- *Corresponding Author:
- Nan Li
Department of Neurosurgery, People's Liberation Army (PLA) Strategic Support Force Characteristic Medical Center, Chaoyang, Beijing 100101, China
E-mail: linan107072@163.com
| Date of Received | 04 July 2023 |
| Date of Revision | 06 December 2023 |
| Date of Acceptance | 09 March 2024 |
| Indian J Pharm Sci 2024;86(2):593-598 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Previous literature has described the significant biological and pharmacological activities of rutin in mammals. Nevertheless, there is no known function or role for Rutin in spinal cord neuroinflammation. In this research, 120 male adult Sprague-Dawley rats were randomly divided into 4 groups (n=30 per group) viz., sham operation (control group), spinal cord injury, methylprednisolone treatment and rutin treatment. Enzyme-linked immunosorbent assay analysis of pro-inflammatory cytokines, malondialdehyde, superoxide dismutase, catalase, and glutathione peroxidase were analyzed using kits. Fibroblast growth factor, neurotrophin 3, brain-derived neurotrophic factor, and nerve growth factor, messenger ribonucleic acid level were examined using real-time fluorescent quantitative polymerase chain reaction. Western blot analysis of p38 mitogen-activated protein kinase protein expression relative to the model group (spinal cord injury group), inflammatory cytokines, malondialdehyde levels, spinal cord water content, and p38 mitogen-activated protein kinase protein levels were reduced in the rutin treatment group, however, the superoxide dismutase, catalase, and glutathione peroxidase levels, fibroblast growth factor, neurotrophin 3, brain-derived neurotrophic factor, and nerve growth factor, messenger ribonucleic acid levels, and the blood-brain barrier score (24, 48, 72 h) were increased. Rutin relieves spinal cord neuroinflammation by inhibiting the p38 mitogen-activated protein kinase signaling pathway.
Keywords
Rutin, spinal cord injury, inflammatory response, p38 mitogen-activated protein kinase signaling pathway
Spinal Cord Injury (SCI) frequently leads to severe dysfunction of the limbs below the injured segment[1]. Owning to the lack of effective treatments, SCI has caused a tremendous economic and social burden worldwide[2]. Generally, the pathophysiology of SCI is classified into two stages; irreversible primary injury and secondary damage arising from oxidative stress and inflammatory response[3]. In fact, the degree of secondary injury largely determines the severity of injury. Emerging evidence indicated that neuroinflammation, the principal secondary change following SCI, was an essential role in modulating SCI pathological progression[4]. Of note, rutin (also known vitamin P) comprises flavonal quercetin and disaccharide rutinose and possessed anti-oxidant, anti-inflammatory, anti-bacterial, and other biological effects[5,6]. Numerous studies have suggested that Rutin Treatment (RT) group might protect against myocardial and kidney damage by anti-oxidative and anti-inflammatory action via Mitogen-Activated Protein Kinase (MAPK) pathway[7,8]. Furthermore, p38-MAPK pathway might mediate the inflammatory response after SCI[9]. Herein, this project focused on whether Rutin might exert the neuroprotective role on SCI via regulating p38 MAPK pathway.
Materials and Methods
Animal:
In this study, 120 male adult rats (aged 2-3 mo, weight 250±20 g) were selected and obtained from Animal Research Center of Shandong University of China (SYXK (Lu) 2020-0001) under controlled conditions.
Reagents and chemicals:
Enzyme-Linked Immunosorbent Assay (ELISA) detection kits and oxidative stress index detection kits were respectively provided by Beyotime (Shanghai, China) and Mlbio (Shanghai, China). Takara (Tokyo, Japan) offered Ribonuclic Acid (RNA)-Trizol and Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR) kits. Baucide Biotechnology (Beijing, China) offered Radioimmunoprecipitation Assay (RIPA) protein lysate and Bicinchoninic Acid (BCA) kits. Besides, Solarbio (Beijing, China) offered Rutin (powder, purity ≥98 %).
Methods:
Preparation of rat SCI model: After being anesthetized, the skin on the back of the rats was completely exposed. Subsequently, a median incision of about 2.5 cm in length was made by the spinous process on the thoracic 10 vertebrae as the center of the incision, followed by separation of the muscles on both sides of the vertebral plate to both sides and full exposure of the vertebral plate. After complete resection of the spinous and vertebral plates of thoracic 9 to 11, the spinal cord of this segment was completely exposed. After that, a guide pin weighing approximately 15 g was dropped from a height of 5 cm above the spinal cord to accurately compress the dura mater, after which the pin was immediately removed. The modeling was successful when the rat showed twitching of the body and both lower limbs and tail wagging, followed by complete relaxation of both lower limbs after a few seconds. The rats were sampled from the 3rd d after successful modeling.
Grouping: 120 Sprague-Dawley (SD) rats were randomly divided into the following groups (30 rats in each group); Sham-operated (control group), consisting of rats undergoing laminectomy but were not subjected to spinal cord compression, was injected with 1 ml Dimethyl Sulfoxide (DMSO); SCI model, composed of SCI rats injected with 1 ml DMSO; Methylprednisolone treatment (MP group), composed of SCI rats intraperitoneally injected with 100 mg/kg MP group; RT group, composed of SCI rats intraperitoneally injected with rutin 100 mg/kg and 1 ml DMSO.
ELISA: After being collected peripheral blood from heart of each group, these samples were centrifuged. Then, the supernatant was collected and detected using Interleukin-1 Beta (IL-1β), IL-6, Tumor Necrosis Factor-Alpha (TNF-α), ELISA kits.
Assessment of oxidative stress: Based on commercial kits, the concentrations of Malondialdehyde (MDA), Superoxide Dismutase (SOD), Catalase (CAT), Glutathione Peroxidase (GSh-Px) were measured in collected supernatant.
RT-qPCR: Total RNAs from these spinal cord tissues were extracted using RNA-Trizol solution. Subsequently, total RNAs were reversely transcribed into complementary Deoxyribonucleic Acid (cDNA), which then was used for RT-qPCR reaction. The cycling condition: 95° pre-denaturation for 5 min, 95° denaturation for 30 s, 60° annealing 30 s, extension at 72° for 30 s. The reaction was performed in 40 cycles. Primer sequences (Sangon Biotech, Shanghai, China) were shown; Fibroblast Growth Factor (FGF): 5’-CTTGACGTCGTGGAACGATCT-3’ (sense), 5’-AGAACGGTCAACCATGCAGAG-3’ (antisense); Neurotrophin-3 (NT-3): 5’-GCGAATTCATGTCACGCGGAGCATAAG-3’ (sense), 5’-GCGGATCCTCAGCCCGGCC-3’ (antisense); Brain-Derived Neurotrophic Factor (BDNF): 5’-TCAAGTTGGAAGCCTGAATGAATG-3’ (sense), 5’-CTGATGCTCAGGAACCCAGGA-3’ (antisense); Nerve Growth Factor (NGF): 5’-TGCCAAGGACGCAGCTTTC-3’ (sense), 5’-TGAAGTTTAGTCCAGTGGGCTTCAG-3’(antisense).
Measurement of spinal cord water content: After all rats were executed, spinal cord samples were subjected to drying for 48 h to examine the dry weights. Based on the formula (wet weight-dry weight)/wet weight×100 %, spinal cord water content was calculated.
Blood-Brain Barrier (BBB) scores: In short, BBB score is a method for evaluating the locomotor function of rats[10], which is divided into 22 grades. In this research, BBB score was calculated for 24, 48, 72 h.
Western blot: After extracting the total protein of spinal cord tissue, the protein concentration was determined by BCA method, followed by high temperature denaturation. After that, these samples were loaded and subjected to Sodium Dodecyl-Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE) separation and transferred to Polyvinylidene Difluoride (PVDF) membrane. Following blocked, the primary antibody at 4° was added to the membrane overnight, which then were incubated with the secondary antibody. At last, signals were assessed using ImageJ software.
Statistical analysis:
Statistics was implemented using Statistical Package for the Social Sciences (SPSS) 22.0 software. Results were expressed as (x±s) and compared with t-test for two groups, one-way Analysis of Variance (ANOVA) for multiple groups, and Least Significant Difference (LSD)-t for inter-groups. p<0.05 was deemed significant.
Results and Discussion
Referring to data exhibited in Table 1, SCI might improve IL-1β, IL-6, and TNF-α relative to control group. Nevertheless, these inflammatory cytokines were apparently decreased in RT group vs. SCI group. As shown in Table 2, SCI might induce MDA content enhancement vs. the control group, while Rutin might reduce MDA level relative to the SCI group. Meanwhile, SCI decreased SOD, CAT, GSH-Px contents relative to control group, whereas Rutin might obviously enhance their levels in comparison with SCI group.
| Groups | IL-1β (pg/ml) | IL-6 (pg/ml) | TNF-α (pg/ml) |
|---|---|---|---|
| Control | 2.07±0.11 | 1.83±0.25 | 99.27±8.14 |
| SCI | 5.27±0.19* | 6.35±1.11* | 278.94±33.25* |
| MP | 3.98±0.25# | 3.46±0.25# | 146.73±25.59# |
| RT | 3.01±0.17## | 2.75±0.33## | 123.77±20.36## |
| F | 273.649 | 275.664 | 392.304 |
| p | 0.000 | 0.000 | 0.000 |
Note: *p<0.05, and #p<0.05 relative to the control group and the SCI group, respectively
Table 1: Anti-inflammatory effects of rutin on SCI (x̄±s, n=9).
| Groups | MDA (mmol/ml) | SOD (U/ml) | CAT (U/ml) | GSH-Px (U/ml) |
|---|---|---|---|---|
| Control | 2.55±0.25 | 23.58±4.18 | 212.39 ±26.83 | 60.35±5.39 |
| SCI | 6.94±0.29* | 7.35±0.82* | 50.37±4.29* | 23.75±3.11* |
| MP | 3.62±0.15# | 18.37±1.84# | 173.94±30.84# | 56.39±4.11# |
| RT | 3.08±0.22## | 20.75±4.83## | 196.74± 29.64## | 58.92±6.20## |
| F | 183.495 | 155.385 | 169.063 | 172.007 |
| p | 0.000 | 0.000 | 0.000 | 0.000 |
Note: *p<0.05, and #p<0.05 in comparison with the control group and the SCI group, respectively
Table 2: Anti-oxidative effects of RUTIN on SCI (x̄±s, n=9).
According to the data shown in Table 3, no significant differences in FGF, NT-3, BDNF, NGF messenger RNA (mRNA) levels were found between control group and SCI group. However, these growth factors were apparently raised after RT compared with the SCI group.
| Groups | FGF mRNA | NT-3 mRNA | BDNF mRNA | NGF mRNA |
|---|---|---|---|---|
| Control | 1.00±0.09 | 1.02±0.11 | 1.00±0.10 | 1.01±0.10 |
| SCI | 1.19±0.24 | 1.19±0.11 | 1.18±0.11 | 1.15±0.13 |
| MP | 1.69±0.33# | 1.58 ±0.25# | 1.77±0.28# | 1.53±0.26# |
| RT | 1.98±0.36## | 2.04±0.17## | 1.95±0.35## | 1.85±0.14## |
| F | 185.365 | 225.873 | 196.703 | 202.337 |
| p | 0.000 | 0.000 | 0.000 | 0.000 |
Note: *p<0.05 and #p<0.05 vs. the control group and the SCI group, respectively
Table 3: Rutin influences nerve growth factors after SCI (x̄±s, n=9).
In comparison with the control group, SCI might improve spinal cord water content. On the contrary, its content was significantly increased after Rutin vs. SCI group (Table 4). Relative to the control group, SCI decreased BBB scores at 24, 48, and 72 h. Besides, Rutin or MP led to an enhancement in the BBB scores compared with the SCI group (Table 5).
| Groups | Spinal cord water content (%) |
|---|---|
| Control | 62.49±5.92 |
| SCI | 93.85±7.10* |
| MP | 73.74±8.36# |
| RT | 70.65±5.63## |
| F | 367.843 |
| p | 0.000 |
Note: *p<0.05 and #p<0.05 vs. the control group and the SCI group, respectively
Table 4: Rutin affects the spinal cord water content (x̄±s, n=9).
| Groups | BBB score (24 h) | BBB score (48 h) | BBB score (72 h) |
|---|---|---|---|
| Control | 18±1 | 20±2 | 22±2 |
| SCI | 8±1* | 7±1* | 9±1* |
| MP | 13±2# | 14±2# | 16±3# |
| RT | 17±3## | 18±4## | 19±4## |
| F | 195.837 | 226.744 | 273.846 |
| p | 0.000 | 0.000 | 0.000 |
Note: *p<0.05 and #p<0.05 compared with the control group and the SCI group, respectively
Table 5: BBB score assessed the effect of rutin on locomotor recovery after spinal cord injury (x̄±s, n=9).
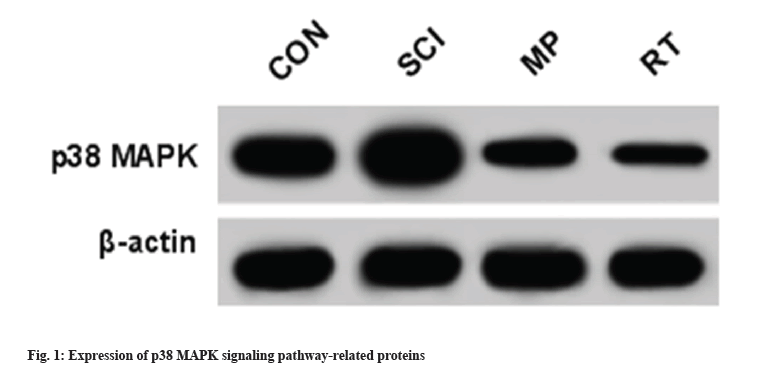
As presented in Table 6 and fig. 1 SCI might substantially elevate p38 MAPK protein level relative to the control group. In addition, its protein expression was clearly hindered by RT or MP treatment vs. the control group.
| Groups | p38 MAPK protein level |
|---|---|
| Control | 1.00±0.09 |
| SCI | 1.56±0.25* |
| MP | 0.88±0.08# |
| RT | 0.62±0.06## |
| F | 137.83 |
| p | 0.000 |
Note: *p<0.05 and #p<0.05 relative to the control group and SCI group, respectively
Table 6: Rutin affects p38 MAPK signal pathway (x̄±s, n=9).
As a debilitating injury, SCI has been considered as a negative impact in human health and the quality of life all over the world. Furthermore, emerging evidence has indicated that SCI is a serious complication of spinal fractures and dislocations, which is categorized into primary SCI and secondary SCI, manifesting as sensory-motor dysfunction of the trunk and extremities[11,12].
Current studies have presented that inflammatory response and oxidative stress after SCI play major pathogenesis roles in secondary damage, and inflammatory cytokines and free radical levels represent indicators for damage extent[13,14]. Therefore, preventing or decreasing inflammatory response have been regarded as the therapeutic efficacy for SCI. Herein, our data found that rutin might apparent reduced IL-1β, IL-6, and TNF-α levels in SCI rat model, implying the repression of rutin on inflammation response. Meanwhile, current work verified that rutin or MP treatment might decrease MDA content and enhance the levels of SOD, CAT, and GSH-Px in the SCI rat model, indicating that rutin might protects cells of the spinal cord via attenuating oxidative stress. Previous studies have suggested that NGF might boost the axonal sprouting of the sensory afferents and achieve better behavioral outcome[15]. It has been reported that the upregulation of NGF might repair SCI in rats, improving hind limb movement[16]. In this work, these NGF (FGF, NT-3, BDNF, NGF) were obviously improved after RT in SCI rat model. In fact, rutin has been substantiated to possess various pharmacological activities. Earlier studies have shown that BBB scores assessed hind limb motor function in rats, and that both MP and Rhodiola rosea glycosides increased hind limb BBB scores in a rat SCI rat model[17,18]. Herein, our data exhibited that rutin might elevate BBB score and decrease spinal cord water content of rats, implying that rutin might attenuate histological alterations and improve exercise recovery.
MAPK signaling pathway plays an important role in development and disease pathogenesis. As a key member of the MAPK family, p38 MAPK might control inflammatory responses. It has been reported that inflammatory cytokines, osmotic stress, and Ultraviolet (UV) irradiation, might trigger p38 MAPK activation through kinase cascades, thereby regulating cell proliferation, differentiation and apoptosis[19-21]. Herein, our data displayed that rutin reduced p38 MAPK protein expression in SCI, indicating that rutin might partly relieve inflammatory response by repressing p38 MAPK pathway.
In summary, rutin might partly protect spinal cord neuroinflammation through regulating the p38 MAPK pathway, providing a new direction for SCI.
Funding:
This work was supported by institute-level project (21XK0112).
Conflict of interests:
The authors declared no conflict of interests.
References
- Eli I, Lerner DP, Ghogawala Z. Acute traumatic spinal cord injury. Neurol Clin 2021;39(2):471-88.
[Crossref] [Google Scholar] [PubMed]
- Liu Y, Yang X, He Z, Li J, Li Y, Wu Y, et al. Spinal cord injury: Global burden from 1990 to 2019 and projections up to 2030 using Bayesian age-period-cohort analysis. Front Neurol 2023;14:1304153.
[Crossref] [Google Scholar] [PubMed]
- Hu X, Xu W, Ren Y, Wang Z, He X, Huang R, et al. Spinal cord injury: Molecular mechanisms and therapeutic interventions. Signal Transduct Target Ther 2023;8(1):245.
[Crossref] [Google Scholar] [PubMed]
- Freyermuth-Trujillo X, Segura-Uribe JJ, Salgado-Ceballos H, Orozco-Barrios CE, Coyoy-Salgado A. Inflammation: A target for treatment in spinal cord injury. Cells 2022;11(17):2692.
[Crossref] [Google Scholar] [PubMed]
- Goyal J, Verma PK. An overview of biosynthetic pathway and therapeutic potential of rutin. Mini Rev Med Chem 2023;23(14):1451-60.
[Crossref] [Google Scholar] [PubMed]
- Muvhulawa N, Dludla PV, Ziqubu K, Mthembu SX, Mthiyane F, Nkambule BB, et al. Rutin ameliorates inflammation and improves metabolic function: A comprehensive analysis of scientific literature. Pharmacol Res 2022;178:106163.
[Crossref] [Google Scholar] [PubMed]
- Wang Y, Zhang Y, Sun B, Tong Q, Ren L. Rutin protects against pirarubicin-induced cardiotoxicity through TGF-β1-p38 MAPK signaling pathway. Evid Based Complement Alternat Med 2017;2017:1759385.
[Crossref] [Google Scholar] [PubMed]
- Salama SA, Arab HH, Maghrabi IA. Troxerutin down-regulates KIM-1, modulates p38 MAPK signaling, and enhances renal regenerative capacity in a rat model of gentamycin-induced acute kidney injury. Food Funct 2018;9(12):6632-42.
[Crossref] [Google Scholar] [PubMed]
- Wu Z, Wang Z, Xie Z, Zhu H, Li C, Xie S, et al. Glycyrrhizic acid attenuates the inflammatory response after spinal cord injury by inhibiting high mobility group box-1 protein through the p38/Jun N-terminal kinase signaling pathway. World Neurosurg 2022;158:e856-64.
[Crossref] [Google Scholar] [PubMed]
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma 1995;12(1):1-21.
[Crossref] [Google Scholar] [PubMed]
- Chen WC, Liu WF, Bai YY, Zhou YY, Zhang Y, Wang CM, et al. Transplantation of mesenchymal stem cells for spinal cord injury: A systematic review and network meta-analysis. J Transl Med 2021;19(1):1-4.
[Crossref] [Google Scholar] [PubMed]
- Li X, Lou X, Xu S, Du J, Wu J. Hypoxia inducible factor-1 (HIF-1α) reduced inflammation in spinal cord injury via miR-380-3p/NLRP3 by Circ 0001723. Biol Res 2020;53(1):35.
[Crossref] [Google Scholar] [PubMed]
- Zhang P. Experimental study on inhibition of inflammatory response by rutin in rats with spinal cord injury and its related mechanism. Shanxi Dayi Hosp 2017;10(2):1-10.
- Deng GY. miR-136-5p affects the expression of inflammatory factors in spinal cord injury by targeting IKKβ and regulating NF-κB signaling pathway. Guangxi Med Univ 2019;50(2):512-524.
- Romero MI, Rangappa N, Li L, Lightfoot E, Garry MG, Smith GM. Extensive sprouting of sensory afferents and hyperalgesia induced by conditional expression of nerve growth factor in the adult spinal cord. J Neurosci 2000;20(12):4435-45.
[Crossref] [Google Scholar] [PubMed]
- Feng SQ, Kong XH, Liu Y, Ban DX, Ning GZ, Chen JT, et al. Regeneration of spinal cord with cell and gene therapy. Orthopaed Surg 2009;1(2):153-63.
[Crossref] [Google Scholar] [PubMed]
- Wang F, Xia Y, Yu H. Effect of P38 MAPK on the expression of GFAP and vimentin in the early stage of glial scar formation after spinal cord injury in rats. Basic Med Clin 2016;36(4):462-7.
- Su YJ. Effect of salidroside on motor function in rats with spinal cord injury by interfering with NF-κB and MAPK signaling pathway. Guangxi Med Univ 2017;234(8):14259-69.
- Falcicchia C, Tozzi F, Arancio O, Watterson DM, Origlia N. Involvement of p38 MAPK in synaptic function and dysfunction. Int J Mol Sci 2020;21(16):5624.
[Crossref] [Google Scholar] [PubMed]
- Li C, Guo Z, Yang R. Protective effect of myricetin on spinal cord injury in rats by regulating NF-κB and MAPK signaling pathway. J Guangxi Med Univ 2020;37(11):7.
- Zeyen L, Seternes OM, Mikkola I. Crosstalk between p38 MAPK and GR signaling. Int J Mol Sci 2022;23(6):3322.
[Crossref] [Google Scholar] [PubMed]