- Corresponding Author:
- Renu Solanki
Lachoo Memorial College of Sciences and Technology, Pharmacy Wing, Sector-A, Shastri Nagar, Jodhpur - 342 003, India
E-mail: solankirenu@yahoo.com
| Date of Submission | 2 Sepetember 2009 |
| Date of Revision | 23 January 2010 |
| Date of Acceptance | 21 May 2010 |
| Indian J Pharm Sci, 2010, 72 (3): 384-387 |
Abstract
A new reverse phase high performance liquid chromatography method for the simultaneous estimation of frusemide and amiloride hydrochloride in tablet formulation is developed. The determination was carried out on a HIQ SIL, C18 (250Χ4.6 mm, 5 μm) column using a mobile phase of 50 mM phosphate buffer solution:acetonitrile (50:50 v/v, pH 3.0). The flow rate was 1.0 ml/min with detection at 283 nm. The retention time for frusemide was 3.038 min and for amiloride hydrochloride 10.002 min. Frusemide and amiloride hydrochloride showed a linear response in the concentration range of 20-200 μg/ml and 10-100 μg/ml, respectively. The results of analysis have been validated statistically and by recovery studies. The mean recoveries found for frusemide was 99.98% and for amiloride hydrochloride was 100.09%. Developed method was found to be simple, accurate, precise and selective for simultaneous estimation of frusemide and amiloride hydrochloride in tablets.
Keywords
Amiloride hydrochloride, frusemide, RP-HPLC, simultaneous determination
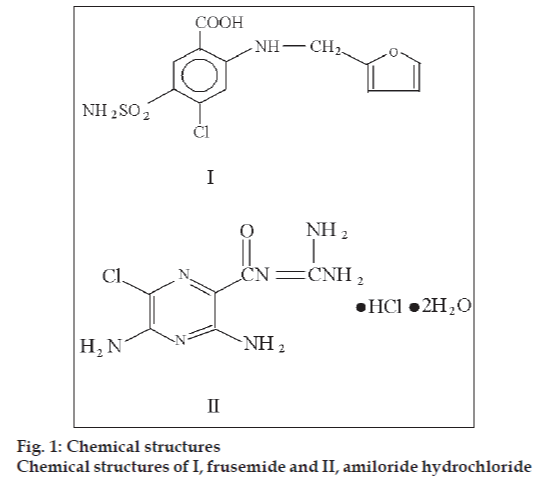
Frusemide (FRM) is chemically 4-chloro-2- furfurylamino-5-sulphamoyl benzoic acid (fig. 1). It is a potent loop diuretic [1]. It acts primarily by blocking sodium and chloride reabsorption in the ascending limb of the loop of Henle. FRM helps to conserve potassium and minimize the risk of alkalosis, in the treatment of oedema associated with hepatic cirrhosis and congestive heart failure. Several analytical methods have been reported for quantitative determination of frusemide individually by UV [2,3], GC [4], TLC [5], HPLC [6,7] and colorimetry [8,9].
Amiloride hydrochloride (AH) is chemically 3,5-diamino-N-(diaminomethylene)-6-chloropyrazinecarboxamide monohydrochloride dihydrate (fig. 1). It is a potassium sparing diuretic [1]. AH in conjunction with loop diuretics such as FRM, reduces overall fluid volume in the body and help to control symptoms of heart disease, kidney and liver disease [10,11]. The individual determination of AH is carried out by UV [12,13], TLC [14] and HPLC [15] methods. FRM is offi cial in IP [16], BP [17] and USP-NF [18] and AH is offi cial in IP [19], BP [20] and USP-NF [21].
In recent years, these two drugs are successfully used in association in the treatment of many diseases related to kidney, liver and heart and the pharmaceutical preparation containing both drugs have been marketed. Although, many methods have been reported in the literature for the estimation of FRM and AH individually, there is no single method reported for simultaneous estimation of these drugs in combined dosage form. Hence, in the present assay, a new simple, sensitive, accurate and specifi c reverse phase high performance liquid chromatography (RP-HPLC) method is developed and validated for simultaneous estimation of FRM and AH in tablet formulation.
Working reference standards of FRM and AH were kindly supplied as gift samples by Elder Pharmaceuticals Ltd., Mumbai, India. Two marketed formulations with brand names, Amifru (Elder Pharmaceutical Ltd., Mumbai, India) and Frumil (Geno Pharmaceuticals, Goa, India) were procured from the local pharmacy. The solvents used were of HPLC/AR grade. Double distilled water was used for analysis.
A gradient HPLC (Water, Germany) with PU-1580 double reciprocating pump, UV-1575 UV detector, and RP-C18 column (5 μm particle size) was used. The RP-HPLC system was equipped with Winchrom software for data processing. Method was developed using a HIQ SIL, C18 (250×4.6 mm, 5 μm) column. Mobile phase was used for preparation of drug samples throughout the analysis. For preparing the mobile phase 50 mM phosphate buffer and acetonitrile were mixed together in the ratio of 50:50% v/v and pH of the resulting solution was adjusted to 3.0. It was filtered before use through 0.45 μ membrane fi lter. Flow rate employed was 1.0 ml/min. Detection was carried out at 283 nm at 25º.
Among the several mobile phases used for the present assay phosphate buffer and acetonitrile in the ratio of 50:50 v/v, pH 3.0 was found to be most suitable. With the above mobile phase a good resolution between FRM and AH was achieved. UV detection was carried out at 283 nm as FRM and AH both showed good absorbance at this wavelength.
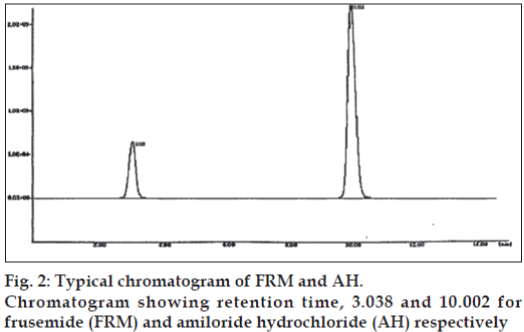
Standard stock solution of FRM (200 μg/ml) was prepared by dissolving 20 mg FRM in 100 ml mobile phase. Standard stock solution of AH (100 μg/ml) was prepared by dissolving 10 mg AH in 100 ml mobile phase. Aliquots of standard stock solutions of FRM and AH were taken in 10 ml volumetric flasks and diluted upto the mark with mobile phase in such a way that fi nal concentrations of FRM and AH were in the range of 20–200 μg/ ml and 10-00 μg/ml, respectively. The standard solutions were further diluted to contain a mixture of 80 μg/ml of FRM and 10 μg/ml of AH. Twenty tablets of Amifru and Frumil each containing 40 mg of FRM and 5 mg of AH were weighed and fi nely powered separately. Powder equivalent to 80 mg FRM and 10 mg AH was weighed and dissolved in 100 ml mobile phase. The solution was sonicated for 15 min and was filtered through a Whatman filter paper no. 40. Further dilutions were made to get a concentration of 80 μg/ml of FRM and 10 μg/ml of AH. These solutions were fi ltered through 0.45 μm membrane fi lter. Ten microlitre solution of the each tablet was injected separately and chromatograms were recorded. A representative chromatogram is shown in fig. 2.
The retention time of FRM and AH was found to be 3.038 min and 10.002 min, respectively. The peak shapes of both the drugs were symmetrical and asymmetry factor was less than 2.0. The proposed method was validated as per the standard analytical procedure. Each sample was repeated 6 times and the same retention time was observed in all the cases. Linearity experiments were performed by giving six replicates for both the drugs and response was found to be linear in the range of 20-200 μg/ml of FRM and 10 -100 μg/ml of AH. Each standard solution (10 μl) was injected into the column after fi ltration using 0.45 μm membrane filter. The calibration curves were constructed by plotting the peak areas versus the corresponding drug concentration. The slope and correlation coeffi cients were determined, which were found to be 0.99995 for FRM and 0.99925 for AH. In precision studies, the injection repeatability showed a RSD of 0.069% for frusemide and 0.400% for amiloride hydrochloride. The intra-day analysis showed a RSD of 0.072% for frusemide and 0.754% for amiloride hydrochloride and the inter-day study showed a RSD of 0.024, 0.015, 0.028% for frusemide and 0.766, 0.693, 0.749% for amiloride hydrochloride for day 1, 2 and 3, respectively. These results indicate good precision of the samples analyzed. System suitability parameters of FRM and AH are given in the Table 1. Accuracy of the method was calculated by recovery studies (n=3) at five levels. Standard drug solutions containing drugs in the concentration range of 80-160 μg/ml for FRM and 10-20 μg/ml for AH were added to previously analyzed test solution containing 80 μg/ml FRM and 10 μg/ml AH. Amount of drug recovered at each level (n=3) was determined. Percent recovery at each level was calculated. The mean % recovery was found to be 99.98% for FRM and 100.09% for AH. Data from the recovery study are shown in the Table 2. The sample recovery in the marketed formulation was in good agreement with the label claim. High percentage recovery showed that the method was free from interference of excipients used in formulations. The data of result of marketed formulation analysis is shown in the Table 3. The results of the study indicate that the proposed HPLC method was simple, accurate, precise and selective. Therefore, the proposed method appears to be suitable for routine analysis of FRM and AH in their combined dosage form.
| Parameter | Frusemide | Amiloride hydrochloride |
|---|---|---|
| Tailing factor* | 1.04 | 1.01 |
| No. of theoretical plate* | 2979 | 9900 |
| Asymmetry factor* | 1.01 | 1.00 |
| Retention time (Min.) | 3.040 | 10.004 |
| Resolution (Rs) | --- | 6.854 |
| Calibration Range | 80-160 μg/ml | 10-20 μg/ml |
*Each value is the mean of 6 determinations (n=6)
Table 1: System suitability parameters
| Drug | Amount added (μg/ml) | Recovery (%)* | Mean ± SD |
|---|---|---|---|
| Frusemide | 80 | 99.87 | 99.98 ± 0.123 |
| 100 | 100.20 | ||
| 120 | 99.99 | ||
| 140 | 99.92 | ||
| 160 | 99.94 | ||
| Amiloride | 10 | 99.95 | 100.09 ± 0.317 |
| hydrochloride | 13 | 99.82 | |
| 15 | 100.44 | ||
| 18 | 100.43 | ||
| 20 | 99.83 |
SD stands for standard deviation, *each value is the mean of 3 determinations (n=3).
Table 2: Recovery studies with sample solution.
| Marketed formulation | Drug | Label claim (mg/tab) | % mean* | ± SD | SEM |
|---|---|---|---|---|---|
| Amifru (Elder Pharmaceutical Ltd.) | FRM | 40 | 99.61 | ± 0.243 | 0.140 |
| AH | 5 | 95.35 | ± 0.455 | 0.262 | |
| Frumil (Geno Pharmaceuticals) | FRM | 40 | 99.78 | ± 0.162 | 0.094 |
| AH | 5 | 95.33 | ± 0.507 | 0.292 |
SEM stands for standard error of the mean, *each value is the mean of 3 determinations (n=3).
Table 3: Result of marketed formulation analysis.
Acknowledgements
The authors are thankful to Elder Pharmaceuticals Ltd., Mumbai for providing the gift samples of FRM and AH.
References
- Tripathi KD. The Kidney and Hypertension. In Essential of Medical Pharmacology. 5th ed. New Delhi: Jaypee Brothers Medical Publishers; 2003. p. 605.
- Salim EF, Haussler A, Vaughan JB. Analytical methodologies for determinations of frusemide. Indian J Pharm Sci 1978;57:640-1.
- Michaela W, Mary T, Malcolm R. Comparison of two extraction methods for determination of propranolol and frusemide in human plasma by mixed-mode chromatography. J Pharm Biomed Anal 1996;14:475-81.
- Ptacek P, Vyhnalek O, Breuel HP, Macek J. Determination of frusemide in plasma and urine by gas chromatography/ mass spectrometry. Arzneimittelforschung 1996;46:277-83.
- Schaefer M, Geissler, Heirnrich E, Mutschler. Thin layer chromatography for estimation of frusemide drug. J Chromatogr 1987;143:636-9.
- Carr K, Rane, Anders, Froelich, Juergen C. Method development by RP-HPLC for estimation of frusemide and its application. J Chromatogr 1988;145:421-7.
- Ghanekar AG, Das Gupta V, Gibbs, Charles W. Stability of frusemide in aqueous systems. J Pharm Sci 1998;67:808-11.
- Felipe S, Eder T, Gomes C. Spectrophotometric determination of furosemide based on its complexation with Fe(III) in ethanolic medium using a flow injection procedure. Indian J Pharm Sci 2006;39:2557-67.
- Prasad TN, Sastry BS, Rao EV, Sastry CS. Simple colorimetric estimation of furosemide in dosage forms. Indian J Pharm Sci 1987;57:126-9.
- Physician’s Desk Reference. Consumersinformations: Frusemide and amiloride. 36th ed. Oradell, NJ: Medical Economics Co; 1999. p. 2030-3.
- Budavari S, editor. The Merck Index. 11th ed. Whitehouse Station, NJ: Merck and Co Inc; 1993.
- Yuan N, Benny L, Douglas E, Bruce N. Photodegradation of amiloride in aqueous solution. Int J Pharm 1999;183:109-16.
- Barrales P, Pellerano G, Vazquez FA, Molina A. Rapid and sensitive determination of amiloride by cation exchange preconcentration and direct solid-phase UV detection. Anal Lett 2002;35:1491-504.
- Reuter K, Knauf H, Mutschler E. Fluorimetric determination of amiloride in human plasma using thin-layer chromatography. J Chromatogr 1982;10:233-6.
- Chang SM, Jung WB, Young SP. Analytical HPLC method validation of amiloride and its pharmacokinetic study in humans. J Liq Chromatogr Relat Technol 2006;13:2455-66.
- Indian Pharmacopoeia. Vol. 1. New Delhi: The Controller of Publications; 1996. p. 332-3.
- British Pharmacopoeia. Vol. 1. London: The British Pharmacopoeia Commission; 1996. p. 262-3.
- United States Pharmacopoeia National Formulary. Vol. 1. Rockville, MD: United States Pharmacopoeial Convention, Inc.; 1990. p. 597.
- Indian Pharmacopoeia. Vol. 1. New Delhi: The Controller of Publications; 1996. p. 39-41.
- British Pharmacopoeia. Vol. 1. London: The British Pharmacopoeia Commission; 1996. p. 113-4.
- United States Pharmacopoeia National Formulary. Vol. 1. Rockville, MD: United States Pharmacopoeial Convention, Inc.; 1990. p. 59