filler blends used. The total floating time for tablets of all batches was found to be more than 12 h.
In designing a swelling controlled drug delivery system for constant drug delivery, apart from system configuration, the hydrophilic polymer employed is of crucial importance, as drug release is controlled by simultaneous aqueous medium diffusion into the matrix, polymer chain relaxation and drug diffusion. Mechanistic drug release kinetics depends on the relative ratio of the rate of the polymer swelling at the glassy/rubbery front to the rate of polymer erosion/drug dissolution at the swollen polymer/dissolution front. Ideally the synchronization of the velocities of the swelling front and erosion front leads to linear release kinetics as long as surface area through which drug is released remains constant [14]. Determination of actual release process and derivation of mass-balance equation for complex polymeric matrices is a subject of different study. It is however appropriate to determine the release kinetics by power law equation given by Ritger and Peppas [15].
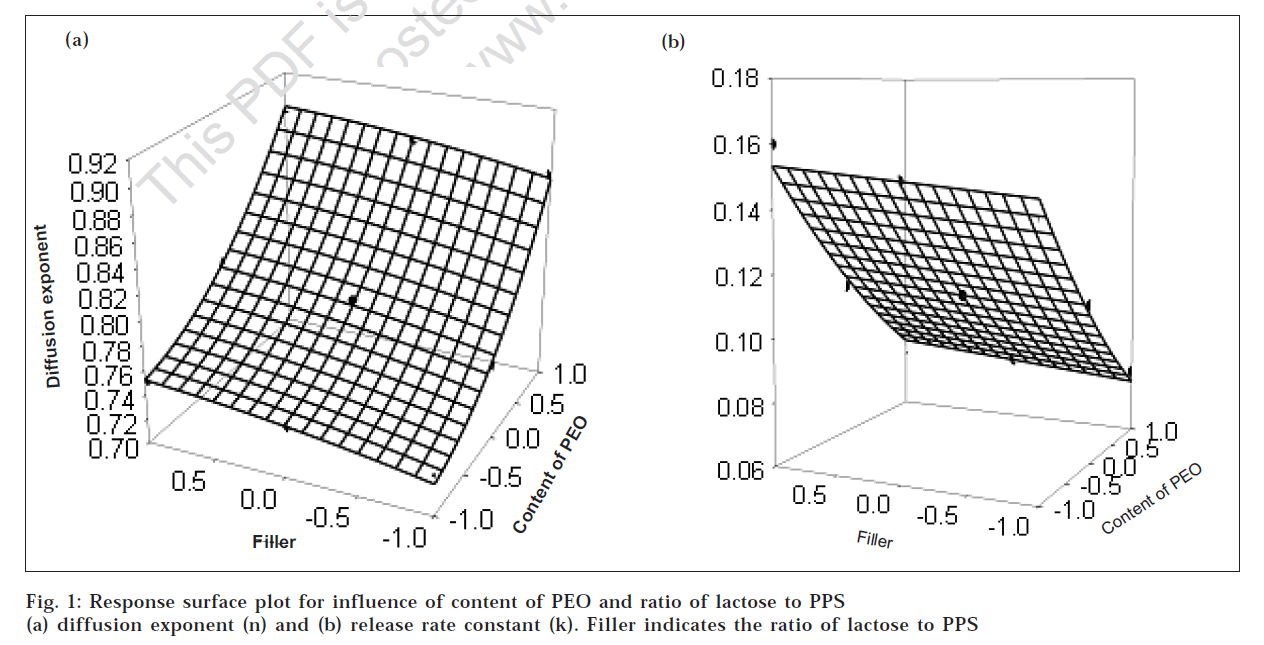
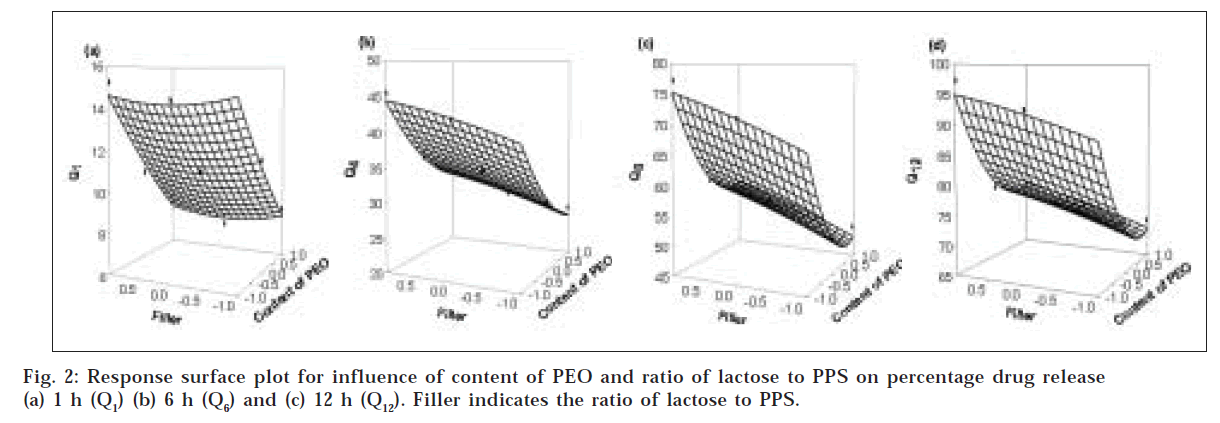
The results of diffusion exponent (n), release rate constant (k) and percentage drug release at 1 h (Q1), 4 h (Q4), 8 h (Q8) and 12 h (Q12) showed wide variation (Table 1). From results of multiple regression analysis, it was found that both factors had statistically significant influence on all dependent variable (P<0.05, Table 2). The high value of multiple correlation co-efficients clearly indicate the response are strongly dependent on factors studied (Table 2, P<0.05). To demonstrate graphically the influence of factors, the response surface plots were generated (figs. 1 and fig. 2) for all dependent variables. To evaluate the relative contribution of different levels of each factor, two way ANOVA was performed followed by Tukey test and results are depicted in Table 3.
| Parameters | Co-efficient of regression parameters | |||||||
|---|---|---|---|---|---|---|---|---|
| b0 | b1 | b2 | b12 | b11 | b22 | r2 | P | |
| Diffusion exponent (n) | 0.786 | 0.068 | 0.015 | - | 0.011 | - | 0.997 | 0.0011 |
| Release rate constant (k) | 0.108 | -0.034 | -0.004 | - | 0.006 | - | 0.998 | 0.0006 |
| Q1 | 10.62 | -3.305 | - | - | - | - | 0.993 | 0.0053 |
| Q4 | 33.18 | -6.756 | 2.556 | -0.099 | 2.468 | - | 0.999 | 0.0002 |
| Q8 | 55.73 | -9.186 | 4.308 | -1.821 | 6.597 | - | 0.999 | 0.0001 |
| Q12 | 76.02 | -9.246 | 6.944 | - | 0.996 | 0.0023 | ||
Q1, Q4, Q8 and Q12 are percentage drug release at 1 h, 4 h, 8 h and 12 h, respectively.
Table 2: Summary of regression output of significant factors for the measured responses.
| Source of variation | DF | SS | MS | F | P |
|---|---|---|---|---|---|
| Diffusion exponent (n) | |||||
| Content of PEO | 2 | 0.02940 | 0.01470 | 386.83 | <0.001 |
| Filler | 2 | 0.00128 | 0.00064 | 16.83 | 0.011 |
| Residual | 4 | 0.00015 | 0.00004 | ||
| Total | 8 | 0.03080 | 0.00385 | ||
| Release rate constant (k) | |||||
| Content of PEO | 2 | 0.00734 | 0.00367 | 328.69 | <0.001 |
| Filler | 2 | 0.00014 | 0.00007 | 6.23 | 0.058 |
| Residual | 4 | 0.00005 | 0.00001 | ||
| Total | 8 | 0.00753 | 0.00385 | ||
| Q1 | |||||
| Content of PEO | 2 | 65.74 | 32.87 | 70.85 | <0.001 |
| Filler | 2 | 0.360 | 0.180 | 0.388 | 0.701 |
| Residual | 4 | 0.00015 | 0.000038 | ||
| Total | 8 | 0.0308 | 0.00385 | ||
| Q4 | |||||
| Content of PEO | 2 | 285.93 | 142.96 | 123.10 | <0.001 |
| Filler | 2 | 39.50 | 19.48 | 17.01 | 0.011 |
| Residual | 4 | 0.00015 | 0.000038 | ||
| Total | 8 | 0.0308 | 0.00385 | ||
| Q8 | |||||
| Content of PEO | 2 | 593.40 | 296.70 | 85.98 | <0.001 |
| Filler | 2 | 111.57 | 55.78 | 16.16 | 0.012 |
| Residual | 4 | 0.00015 | 0.000038 | ||
| Total | 8 | 0.0308 | 0.00385 | ||
| Q12 | |||||
| Content of PEO | 2 | 609.75 | 304.87 | 57.25 | 0.001 |
| Fille | 2 | 65.13 | 32.56 | 6.12 | 0.061 |
| Residual | 4 | 0.00015 | 0.000038 | ||
| Total | 8 | 0.0308 | 0.00385 |
Q1, Q4, Q8 and Q12 are percentage drug release at 1 h, 4 h, 8 h and 12 h, respectively. Filler indicates the ratio of lactose to PPS, DF is degree of freedom, SS is sum of square, MS is mean sum of square and F is Fischer’s ratio.
Table 3: Result of two way anova for dependent variables.
Fig. 1a shows the influence of content of PEO and ratio of lactose to PPS on diffusion mechanism (n). For diffusion exponent although the content of polymer and ratio of lactose to PPS had significant influence (P<0.05), the diffusion exponent ranges from 0.732 to 0.890 indicating drug release mechanism ranges from anomalous drug release to zero order drug release showing involvement of swelling, diffusion and/or erosion of the matrices which might be due to poor solubility of dipyridamole, high swelling tendency of PEO and blends of two filler employed. Results of Tukey test showed that difference was statistically significant amongst all levels of content of polymer studied (P<0.05) while in case of ratio of lactose to PPS significant difference was only observed between matrices prepared either using plain lactose or PPS. The mechanism of drug release was more dependent upon content of PEO as it was observed that as content of PEO increased the diffusion exponent value is also increased. This observed effect may be explained by assuming that as polymer levels in the matricesincreased, it leads to stronger gel layer formation becausethe particles had more intimate contact with each other.This leads to an increase in diffusion path length for thedrug molecule. With respect to the influence of filler itwas observed that formulation prepared with lactose of yielded mechanism of drug release nearer to zero order as it being a small molecule having high aqueous solubility compared to PPS. Lactose can diffuse readily in constant manner and had negligible contribution in gel formation except it accelerate the penetration of medium with reference to PPS which has swelling properties and may significantly influence the swelling tendency of polymeric matrices. The PPS may synergistically form stiff gel in conjugation with PEO, resulting in formation of matrices which are less prone to erosion, gave mechanism of drug release towards diffusion type or anomalous behavior.
Fig. 1b shows influence of content of PEO and ratio of lactose to PPS on release rate constant (k). From results of multiple regression analysis and two way ANOVA, it was cleared that both factors significantly contributed to the release rate constant (P<0.05, Table 2 and Table 3). It was observed that as polymer level increased, the release rate constant decreased. Increasing polymer level in matrices increases the tortuosity and consequently the possibility of interaction between molecules of dipyridamole and swollen PEO particles is higher. Increase in polymer level also leads to formation of a tightly swollen gel layer due to more intimate contact between the particles of PEO. This results in decreased mobility of insoluble drug particles in swollen matrices thereby causing decreased drug release. Amongst the filler blend evaluated lactose gave higher release rate compared to PPS might be due to the difference in characteristics of fillers as stated previously. Slower drug release from matrices containing PPS may be due to slower penetration of dissolution medium front towards central core of the matrix. Thus, the diffusion path length would become more convoluted and the drug release rate would decrease. The results are in good agreement with study reported by Michailova and co-workers [16]. They characterized HPMC/pregelatinized starch hydrogel as ‘filled’ composite system where starch filler functions as a supporting frame, while the linear hypromellose forms the continuous dispersion medium. The pregelitinized starch hydrates to a considerable lower degree due to the formation of intramolecular hydrogen bonds in the highly branched amylopectin which hindered the degree of hydration of polymer/PPS resulting in reduced gel layer diffusivity and decreased drug velocity from matrices containing pregelatinized starch [17].
To describe entire dissolution profile, four time points were selected. Percentage drug release at 1 h (Q1), 4 h (Q4), 8 h (Q8) and 12 h (Q12) were selected as dependent variables. Fig. 2a shows the influence of content of PEO and ratio of lactose to PPS on percentage drug release at 1 h which is generally termed as period of burst release. From results of multiple regression analysis and two way ANOVA, it appeared that only content of PEO had statistically significant influence on Q1 while ratio of lactose to PPS did not play an important role (P<0.05, Tables 2 and 3). The results obtained indicate that content of PEO dominated for Q1 by rapid establishment of gel layer that controlled the release at initial periods depending upon the content of polymer irrespective of filler blend used. This effect was evident from response surface plot (fig. 2a) where the response was linear with respect to content of PEO while a flattening of the curve was seen with respect to type of filler.
Fig. 2b shows the influence of content of PEO and ratio of lactose to PPS on percentage drug release at 4 h (Q4). From results of regression analysis and two way ANOVA, it was observed that both factors significantly contributed to Q4 (P<0.05, Table 2 and Table 3). The drug release at 4 h decreased as content of PEO increased and it was higher when lactose was used as filler compared to PPS alone or in combination with lactose. This period is generally termed as period of establishment of fully swollen gel layer depending upon the concentration of polymer present and other component of the system mainly filler. As stated previously it is expected that the percentage drug release may be higher in tablets containing lactose as filler because it accelerates the diffusion of aqueous medium inside the matrices and opens up the channel for drug to get diffuse out. From results of Tukey test, it was found that the difference was significant between all levels of content of PEO while in case of ratio of lactose to PPS, the difference was only significant between the formulation containing either lactose or PPS as filler (P<0.05).
Fig. 2c shows the influence of content of PEO and ratio of lactose to PPS on percentage drug release at 8 h (Q8) From results of multiple regression analysis and two way ANOVA, it was observed that both factors had significant contribution for Q8 (P<0.05, Tables 2 and 3). The drug release at 8 h decreased as a function of increasing polymeric content in matrices and it was higher when lactose was employed as filler. This period may be generally termed as period of dominance of erosion as it is expected that the water-front may reach the central core of the matrices for rapidly hydrating the highly hydrophilic polymer. The degree of erosion was dependent on the strength of the gel layer formed depending upon the content of PEO and the filler. The matrices containing PPS as described previously formed a stiff gel and expected to erode slowly leading to slower drug release as compared with lactose as filler. From results of Tukey test it was observed that the difference was not significant between matrices prepared using either 30% or 40% of PEO (P>0.05). This observed effect can be explained by assuming that after a certain threshold level of polymer content, further increase in polymer level may actually reduced the drug release but not to a significant extent. This effect is also evident from response surface plot (fig. 2c) as there is little curvature at the terminal portion of the curve indicates non linearity of the response with respect to content of polymer. Among the ratio of lactose to PPS, similar results are obtained as observed for Q4.
Fig. 2d shows the influence of content of PEO and ratio of lactose to PPS on percentage drug release at 12 h (Q12) which is end phase of dissolution profile. From results of multiple regression analysis and two way ANOVA, it was observed that both factors had significant contribution for Q12 (P<0.05, Table 2 and Table 3). From results of Tukey test, it was observed that statistically no significant difference was observed between the formulation containing either 30% or 40% of PEO (P>0.05, Table 4). Above certain threshold level of polymer content further increase in its content did not produce marked effect. For ratio of lactose to PPS, similar results were obtained as seen for Q4 and Q8. The results indicated that PEO may play a dominant role in controlling drug release due to faster and stronger formation of gel layer which may nullify the influence of other variables.
In conclusion, PEO levels may be responsible for critical performance of hydrophilic matrices as compared to ratio of lactose to PPS although the later had certain degree of contribution to the kinetics of drug release.
References
- Hirtz, J., Brit. J. Clin. Pharmacol. , 1985, 19, 77S.
- Davis, S.S., Drug Discovery Today , 2005, 10, 249.
- Arora, S., Ali, J., Ahuja, A., Khar, R.K. and Baboota S ., AAPS Pharm. Sci. Tech., 2005, 6, article 47.
- Yeole, P.G., Khan, S. and Patel, V.F., Indian J. Pharm. Sci. , 2005, 67, 265.
- Pillay, V. and Fassihi, R., J. Pharm. Sci. , 1999, 88, 1140.
- Pinto, J.F., Wunder, K.F. and Okoloekwe, A., AAPS Pharm. Sci., 2004, 6, article 15.
- Dhawan, S., Dhawan, K., Varma, M. and Sinha, V.R., Pharm. Tech., 2005, 29, 72.
- Levina, M. and Rajabi-Siahboomi, A.R., J. Pharm. Sci., 2004, 93, 2746.
- Bravo, S.A., Lamas, M.C. and Salomon, C.J., J. Pharm. Pharm. Sci., 2002, 5, 213.
- Kohri, N., Miyata, N., Takechi, S. and Nomura, A., Int. J. Pharm., 1992, 81, 49.
- He, X., Kadomura, S., Takekuma, Y., Sugawara, M. and Miyazaki, K., J. Pharm. Sci. , 2004, 93, 71.
- Pillay, V. and Fassihi, R., J. Control. Release, 2000, 67, 67.
- Pillay, V. and Fassihi, R., J. Control. Release, 2000, 67, 55.
- Yang, L. and Fassihi, R., J. Pharm. Sci., 1996, 85, 170.
- Ritger, P.L. and Peppas, N.A., J. Control. Release, 1987 , 5, 23.
- Michailova, V., Tiveta, S., Kotsilkova, R., Krusteva E. and Minkov, E., Int. J. Pharm., 2001, 222, 7.
- Hanselmann, R., Burchard, W., Ehrat, M. and Widmer, H.M., Macromolecules, 1996, 29, 3277.