- Corresponding Author:
- A. N. Bajaj*
C. U. Shah College of Pharmacy, S. N. D. T. Women?s University, Juhu campus, Santacruz (west), Mumbai-400 049, India
E-mail: bajajamrita@rediffmail.com
Abstract
Dry powder inhalation formulations of rifampicin were prepared. Spray drying was used to prepare pulmospheres and their physicochemical characteristics were evaluated. Spray dried pulmospheres containing rifampicin were mixed with inhalable lactose for preparing dry powder inhalation formulations. These formulations were further characterized to evaluate the feasibility of developing effective treatments for pulmonary tuberculosis.
Keywords
Rifampicin pulmospehres, dry powder inhalations, inhalable lactose, entrapment effi ciency, rotahaler
Tuberculosis is most commonly caused by the deposition of bacteria, Mycobacterium tuberculosis in the lungs. Pulmonary tuberculosis is characterized by alveolar macrophages containing large number of bacilli that are typically about 5 µm in length, facilitating their entry into the lower airways. Antitubercular drug delivery systems can be administered by the pulmonary route to avoid frequent dosing. It could be helpful in direct drug delivery to the lungs, drug targeting to alveolar macrophages, reducing systemic toxicity of the drug. Biodegradable polymers like PLGA are capable of sustained drug release over days to several weeks. Therefore in present study, an attempt was made to develop dry powder inhalation (DPI) formulations of rifampicin (RIF) for pulmonary delivery. DPI formulations of spray dried RIF and of RIF pulmospheres with Poly(DL-lactide-co-glycolide) polymer (75:25) have been developed
Materials and Methods
Rifampicin (Lupin Ltd., Mumbai, India); Pharmatose 325 (DMV international) and Lactohale (Borculo) were generous gift samples. Biodegradable polymer PLGA (75:25) with viscosity of 0.19 dl/g in chloroform as reported by the manufacturer was procured from Birmingham polymers.
Development of conventional spray dried RIF
RIF solutions of concentration of 2-40 mg/ml were prepared in dichloromethane and spray dried using Labultima Mini Spray Dryer. Process parameters were optimized using 22 factorial design. Effect of process parameters on particle size was studied (fig. 1).
Preparation and characterization of RIF-loaded pulmospheres
Spray drying technology was used to prepare pulmospheres. The solution of polymer containing drug was spray dried to generate polymer-coated particles. Developed pulmospheres were evaluated for physiochemical properties like particle size, surface characteristics, % drug loading and entrapment effi ciency. DSC studies were performed.
Formulation development of DPI
Spray dried RIF/pulmospheres of RIF were mixed with inhalable lactose in varying ratios and developed DPI formulations were characterised (Table 1). The optimum ratio of coarse lactose to fi ne lactose that gives maximum respirable fraction was selected (fig. 2). In vitro aerolisation behaviour of the formulations was evaluated using a Rotahaler, and the performance was characterised based on uniformity of emitted dose and aerodynamic particle-size distribution (respirable fraction (RF), as a percentage of nominal dose (RFN) and emitted dose (RFE). DPI formulations of spray dried RIF and PLGA-based RIF Pulmospheres were compared in terms of respirable fraction delivered (fig. 3).
| Batch code | Assay (%w/w) | bulk density (gm/cc) | Moisture content (% w/w) | % deposition in stage ii |
|---|---|---|---|---|
| D1 | 82 | 0.4 | 0.2 | 26.49 |
| D2 | 85 | 0.32 | 0.38 | 29.15 |
| D3 | 90 | 0.41 | 0.5 | 32.3 |
| D4 | 95 | 0.38 | 0.8 | 33.4 |
| D5 | 98 | 0.33 | 0.5 | 36 |
Table 1: Quality Control Tests Performed on Conventional Rif Dpi Formulations
In vitro release studies
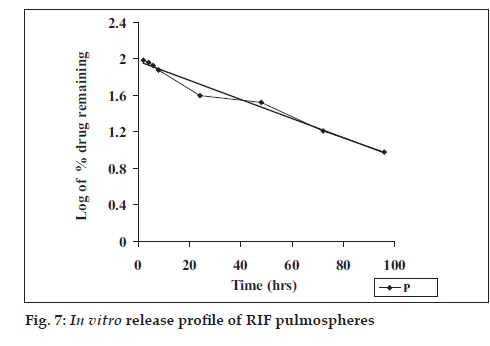
Release studies were performed by dialysis method. The diffusion cell was kept at 37° with continuous stirring at 100 rpm and RIF was analyzed spectrophotometrically at 475 nm. Coefficient of correlation from plots of Q vs. t, (cumulative % drug release vs. time), log of percent drug retained vs. t and Q vs. square root of t were calculated to determine drug release.
Results and Discussion
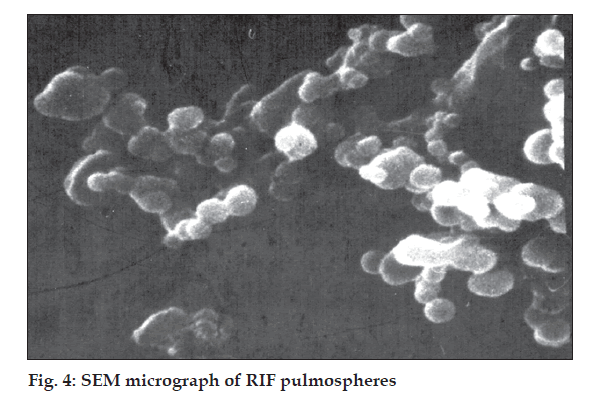
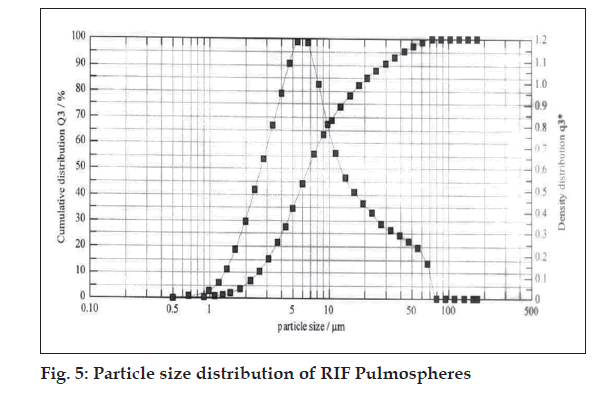
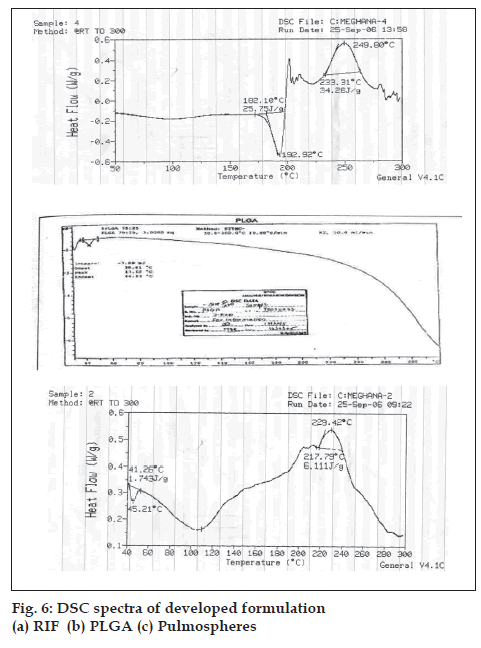
Optimum process parameters for spray dried RIF were inlet temperature (55o), aspirator rate (60 l/min), feed rate (10 ml/min) and optimum concentration was 2 mg/ml (fig. 1). DPI formulations were developed using various grades of inhalable lactose like pharmatose and Lactohale in various combinations. The effect of particle size of excipients on respirable fraction of RIF was assessed (Table 1, fig. 2). Pulmospheres of RIF were prepared with PLGA (1:1, drug:polymer ratio) with 96% v/v entrapment efficiency. Developed PLGA porous pulmospheres were characterized by SEM analysis (fig. 4) and for particle size (fig. 5), % drug entrapment and %FPF (Table 2). DSC studies confirmed no interaction between drug and polymer (fig. 6). Regression coeffi cients (near to 1) for zero order, first order and Higuchi’s model equations confi rmed release by fi rst order (R2=0.97887) (fig. 7). Spray dried RIF and Pulmospheres exhibited excellent flow and dispersion from passive DPIs while pulmospheres released for longer period of time. In vitro characterization has predicted highly efficient lung delivery of RIF for treatment of pulmonary tuberculosis.
| Parameters | Observation | |
|---|---|---|
| Spray Dried RIF | Pulmospheres | |
| Appearance | Spherical | Hollow porous |
| Pariticle size | 1-10μm | 1-10μm |
| %Yield | 48 | 45.6 |
| % moisture content | 0.5 | 0.75 |
| % drug entrapment | - | 96 |
| %FPF | 36 | 32 |
Table 2: Results of Optimised Dpi Formulations Prepared by Spray Drying Technology
 0-10,
0-10,  10-20,
10-20,  20-30,
20-30,  30-40,
30-40,  40-50 and
40-50 and  50-60.
50-60.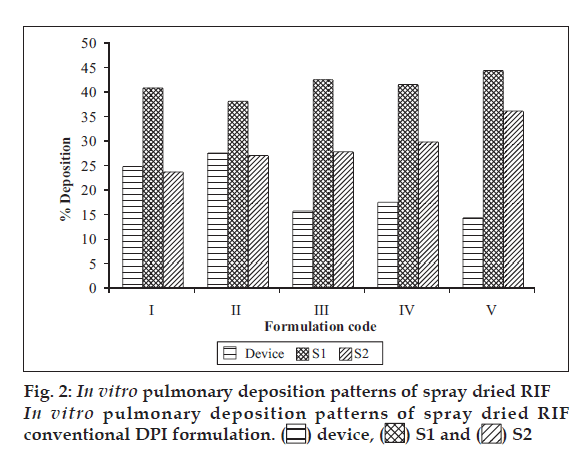
 device,
device,  S1 and
S1 and  S2
S2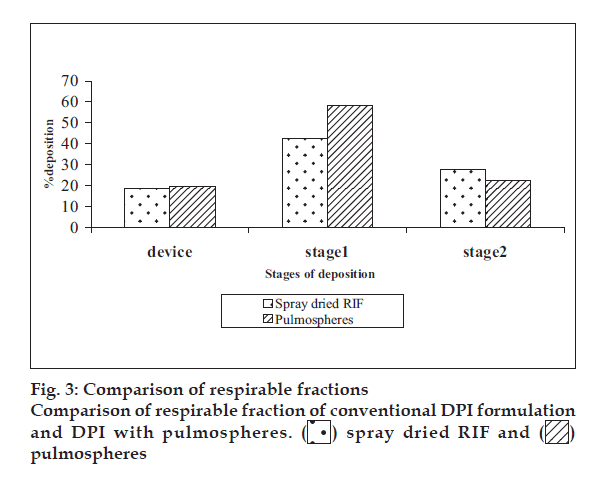
 spray dried RIF and
spray dried RIF and