- *Corresponding Author:
- S. Kumar
Department of Pharmaceutical Sciences and Drug Research, Punjabi University, Patiala-147 002, India
E-mail: thakur_pu@yahoo.com
| Date of Submission | 23 August 2016 |
| Date of Revision | 04 January 2017 |
| Date of Acceptance | 18 April 2017 |
| Indian J Pharm Sci 2017; 79(3):395-401 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Baptisia tinctoria (Wild indigo; Fabaceae) has long tradition of use in Indian systems of medicine for the treatment of depression. This investigation deals with the evaluation of the antidepressant activity of various extracts and fractions of B. tinctoria roots and estimation of the content of bioactive principle using thin-layer chromatography densitometry. Roots of B. tinctoria were successively extracted to obtain n-hexane, chloroform, methanol and water extracts. The chloroform, methanol and water extracts were screened for antidepressant activity at doses of 200 or 400 mg/kg, p.o., in mice subjected to forced swim test. Methanol extract showed significant activity at a dose of 400 mg/kg. The methanol extract was further fractionated successively to obtain ethyl acetate and 1-butanol fractions. Antidepressant activity of these fractions was assessed using forced swim test at the doses of 90 and 80 mg/kg, p.o., respectively. The ethyl acetate fraction showed significant antidepressant activity at the dose of 90 mg/kg in mice. Further, specific antidepressant activity without psychostimulant effects of bioactive methanol extract and ethyl acetate fraction was confirmed through evaluation of locomotor behaviour in mice using open field test. Phytochemical screening showed the presence of flavonoids as major class of phytoconstituents in the methanol extract. Comparative thin-layer chromatography fingerprint studies confirmed the presence of hesperitin in methanol extract. Hesperitin was used as a chemical marker to standardize B. tinctoria roots using validated thin-layer chromatography densitometric method and the content of hesperitin was found to be 0.0085% w/w.
Keywords
Antidepressant, Baptisia tinctoria, Fabaceae, TLC densitometry, wild indigo
Depression is a state of low mood and aversion to activity that can affect a person’s thoughts, behaviour, feelings and sense of well-being. Approximately 15% of the population experiences a major depressive episode at some point of life [1]. Research also suggests that maternal depression may be a risk factor for poor growth in young children [2]. Depression is a significant contributor to the global burden of disease and affects people in all communities across the world [3]. It is estimated that about 350 million people worldwide suffer from depression. The World Mental Health Survey conducted in 17 countries found that on average about 1 in 20 people reported having an episode of depression in the previous year. Depressive disorders often start at a young age; they reduce people’s functioning and often are recurring. The pharmacotherapy of depression includes tricyclic antidepressants, tetracyclic antidepressants, monoaminoxidase inhibitors and selective serotonin reuptake inhibitors [4,5]. These antidepressant drugs frequently produce side effects such as dry mouth, mydriasis, constipation, temporary fatigue, restlessness and headaches [6]. The increased risk of side-effects, drug-drug and drug-food interactions have raised the need for efficacious and relatively safer drugs to cure depression. In the light of adverse effects associated with these synthetic drugs, researchers worldwide are searching for newer, safer and more efficacious alternatives [7,8]. Herbs prove to be useful, safe and potent therapeutic agents to treat various CNS disorders including depression [9]. A wide variety of plants exist in nature popularly reputed to have medicinal properties. Baptisia tinctoria (L.) R. Vent. is one of such plants.
B. tinctoria (Wild Indigo; family-Fabaceae) is indicated in wandering feeling, inability to think, mental confusion, delirium, melancholia with stupor and threatened miscarriage from mental depression [10-12]. The plant has been reported to contain various groups of phytoconstituents- alkaloids, phenols, flavonoids, coumarins and triterpenes. The polysaccharides isolated from B. tinctoria, showed significant immune stimulating activity as assessed by the in vitro granulocyte test and in vivo carbonclearance test [13]. Glycoproteins and arabinogalactan proteins of the plant stimulated enhanced secretion of the endogenous immunostimulant interleukin [14]. B. tinctoria showed significant phagocytic activity in the granulocyte carbon-clearance tests when administered in combination with Echinacea angustifolia DC. [15]. Literature search revealed that B. tinctoria has not been scientifically investigated for antidepressant activity. Thus, it was planned to investigate antidepressant activity of various extracts and fractions of B. tinctoria roots and to estimate the content of hesperitin in the plant using thin-layer chromatography (TLC) densitometry.
Materials and Methods
B. tinctoria roots were procured form Himalaya Herbs Store, Madhav Nagar, Saharanpur, UP, India in September, 2015. The plant was identified in the Department of Botany, Punjabi University, Patiala, India (Reference No. SPL-105/Bot, dated 12-10- 2015). LR grade solvents, n-hexane, chloroform, methanol, ethyl acetate, 1-butanol procured from E Merck, Delhi, India, were used for the preparation of plant extracts and fractions. Toluene, ethyl acetate, methanol and glacial acetic acid, of AR grade were employed for TLC studies. Quercetin, naringenin, hesperitin, apigenin, and chrysin procured from Sigma- Aldrich Company, St. Louis MO, USA were used for establishing TLC fingerprint profiles. Instruments used were, rotary vacuum evaporator (Buchi, Switzerland), digital weighing balance (Ohaus, USA), hot air oven (Universal Instrument, Bangalore, India), Soxhlet apparatus, water bath (Perfit, Ambala, Haryana, India), TLC densitometer (Camag, Switzerland) and UV Light Chamber (254/366 nm, Gupta Scientific Store, Ambala, Haryana, India) were used to perform experiments of present research work. TL chromatograms were taken on the precoated aluminium based TLC plates (E Merck, Delhi, India).
Preparation of extracts and fractions:
The roots of B. tinctoria were powdered in a grinder. Dried powdered plant material (0.5 kg) was placed in thimble, made up of fine filter paper. The plant was then extracted in a Soxhlet apparatus with n-hexane (2 l) exhaustively till few drops collected from siphoning tube on watch glass did not leave any residue after evaporation. The marc was dried, packed in a thimble and extracted exhaustively in a Soxhlet apparatus using chloroform (2 l) to get chloroform extract (CE). After complete extraction with chloroform, the same procedure was adopted to get methanol extract (ME) using methanol (2 l). The water extract (WE) was prepared by boiling the marc of plant material with distilled water (2 l) for 2 h on a hot plate. The solvents from crude extracts were recovered under reduced pressure using a rotary vacuum evaporator to get n-hexane extract (HE), CE, ME and WE.
The ME (25 g) was taken in a round bottom flask, and 50 ml of distilled water was added to the extract. A suspension of the extract in distilled water was prepared by triturating the material for 30 min with a glass rod. It was then partitioned with 50 ml of ethyl acetate by heating at 50° for 30 min along with continuous stirring. The contents were cooled and ethyl acetate layer (upper layer) was separated. To the extract 50 ml of fresh ethyl acetate was added and the procedure was repeated Fractional extraction with ethyl acetate was repeated till a few ml of ethyl acetate layer did not leave any significant residue on a watch glass after evaporation. All the separated layers of ethyl acetate were pooled and concentrated under reduced pressure to finally obtain ethyl acetate fraction (EAF). Similar procedure was adopted on the remaining bioactive extract (RBE) to get 1-butanol fraction (BF). After successive partitioning with ethyl acetate and 1-butanol, the RBE was also concentrated. All the extracts of plant and fractions of the bioactive extract were screened for the presence of various groups of phytoconstituents [16].
Antidepressant activity:
Laca mice (either sex, body weight 20-25 g) were used for pharmacological activities. The animals were purchased from the Central Research Institute, Kasauli, India. Normal laboratory pellet diet and water ad libitum were given to mice. The approval was taken from Institutional Animal Ethics Committee (IAEC) of Punjabi University, Patiala before carrying out animal studies (107/99/CPCSEA-2016-30, dated 27-05-2016). Animals were acclimatized to laboratory conditions daily for 1 h for seven days before starting the experiment. Groups of six animals were used in all sets of experiments. The test drugs were administered orally with the help of an oral cannula fitted on a tuberculin syringe. The antidepressant and locomotor activity studies were investigated using well established models such as forced swim test (FST) and open field test, respectively, as per standard procedures [9]. Distilled water+Tween 80 (2%) was used as a vehicle for preparing various test doses of crude extracts, fractions and the standard drug. Imipramine (Triko Pharmaceuticals, Rohtak, Haryana, India; 15 mg/kg, p.o.), was used as standard antidepressant drug.
Experimental protocol:
Three experimental protocols were designed consisting of 16 groups of mice. Experimental protocol I, comprising groups 1 to 8, was designed to assess antidepressant activity of various crude extracts of B. tinctoria roots. Control group (group 1) received vehicle (0.25 ml, p.o.); standard group (group 2) received imipramine (15 mg/kg, p.o.); test group 3 and 4 received 200 and 400 mg/kg doses of CE, respectively; test group 5 and 6 received 200 and 400 mg/kg doses of ME, respectively and test groups 7 and 8 received 200 and 400 mg/kg doses of WE.
Experimental protocol II, comprising groups 9 to 12, was designed to assess antidepressant activity of various fractions of B. tinctoria roots. Control group (group 9) received vehicle (0.25 ml, p.o.); standard group (group 10) received imipramine (15 mg/kg, p.o.); test group (group 11) received 90 mg/kg dose of EAF and test group 12 received 80 mg/kg dose of BF.
Experimental protocol III, comprising groups 13 to 16, was designed to assess locomotor activity of bioactive extract and fraction of B. tinctoria roots. Control group (Group 13) received vehicle (0.25 ml, p.o.); standard group (group 14) received imipramine (15 mg/kg, p.o.); test group (group 15) received 400 mg/kg dose of ME and test group 16 received 90 mg/kg dose of EAF.
Statistical analyses:
The results were expressed as mean±standard deviation (SD). The test samples were compared with standard drug and control by one way analysis of variance (ANOVA) followed by Student Newman Keul’s test [17].
TLC fingerprint profiles:
A fixed volume, 5 μl of bioactive extract of the plant and standard flavonoids was applied on 20×10 cm precoated TLC plate using Camag Linomat 5. The plate was developed using solvent system, toluene:ethyl acetate:glacial acetic acid (15:11:2) in a TLC chamber, to a distance of 8 cm. The plate was dried under the current of hot air and visualised under ultraviolet light (254 nm and 366 nm) in a UV/Vis cabinet.
TLC densitometric method development studies:
The coarsely powdered root (10 g) was exhaustively extracted with methanol in a Soxhlet apparatus as described earlier. The extract was filtered, concentrated under reduced pressure and the volume was adjusted to 10 ml with methanol. Accurately weighed quantity of the marker compound (5 mg) was dissolved in 5 ml of methanol. The stock solution of the marker compound was diluted with methanol to get six dilutions of different concentrations (4, 8, 12, 16, 20 and 24 μg/ml). An aliquot of 10 μl from each dilution was applied, in triplicate, on a pre-coated TLC plate. The plate was developed in the solvent system, toluene:ethyl acetate:methanol (10:7:3) in a chamber, to a distance of 8 cm. The developed plate was dried in a current of hot air and then scanned in a Camag TLC scanner at 278 nm. The AUC of the peak corresponding to marker compound was noted in each track.
Test solutions (10 μl) of ME was applied in triplicate on a pre-coated TLC plate (5×10 cm). The plate was developed and scanned following the same procedure as described previously. The average AUC of the peak corresponding to marker compound was noted at 278 nm in the test sample and its concentration was calculated from the standard plot. The developed TLC densitometric method was validated for the parameters such as linearity, range, limit of detection, limit of quantification, inter-day precision, intra-day precision, accuracy, repeatability and specificity as per ICH guidelines [9].
Results and Discussion
Percent yields of HE, CE, ME and WE were found to be 0.40, 2.32, 13.02 and 5.00% w/w, respectively. The results of chemical tests of various extracts showed fixed oils in HE; alkaloids, steroids, triterpenoids and coumarins in CE; proteins, carbohydrates, flavonoids, tannins and coumarins in ME; proteins, carbohydrates and tannins in WE. It is clearly evident from the results of phytochemical screening that HE did not show presence of any main class of phytoconstituents. Therefore, only CE, ME and WE were screened for antidepressant activity in mice using FST. In this test, mice are forced to swim in a limited area from which they cannot escape. Initially, mice try to escape from the restricted space and remain in mobile state. After few seconds, mice adapt to characteristic behaviour of immobility. The duration of immobility is recorded as assessment parameter of antidepressant activity of the test substances [18]. The duration of immobility exhibited by mice after acute administration of 200 or 400 mg/ kg, p.o., doses of crude extracts, imipramine (15 mg/ kg, p.o.) and the control (vehicle, p.o.) is shown in Table 1. Amongst various extracts, only ME exhibited significant antidepressant activity with respect to control. Though the ME (200 mg/kg) significantly reduced immobility time in mice with respect to control, but could not produce activity comparable to that produced by imipramine. At a higher dose of 400 mg/kg, the effect produced by ME was similar to that produced by imipramine. It is clearly evident from Table 1 that ME significantly reduced time spent by the mice in the immobile state (antidepressant activity) at the dose of 400 mg/kg, which is statistically not different to that shown by the mice treated with imipramine. WE was found to be devoid of antidepressant activity whereas CE exhibited mild antidepressant activity. CE of plant significantly reduced duration of immobility in mice at the doses of 200 or 400 mg/kg with respect to control, which is much lower than that produced by imipramine. These results demonstrate that the ME is the most active extract of B. tinctoria roots.
| Treatment | Dose (mg/kg) | Meann immobility time (s)±SD |
|---|---|---|
| Control | Vehicle | 278.00±8.78a |
| Imipramine | 15 | 62.33±9.20* |
| CE | 200 400 |
169.17±10.68*a 130.00±8.94*a |
| ME | 200 400 |
85.83±10.20*a 65.00±8.94* |
| WE | 200 400 |
266.83±7.91a 269.16±14.28a |
Table 1: Antidepressant activity of crude extracts of B. Tinctoria roots using fst
The bioactive ME was fractionated successively using solvents in order of increasing polarity, ethyl acetate and 1-butanol for further fractionation. Percent yields of EAF, BF and RBE were found to be 22.45, 19.92 and 57.63% w/w, respectively, in relation to ME. Phytochemical screening of various fractions showed presence of flavonoids, tannins and coumarins in EAF; proteins, carbohydrates, tannins and coumarins in BF; proteins and carbohydrates in RBE. It was clearly evident from results of phytochemical screening that RBE did not show presence of any main class of phytoconstituents. Therefore, EAF and BF were further screened for antidepressant activity in mice using FST. Based on relative percentage yields of EAF and BF to the ME, a single dose of fractions was selected for antidepressant activity evaluation. Table 2 showed the mean time spent by mice in immobile state after administration of EAF (90 mg/kg, p.o.), BF (80 mg/kg, p.o.), imipramine (15 mg/kg, p.o.) and the control (vehicle, p.o.). Only EAF exhibited significant antidepressant activity at the dose of 90 mg/kg with respect to control and the activity was statistically equivalent to that of imipramine. BF reduced significantly immobility time in when compared to the control, but this reduction is much lower than that produced by imipramine. This observation confirms mild antidepressant activity of BF.
| Treatment | Dose (mg/kg) | Meann immobility time (s)±SD |
|---|---|---|
| Control | Vehicle | 278.33±11.25a |
| Imipramine | 15 | 63.33±5.20* |
| EAF | 90 | 62.66±5.24* |
| BF | 80 | 163.16±10.77*a |
Table 2: Antidepressant activity of various fractions obtained from bioactive me of B. Tinctoria roots using fst
The exploratory behaviour of mice in terms of number of squares crossed and rearings in open field apparatus was observed to assess locomotor activity of ME (400 mg/kg, p.o.), EAF (90 mg/kg, p.o.), imipramine (15 mg/kg, p.o.) and the control (vehicle, p.o.). ME and EAF did not exhibit any effect on the number of crossing and rearing in the open field test at 400 and 90 mg/kg, respectively (Table 3). ME and EAF did not show any effect on locomotor activity of mice in open field test, thus, suggested that the decrease in the immobility elicited by ME and EAF in the FST was not related to a psychostimulant effect. These observations confirmed specific antidepressant activity of ME of B. tinctoria roots, and it’s EAF.
| Treatment Group |
Dose (mg/kg) |
Meann number of squares crossed±SD | Meann number of rearings±SD |
|---|---|---|---|
| Control | Vehicle | 57.85±7.01 | 16.24±2.99 |
| Imipramine | 15 | 56.87±6.58 | 16.05±3.87 |
| ME | 400 | 58.44±7.88 | 17.10±3.25 |
| EAF | 90 | 57.25±8.49 | 16.74±2.01 |
Table 3: Locomotor activity of me and eaf of B. Tinctoria roots using open field test
Several studies reported that flavonoids exhibited CNS activities [19]. Therefore, an attempt was made to characterize crude ME of B. tinctoria roots by comparing TLC profiles of reference flavonoids (quercetin, naringenin, hesperitin, apigenin, chrysin) with crude extract. The results of comparative TLC studies showed that ME contains hesperitin. Hesperitin is being reported for the first time in this plant. A perusal of literature revealed that hesperitin and its glycoside hesperidin exhibited strong antidepressant activity. Hesperidin has been reported to exhibit antidepressant activity at acute and chronic doses of 0.1, 0.3 or 1 mg/ kg, i.p., and this effect appeared to be mediated by the inhibition of l-arginine-NO-cGMP pathway and elevation of BDNF levels in hippocampus [20]. The antidepressant activity of hesperidin has been reported at the doses of 0.1, 0.3 or 1 mg/kg, i.p., using FST and tail suspension test via interaction with the κ-opioid receptor and serotonergic 5-HT(1A) receptors [21,22]. In an another study, antidepressant activity of hesperidin was reported at the dose of 0.7 mg/kg, i.p. using FST [23]. These reports support the conclusion that hesperitin, found to be one of the constituents of B. tinctoria roots could be responsible for the antidepressant effects of the plant. Thus, hesperitin was taken as a chemical marker to standardize B. tinctoria using validated TLC densitometric method.
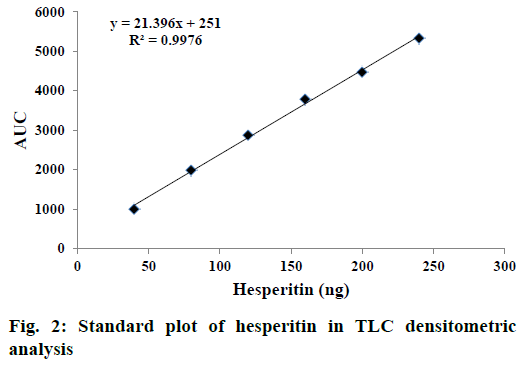
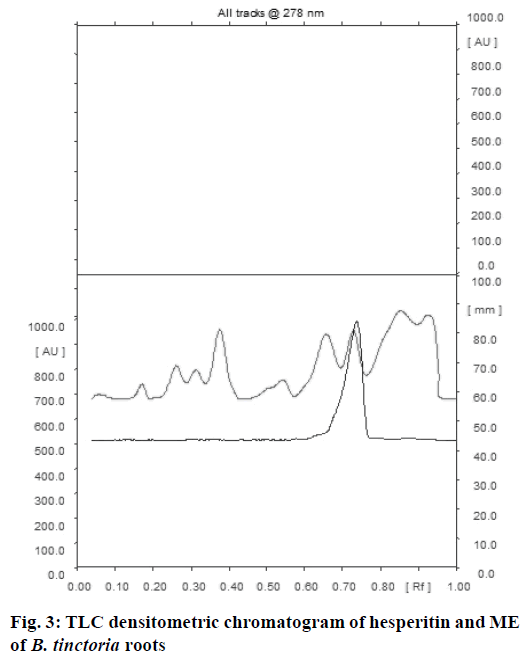
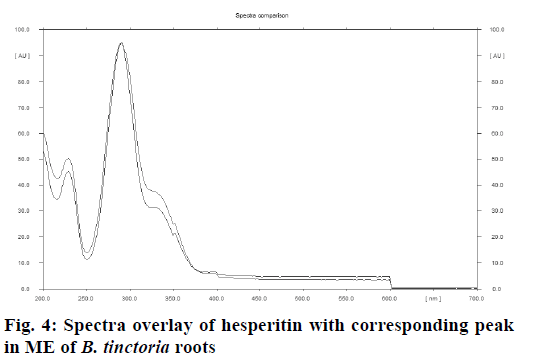
Comparative fingerprint profile of ME and hesperitin of B. tinctoria roots visualized under ultraviolet light at 254 nm is presented in Figure 1. A standard plot was prepared between different concentrations of hesperitin and their peak areas after scanning at 278 nm (Figure 2). Linearity of calibration plot of hesperitin was achieved between 40 to 240 ng. The content of hesperitin in B. tinctoria roots was found to be 0.0085±0.00002% w/w. ICH guidelines were followed to validate the developed TLC densitometric method for estimation of hesperitin in B. tinctoria roots. The prescribed limits of validation parameters such as instrumental precision, intra and inter-day precision and repeatability are less than 2% CV (Tables 4 and 5). When thin layer chromatograms (Figure 3) and UV spectra (Figure 4) overlays of standard and sample were studied, no interference was observed. This observation inferred that developed TLC densitometric method was specific for estimation of hesperitin. In accuracy studies, average recovery was found to be 98.9%. These observations infer that the developed method for estimation of hesperitin in B. tinctoria roots is precise, accurate, reproducible and specific.
| Parameter | Hesperitin |
|---|---|
| Instrumental precision (% CV, n=7) | 0.55 |
| Repeatability (% CV, n=5) | 0.10 |
| Coefficient of determination (r2) | 0.9976 |
| Linearity range (ng) | 40-240 |
| LOD (ng) | 4 |
| LOQ (ng) | 14 |
| Intra-day precision (% CV, n=9) | 0.40 |
| Inter-day precision (% CV, n-=9) | 0.55 |
| Accuracy (average % recovery) | 98.90 |
| Specificity | Specific |
Table 4: Method Validation Parameters of Hesperitin in Tlc Densitometric Analysis
| Compound | Amount of marker present (µg) | Amount of marker added (µg) | Amount of marker found (µg) (meann±SD) | Recovery (%) | Average recovery (%) (meann±SD) |
|---|---|---|---|---|---|
| Hesperitin | 170 170 170 |
136 (80%) 170 (100%) 204 (120%) |
302.41±1.95 335.65±2.64 370.86±3.47 |
98.83 98.72 99.16 |
98.90±0.23 |
Table 5: Recovery Studies of Hesperitin
Acknowledgement
Authors duly acknowledge Prof. R. C. Gupta, Coordinator of DBT-IPLS project for providing access to instrumentation facilities at Punjabi University, Patiala and thank Dr. Avneet Pal Singh, Assistant Professor, Department of Botany, Punjabi University, Patiala for authenticating the plant material.
Conflict of interest
The authors declare no conflict of interest with respect to this work.
Financial support and sponsorship
Nil.
References
- Kendler KS, Gardner CO, Neale MC, Prescott CA. Genetic risk factors for major depression in men and women: similar or different heritabilities and same or partly distinct genes. Physiol Med 2001;31:605-16.
- Rahman A, Patel V, Maselko J, Kirkwood B. The neglected ‘m’ in MCH programmes - why mental health of mothers is important for child nutrition. Trop Med Int Health 2008;13:579-83.
- http://www.who.int/mental_health/management/depression/wfmh_paper_depression_wmhd_2012.pdf.
- Baldessarini RJ. Drugs and the treatment of psychiatric disorders. In: Hardman JG, Limbird, LE, Gilman AG, editors. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. New York: McGraw-Hill; 2001. p. 987-96.
- Majeroni BA, Hess AD. The pharmacologic treatment of depression. J Am Board Fam Med 1998;11:127-39.
- Dziukas LJ, Vohra J. Tricyclic antidepressant poisoning. Med J Aus 1991;154:344-50.
- Arnold AH, Gulumian M. Pharmacopoeia of traditional medicine in Venda. J Ethnopharmacol 1984;12:35-74.
- Zolla C. Traditional medicine in Latin American with particular reference to Mexico. J Ethnopharmacol 1980;2:37-51.
- Randhawa K, Kumar D, Jamwal A, Kumar S. Screening of antidepressant activity and estimation of quercetin from Cocciniaindicausing TLC densitometry. Pharm Biol 2015;53:1867-74.
- Bergner P. Nervous depression and homeopathic indications of herbs. Med Herb 1995;9:12.
- http://www.homeoint.org/books/boericmm/b/bapt.html.
- http:/www.herbaltransitions.com/materiamedica/Baptisia.html.
- Wagner H, Proksch A, Riess-Maurer I, Vollmar A, Odenthal S, Stuppner H, et al. Immunostimulant action of polysaccharides (heteroglycans) from higher plants. Med Res 1984;34:659-61.
- Beuscher N, Beuscher HU, Bodinet C. Enhanced Release of Interleukin-1 from mouse macrophages by glycoprotein and polysaccharides from Baptisiatinctoriaand Echinacea species. Planta Med 1989;55:660.
- Wagner H, Jurcic K. Immunological studies of plant extract combination/in vitroand in vivostudies on the stimulation of phagocytosis. Med Res 1991;41:1072-6.
- Farnsworth NR. Biological and chemical screening of plants. J PharmaSci 1966;55:225-76.
- Scheffer WC. Statistics for the biological sciences. 32nd ed. Philippines: Addison-Wesley Publishing Company; 1980. p. 121-41.
- Weiss JM, Goodman PA, Losito GO, Corrigan S, Carris JM, Bailey WH. Behavioural depression produced by uncontrollable stressor: Relationship to norepinephrine, dopamine and serotonin levels in various regions of rat brain. Brain Res 1981;3:167-205.
- Butterweck V, Jurgenliemk G, Nahrstedt A, Winterhoff H. Flavonoids of Hypericumperforatumshow antidepressant activity in forced swim test. Planta Med 2000;66:291-5.
- Donato F, de Gomes MG, Goes AT, Filho CB, Del Fabbro L, Antunes MS, et al. Hesperidin exerts antidepressant-like effects in acute and chronic treatments in mice: possible role of l-arginine-NO-CGMP pathway and BDNF levels. Brain Res Bull 2014;104:19-26.
- Filho CB, Del Fabbro L, de Gomes MG, Goes AT, Souza LC, Boeira SP, et al. Kappa-opioid receptors mediate the antidepressant-like activity of hesperidin in the mouse forced swimming test. Eur J Pharmacol 2013;698:286-91.
- Souza LC, de Gomes MG, Goes AT, Del Fabbro L, Filho CB, Boeira SP, et al. Evidence for the involvement of the serotonergic 5-HT(1A) receptors in the antidepressant-like effect caused by hesperidin in mice. ProgNeuropsychopharmacolBiol Psych 2013;40:103-9.
- Herrera-Ruiz M, Zamilpa A, Gonzalez-Cortazar M, Reyes-Chilpa R, Leon E, Garcia MP, et al. Antidepressant effect and pharmacological evaluation of standardized extract of flavonoids from Byrsonimacrassifolia. Phytomedicine 2011;18:1255-61.