- *Corresponding Author:
- H. G. Gunjal
Department of Pharmaceutical Sciences, School of Pharmaceutical Sciences, Sandip University, Nashik, Maharashtra 422213, India
E-mail: hggunjal.99@gmail.com
| Date of Received | 13 May 2023 |
| Date of Revision | 28 February 2024 |
| Date of Acceptance | 26 August 2024 |
| Indian J Pharm Sci 2024;86(4):1277-1287 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The topical lotion of clobetasol propionate was approved for the treatment of psoriasis. Commercially, it is available in the various dosage forms like ointment, cream, foam gel, solution, in various quantities ranges from 0.05 %-0.5 % w/w. The drug is reported in all official pharmacopoeias with their 13 related substances. For the purpose of estimating clobetasol propionate in bulk and formulations using the reversed-phase high performance liquid chromatography technology, an accurate and reliable method was devised. Agilent 1260 Infinity II type high performance liquid chromatography with diode array detector and Phenomenex Luna-C18 column, measuring 250×4.6 mm, 5 µm was utilized in the method. Ammonium acetate buffer, acetonitrile and methanol (60:20:20) made up the mobile phase A combination; acetonitrile and ammonium acetate buffer (20:80) made up the mobile phase B combination. The optimum peak was obtained at retention duration of 15.73 min by maintaining a flow rate of 1.0 ml/min at a wavelength of 240 nm throughout. The percentage error for the instrument, method and intermediate precision was 0.01 %, 0.01 % and 0.02 % respectively. For both method and intermediate precision, the total percentage relative standard deviation was 0.02 %. The procedure was linear and accurate with correlation coefficient of 0.9997 for concentration ranges of 0.05-120 μg/ml with accuracy levels of 0.08 %, 0.02 % and 0.03 % for relative standard deviation of 80 %, 100 % and 120 %. When the drug's stress stability was examined, it was discovered to be unstable under basic conditions, degrading at 99.34 % and under heat conditions, degrading at 14.40 %. Since the established approach is relatively linear and the limits of detection and quantification for clobetasol propionate are very low at 0.93 μg/ml and 2.81 μg/ml respectively, it may be employed in a commercial setting. Retentions were verified and impurities were found for injecting the reference standard. Impurities A, B, J, L and M were found and their retention times were 7.91, 9.28, 18.75, 21.35 and 27.07 min. It was discovered that the approach was accurate and robust.
Keywords
Impurity profiling, stability-indicating reversed-phase high performance liquid chromatography, clobetasol propionate, forced degradation
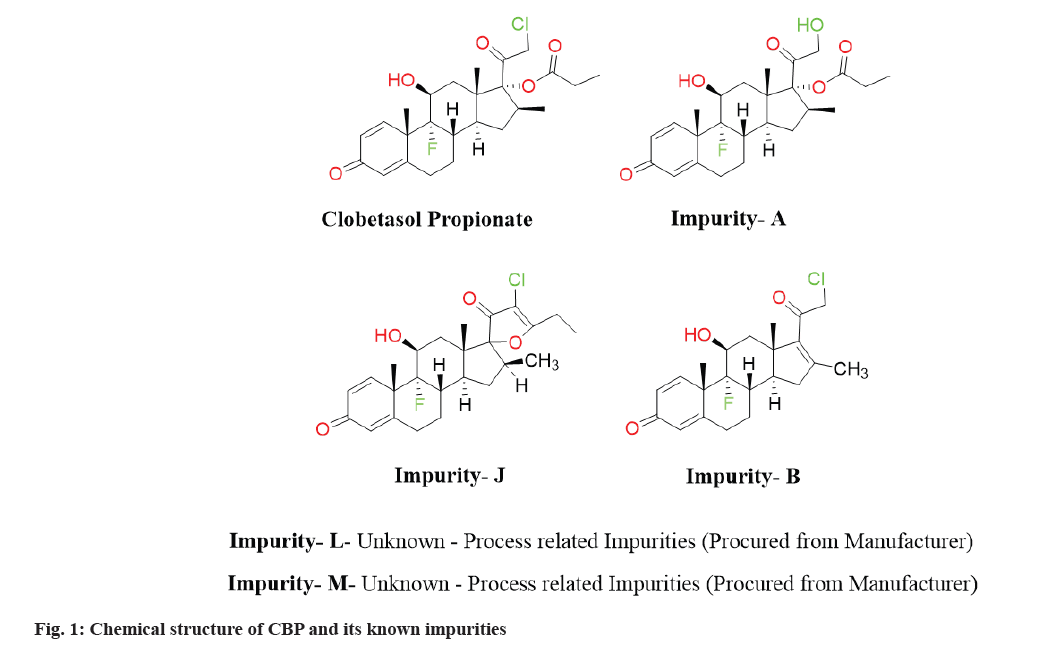
A glucocorticoid, Clobetasol Propionate (CBP) (fig. 1) was approved in 2003 for the treatment of almost all forms of dermatoses, either alone or in combination of prednisolone[1-3]. In the later studies, it was found potent in treatment of scalp diseases by inflammation and fibrosing alopecia (via Koebner phenomenon)[4-6]. The presence of two halogens (-Cl and -F) makes the CBP more active than its analogue of prednisolone and hydrocortisone, but the ester derivative are more likely get degraded by acid or alcohol[7,8]. The drug CBP officially reported with its 13 impurities (Imp-A-M) from those two impurities (Imp-L and Imp-M) are process related impurities and their structure is unknown[9]. The impurity identification is the required parameter to ensure the safety and efficacy of any drug[10,11]. Impurities found within drugs possess the capability to influence their effectiveness by causing shifts in the stability, solubility or bioavailability of the medication.
Such alterations have the potential to result in a decrease in potency or modifications to the drug's pharmacokinetic characteristics, consequently impacting its overall efficacy. Furthermore, the presence of impurities can introduce complications throughout the formulation and utilization processes of these substances, ultimately leading to a reduction in their shelf life. The references including United States pharmacopoeia illustrate the liquid chromatography analysis for bulk and pharmaceutical dosage forms (0.05 % w/w) for CBP. The known impurities (except Imp-L and Imp-K) are mentioned in (fig. 1). Therefore, the goal of the current study was to create and validate the approach for CBP and its forced degradation study, which found the majority of CBP's impurities using a relatively quick run-time method.
Materials and Methods
Instrumentation:
Agilent OpenLab EZChrom software was used to facilitate the creation and validation of the tool, an Agilent 1260 Infinity II equipped with a diode array detector and quaternary pump. The method was developed using an Aczcet analytical balance, Digital Systronic pH meter, Labman ultra-sonicator and Millipore vacuum filter pump with a Merck Millipore Nylon filter (0.45 μm) for filtration.
Materials and reagents:
Aadhaar Life Sciences Pvt. Ltd. provided a gift sample of the pharmaceutical grade reference standards of CBP as well as process-related impurities (Imp-L and Imp-M) (Solapur, Maharashtra, India). For the current investigation, Merck in Mumbai, India provided all the chemicals, including methanol and Acetonitrile (ACN) of High-Performance Liquid Chromatography (HPLC) quality. MilliQ water was likewise obtained from Mumbai, India. Calibrated National Accreditation Board for Testing and Calibration Laboratories standards were used for all weight measurements. Using the analytical balance and Type A glassware, samples were created.
Chromatographic conditions:
Mobile phases A and B were coupled as buffer:ACN:methanol (60:20:20 % v/v/v) and buffer:ACN (20:80 % v/v) at a flow rate of 1.0 ml/ min, respectively. Using an injection volume of 5 μl, the wavelength detection was carried out with consideration for 240 nm. The robustness of the system allows for the column temperature to be maintained at 35° (±2°) throughout the analysis.
Preparation of mobile phase:
Mobile phase A: Buffer:ACN:methanol (60:20:20 v/v/v). After precisely measuring 600 ml of ammonium acetate buffer, 200 ml of ACN and 200 ml of methanol, the blend was passed through a 0.45 μm nylon filter and subjected to a short sonication to eliminate any remaining gas.
Mobile phase B: Buffer:ACN (20:80 v/v). For preparation of mobile phase B, 200 ml of ammonium acetate buffer and 800 ml of ACN were previously measured were transferred into a suitable container. The content was then filtered through 0.45 μm nylon membrane and briefly sonicated to get rid of any leftover gas.
Preparation of diluent/blank: Throughout, a 500 ml of ACN and 500 ml methanol was separately measured and mixed well. It is pre-filtered via a 0.45 μm nylon filter following a sonication for 5 min.
Readying stock standard solution:
CBP 500 μg/ml solution: Before being used, the drug's part was first desiccated under normal circumstances, which were 78° and 3 h. Next, a 50 ml Volumetric Flask (VF) was filled with the weighed 25 mg CBP reference standard. After adding the necessary amount of diluent to make the medication soluble, it was sonicated. After reaching equilibrium, diluent was used to adjust the volume to 50 ml.
Preparation of working standard solution (100 μg/ml):
When kept at room temperature, the working solution was shown to be stable for 8 d. 10 ml VF was filled with 2.0 ml of stock standard and solution volume was revamped with diluent after degassing.
Readying formulation of CBP lotion (0.05 % United States Pharmacopeia) for assay (100 μg/ml):
In a 10 ml VF, 2 g of sample was transferred followed by 5 ml of diluent to mix well. The volume was then adjusted using the same diluent and the sample was vortexed for approximately 1 min. Sample solutions were kept at room temperature and were found to be stable for 4 d.
Forced degradation study:
In compliance with International Council for Harmonisation (ICH) guidelines Q1A (R2) and Q1B[12], CBP was used for the degradation investigations. The spectrum of the chromatogram was analysed according to the position of the drug peak and the appearance of secondary peaks. Any modification to the size and shape of secondary peaks was regarded as deterioration.
Ultraviolet (UV)/photolytic degradation:
Exposing the material to UV light at 254 nm and white light at 1.2 million lux h in a petri dish for 12 h caused photolytic breakdown.
Thermal degradation: To ascertain the drug's thermal properties, the sample was placed in oven for 4 h at a temperature of around 80°. Using diluent, around 5 ml of this stressed solution were diluted.
Acid degradation: By using a 200 μl solution of 1 N Hydrochloric acid (HCl), acid degradation was monitored at 60° after refluxing the content with diluent for 30 min.
Alkali degradation: The alkali degradation studies were performed by refluxing 100 μl solution of 1 N Sodium hydroxide (NaOH) at 60° for 30 min with diluent.
Peroxide degradation: Refluxing 3 % v/v (1 ml) Hydrogen peroxide (H2O2) solution in the water bath at 60° for 30 min allowed the standard solution to degrade oxidatively. Diluent was then added to treat the solution.
Method validation:
Using defined factors for validation, the proposed technique was validated in accordance with ICH Q2 (R1) and United States Food and Drug Administration requirements[13].
Solution stability:
The working standard was examined at various points in time to determine how long the prepared solution was stable and the result was 8 d. The data displayed in Table 1 supports the findings that were examined at five distinct intervals.
| Days | Sample ID | Solution Stability | |||
|---|---|---|---|---|---|
| Area | % assay | % RSD | Cummulative % RSD | ||
| Control | WS | 8152654 | 100.00 | - | - |
| 0th d | 8151432 | 99.99 | 0.01 | - | |
| 1st d | 8025134 | 98.44 | 1.11 | 0.90 | |
| 3rd d | 7954238 | 97.57 | 1.74 | 1.22 | |
| 5th d | 7948571 | 97.5 | 1.79 | 1.26 | |
| 8th d | 7741384 | 94.96 | 3.66 | 1.92 | |
Table 1: Stability of CBP in Solution
Working standard:
Keeping solution in the VF at ambient temperature and in a dry, dark area away from light, the cumulative Relative Standard Deviation (RSD) was 1.92 %. Because the specification limitations are 2 %, the working standard is stable for 8 d.
Results and Discussion
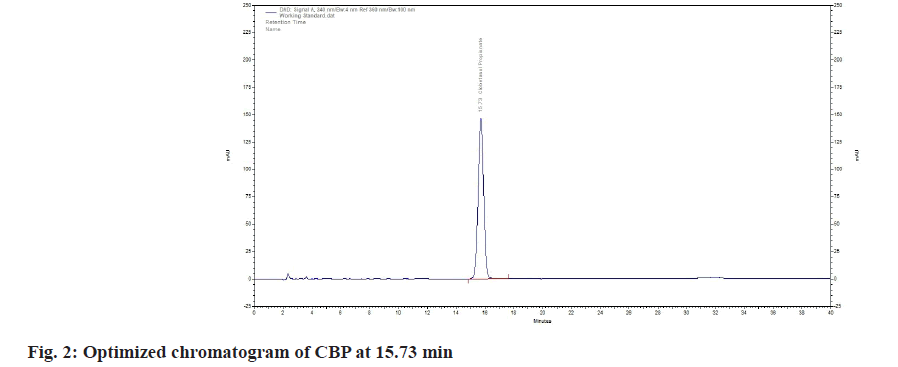
To achieve an effective separation of CBP, several combinations of ammonium acetate buffer and ACN as mobile phase were investigated. The optimized chromatogram and conditions are mentioned in fig. 2 and Table 2 respectively. Using a series of tests, the applicability cum performance of the system was examined. It is discovered that the purity of peak, plate count and tailing factor are all within the guideline’s permitted values.
| Specifications | Condition |
|---|---|
| Instrument (HPLC) | Agilent 1260 Infinity II |
| Column | Phenomenex, Luna C18(2), (Part # 00G-4252-E0) (250 x 4.60 mm), 100°A |
| λmax | 240 nm |
| Mobile Phase | Mobile Phase A: 50 % |
| Mobile Phase B: 50 % | |
| Diluent | ACN: Methanol (50:50) v/v |
| Retention time | 15.73 minutes |
| Run time | 40 minutes |
| Injection volume | 5 µl |
| Column oven Temperature | 35° |
| Flow rate | 1.0 ml/min |
| Column temperature | 35° (±2° allowed by Robustness) |
Table 2: Optimization of Final Chromatographic Conditions
Operational linearity denotes the capability of an analytical performance to produce outcomes that correspond proportionally to the substance concentrations which being analyzed within a defined range. By proving linearity using the five sets of standard solutions, the calibration curve's peak area was plotted against the standard solution's concentration to assess the regression equation. The least-squares method was employed to ascertain the slope, intercept and correlation coefficient.
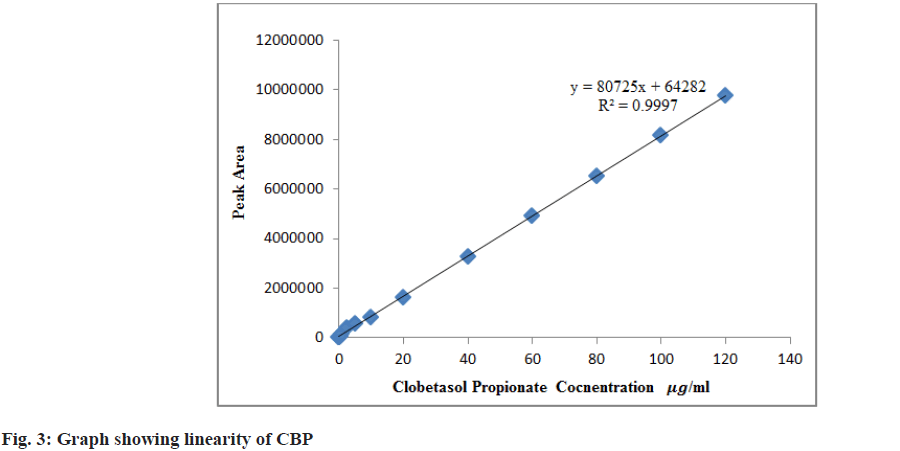
Linearity was evaluated across various levels, encompassing a minimum concentration range from 0.05-120 μg/ml. The linearity (fig. 3) with correlation coefficient (r2) was plotted considering the peak area and CBP concentration. The linearity of impurities (A, B, and J) was evaluated and r2 was found to be 0.9999, 0.9998 and 0.9994 respectively.
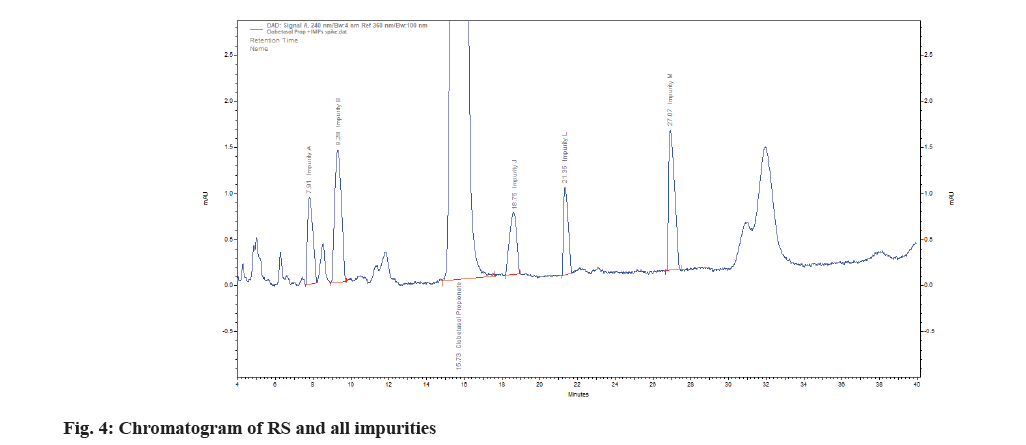
The linearity assessment range (0.05-120 μg/ ml) was established by measuring different concentrations of CBP-containing standard solutions. The successive evaluation of the analyte without the impact of impurities or degradants is termed as specificity; this was confirmed by contrasting the CBP chromatograms with those of a blank sample. The Related Substances (RS) were identified by executing the operational requirements of every single impurity (Impurity A, B, J, L and M) as depicted in (Table 3). All the RS are mentioned in United States pharmacopoeia as reference.
| Solution Name and ID | Retention Time of Major Peaks in Min | |||||
|---|---|---|---|---|---|---|
| Imp-A | Imp-B | CBP | Imp-J | Imp-L | Imp-M | |
| Diluent | ||||||
| ID_Placebo | ||||||
| ID_Imp-A (Impurity A) | 7.91 | |||||
| ID_Imp-B (Impurity B) | 9.28 | |||||
| ID_Clobetasol Propionate | 15.73 | |||||
| ID_Imp-J (Impurity J) | 18.75 | |||||
| ID_Other Known Imps | 7.91 | 9.29 | 15.72 | 21.35 | 27.07 | |
| ID_ISS | 7.91 | 9.28 | 15.73 | 18.76 | ||
| (Impurity spiked sample) | ||||||
| ID_FP (Finished product) | 7.92 | 9.29 | 15.75 | |||
| Working Standard ID | 15.73 | |||||
Table 3: Specificity of API and its Related Substances
After being administered independently of the blank sample, the CBP sample retained its integrity for 15.73 min (fig. 4) without any interference from the blank peaks. The related substances run were used to determine the peaks. The active pharmaceutical ingredient's specificity and related chemicals are listed below (Table 3).
A technique's accuracy may be assessed by looking at how closely the results of its tests match the real value. Three different concentration (80 %, 100 % and 120 %) levels were assessed in the recovery experiments as depicted in Table 4. Three replicate injections were conducted at each level and the quantity of drug retains, % recovery and the associated standard deviation were computed. Accuracy was assessed to evaluate the method's capability to precisely analyze varying concentrations range of the API. The RSD for concentrations of 80 %, 100 % and 120 % were 0.08 %, 0.0 2%, and 0.03%, respectively.
| % Level | Reps | Spiked concentration (µg/ml) | Area | Amount recovered (µg/ml) | % Recovery | Average | SD | % RSD |
|---|---|---|---|---|---|---|---|---|
| 80 % | Rep 1 | 79.92 | 6521024 | 79.91 | 99.99 | 100.06 % | 0.0815 | 0.08 |
| Rep 2 | 79.92 | 6531524 | 80.04 | 100.15 | ||||
| Rep 3 | 79.92 | 6524813 | 79.96 | 100.04 | ||||
| 100 % | Rep 1 | 99.9 | 8151264 | 99.89 | 99.99 | 100.00 % | 0.0183 | 0.02 |
| Rep 2 | 99.9 | 8154234 | 99.92 | 100.02 | ||||
| Rep 3 | 99.9 | 8152412 | 99.9 | 100.00 | ||||
| 120 % | Rep 1 | 119.88 | 9756124 | 119.55 | 99.73 | 99.71 % | 0.0258 | 0.03 |
| Rep 2 | 119.88 | 9751246 | 119.49 | 99.68 | ||||
| Rep 3 | 119.88 | 9754812 | 119.54 | 99.71 |
Table 4: Result of Accuracy Showing % RSD
The precision of analysis relies on how closely individual test results agree with each other. Several samples from a homogeneous source were scrutinized to evaluate precision. Repeatability, intra-day and inter-day variabilities were employed to gauge the precision of the method under consideration. Validation of this parameter involved analyzing samples obtained at different times throughout the day and on multiple days. Precision was assessed through three aspects; instrument precision, which evaluates the consistency of consecutive injections of the same concentration by the instrument; method and intermediate precision, wherein two analysts inject six individual distinct samples with varying drug concentrations and the % RSD of a total of 12 injections, is confirmed.
The applicability of the HPLC equipment was evaluated, and according to the limits specified in Table 2, the instrument was deemed appropriate to proceed with the validation process. Following system suitability testing, all three types of precision assessments were conducted. The resulting data indicates that the RSD for Instrument Precision (IP), Method Precision (MP) and Intermediate Precision (ITP) were 0.01 %, 0.01 % and 0.02 %, respectively (Table 5). The RSD between MP and ITP was 0.02 %. This % RSD shown in this method was very much precise and robust with respect to different analyst and numerous sample preparations for defined concentration.
| System applicability-clobetasol propionate | Peak area | |||||
|---|---|---|---|---|---|---|
| Repetitions | RT | Asymmetry | Theoretical plates | IP | MP | ITP |
| Rep 1 | 15.73 | 1.01 | 7925 | 8152674 | 8152416 | 8155845 |
| Rep 2 | 15.73 | 1.00 | 7984 | 8153254 | 8154321 | 8156584 |
| Rep 3 | 15.73 | 1.01 | 7885 | 8154265 | 8153241 | 8156874 |
| Rep 4 | 15.73 | 1.00 | 7832 | 8154876 | 8152394 | 8154267 |
| Rep 5 | 15.73 | 1.00 | 7623 | 8152364 | 8154231 | 8152416 |
| Rep 6 | 15.73 | 1.01 | 7864 | 8153254 | 8152132 | 8157459 |
| Average | 15.73 | 8153448 | 8153123 | 8155574 | ||
| % RSD | 0.00 | 0.01 | 0.01 | 0.02 | ||
Table 5: System Applicability and Precision Results for Cbp
Robustness testing is performed to determine the method's degree of deviation from its critical parameters. Before utilization, the essential and obligatory step involved calibrating the equipment. However, modifications were implemented to both the column temperature and the mobile phase to verify the method's resilience (Table 6). All the runs were done in triplicates for working standard.
| Rep | Area | RT | TP | Asymmetry | Area % RSD | |
|---|---|---|---|---|---|---|
| Temperature variation | ||||||
| Increase (37) | Rep 1 | 8174298 | 15.89 | 7841 | 1.00 | 0.07 |
| Rep 2 | 8174932 | 15.89 | 7853 | 1.00 | ||
| Rep 3 | 8184573 | 15.89 | 7847 | 1.01 | ||
| Normal (35) | Rep 1 | 8152674 | 15.73 | 7925 | 1.01 | 0.01 |
| Rep 2 | 8153215 | 15.73 | 7865 | 1.00 | ||
| Rep 3 | 8153648 | 15.73 | 7945 | 1.01 | ||
| Decrease (33) | Rep 1 | 8146954 | 15.66 | 7936 | 1.00 | 0.08 |
| Rep 2 | 8134965 | 15.66 | 7955 | 1.01 | ||
| Rep 3 | 8137421 | 15.66 | 7912 | 1.01 | ||
| Variations in mobile phase | ||||||
| Increase MP A | Rep 1 | 8100659 | 15.62 | 8120 | 1.00 | 0.06 |
| Rep 2 | 8101274 | 15.62 | 8102 | 1.01 | ||
| Rep 3 | 8109843 | 15.62 | 8154 | 1.00 | ||
| Normal | Rep 1 | 8152674 | 15.73 | 7925 | 1.01 | 0.01 |
| Rep 2 | 8154754 | 15.73 | 7645 | 1.01 | ||
| Rep 3 | 8153678 | 15.73 | 7852 | 1.01 | ||
| Decrease MP A | Rep 1 | 8169352 | 15.86 | 7648 | 1.00 | 0.01 |
| Rep 2 | 8167828 | 15.86 | 7631 | 1.01 | ||
| Rep 3 | 8167425 | 15.86 | 7512 | 1.00 |
Table 6: Robustness Showing Variation in Column Temperature and Variation in Mobile Phase
Concerning variations in column temperature and mobile phase concentration, there were no observable alterations in theoretical plate count, asymmetry or peak purity. The shifting of retention time, (ascribed to the polarity contrast between ACN and the drug), was observed by decreasing the amount of ACN (a constituent of mobile phase A) to a minor postponement in the retention time of the CBP peak, causing it to elute later. Conversely, elevating the proportion of mobile phase A caused the peak to elute earlier.
The linearity concentration ranges were used from 0.05 %-120 % and the observed r2 was 0.9997. Based on the precision and linearity data (fig. 3), the detection and quantification limits were identified as 0.93 and 2.81 μg/ml respectively for Limit of Detection (LOD) and Limit of Quantification (LOQ). The approach appears to be highly effective in identifying low concentrations of drug, as seen by the considerably low LOD and LOQ. When conducting cleaning validation in the industry, organizations could utilize the values of LOD and LOQ to determine whether the manufactured vessel or equipment was clear of stains from APIs. Based on the linearity data (fig. 3), the LOD and LOQ were determining for CBP and its related compounds (Table 7).
| Name | Specification | LOD concentration | LOQ concentration | ||
|---|---|---|---|---|---|
| (µg/ml) | % wrt sample | (µg/ml) | % wrt sample | ||
| Impurity A | NMT 1.0 % | 0.048 | 0.05 | 0.048 | 0.05 |
| Impurity B | 0.048 | 0.05 | 0.096 | 0.10 | |
| Impurity J | 0.05 | 0.05 | 0.125 | 0.13 | |
| Clobetasol Propionate | NMT 0.50 % | 0.93 | - | 2.81 | - |
Note: NMT: Not MOre Than
Table 7: Combined Data of Lod and Loq for Cbp and its Related Compounds
A CBP lotion 0.05 % (w/w) test was conducted using the approved technique. The assay for the commercial formulation was discovered to be at 96.44 %. The results are depicted in Table 8.
| Parameters | Results |
|---|---|
| Standard area | 8152145 |
| 8152635 | |
| 8153241 | |
| 8152394 | |
| 8152234 | |
| 8153198 | |
| Mean area | 8152641 |
| Sample area | 7862541 |
| Amount found | 0.4822 mg clobetasol propionate per 1 g of lotion |
| % assay | 96.44 % |
Table 8: Result of Assay
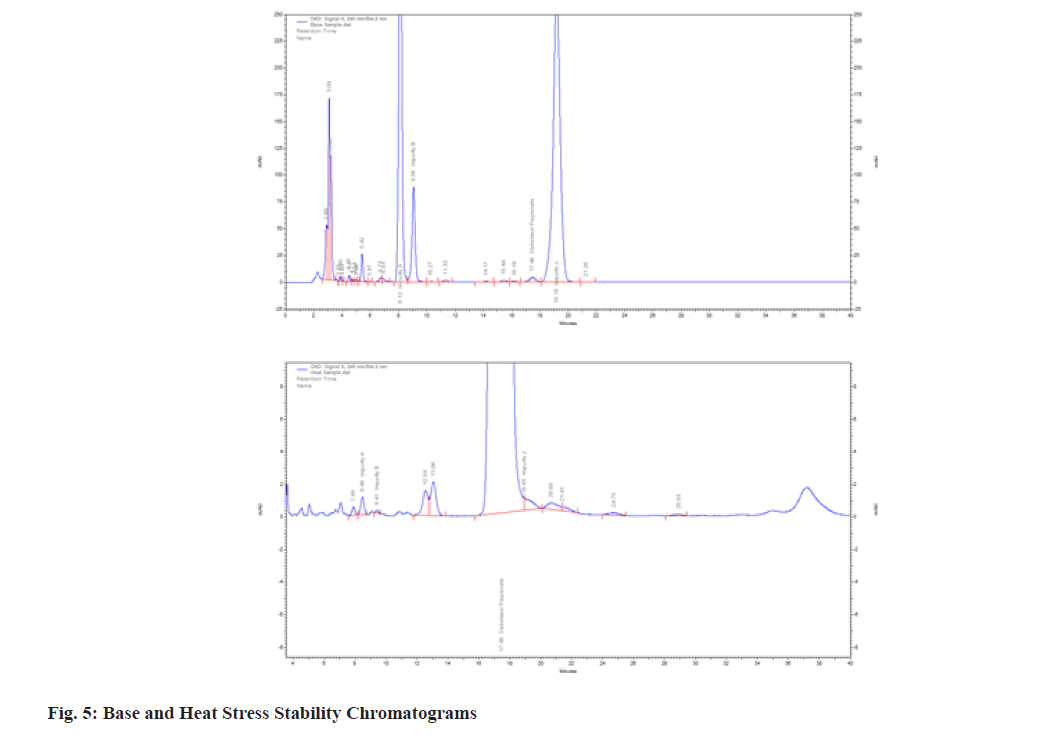
These methods explore how stressors (pH, acid and base) and other environmental factors contribute to our comprehension of drug stability. The studies were carried out as per the ICH criteria and procedures outlined in forced degradation studies and the results are presented in Table 9 and Table 10. The degradation was observed only in the base and photolytic conditions (fig. 5) whereas in the acid, peroxide and UV degradation the drug was found to be stable.
| Accelerated stability condition | Sample ID | Clobetasol propionate | ||
|---|---|---|---|---|
| % Assay | Peak purity | % Degradation | ||
| Control | Working standard | 100.00 | 1 | - |
| Acid | 106.06 | No degradation | ||
| Base | 0.66 | 99.34 | ||
| Peroxide | 109.97 | No degradation | ||
| UV | 100.80 | No degradation | ||
| Heat | 85.60 | 14.40 | ||
Table 9: Accelerated Stability Testing Assay Results
| Condition | Impurity A | Impurity B | Impurity J | Impurity L | Impurity M |
|---|---|---|---|---|---|
| Control | 0.02 | ND | 0.07 | ND | ND |
| Acid | 0.01 | 0.07 | |||
| Base | 31.39 | 45.82 | |||
| Peroxide | ND | 0.07 | |||
| UV | ND | 0.07 | |||
| Heat | 0.02 | 0.07 | 0.02 | 0.01 |
Note: *ND: Not Developed
Table 10: Forced Degradation Impurity Profiling
Although there have been techniques for analyzing CBP in gel formulation, cosmetic items, cream, ointment, nanocapsule suspension and gel formulation documented in the literature[14-19], none of them have been tested for contaminants. The CBP and its associated compounds, particularly its commercial version known as CBP lotion (0.05 % w/w), were the main subjects of the current investigation. The proposed method of extraction and quantitation of CBP has been applied to the analysis of commercial formulations, commercially available on the European market[9]. Currently, we used the Imp-A, B, J, L and M, from which the two impurities (Imp-L and M) are unknown (as mentioned in European Pharmacopoeia) and samples of process-related impurities were procured from the manufacturer. After several trials, the CBP was eluted at 15.73 min using mobile phases A and B as buffer:acetonitrile:methanol (60:20:20 v/v/v) and buffer:ACN (20:80 v/v) respectively, using ACN:methanol (50:50 %) as diluent. The quantification via analytical methods is accountable for the mannerism between standard drugs and their degradant generated under specific conditions. The medication was exclusively degraded under photolytic and basic conditions, according to stress degradation experiments. The mass balance ratio revealed that the degradation rates for the base and photolytic conditions were, respectively, 99.34 % and 14.40 %. For acid, peroxide and ultraviolet degradation investigations, the existing methodology did not show any discernible degradant peaks. When comparing the percentage impurity of related compounds, the base sample of Imp-J had the highest impurity (45.82 %), while the degradation of Imp-M and Imp-A by heat stress and acid showed the lowest impurity (0.01 %), respectively. One medication that is frequently used to treat severe dermatoses is the glucocorticoid derivative CBP. Any conventional drug's impurity may have a similar impact, leading to negative side effects and toxicity-related issues. Therefore, it is important to periodically assess these contaminants (in all dose forms) using commercially available routine analytical techniques. The CBP literature reveals that while there are a number of techniques available for quantifying CBP, none of them have been specifically designed to target the contaminants that have been identified. In the present investigation, commercial CBP lotion with a 0.05 % (w/w) concentration was obtained and its claimed impurities (imp-A, B, and J) were assessed. It's interesting to note that the manufacturer also provided the unknown impurities (Imp-L and M) for evaluation as process-related impurities. The estimate of all the data demonstrates the accuracy and precision of the approach in terms of both drug and impurity identification. The stock solution demonstrated the maximum system applicability for eight days. The r2 0.9997 is displayed throughout the whole linearity range of CBP, which is 0.05-120 μg/ml. The correctness was confirmed by calculating the percentage recovery, which runs from 99.68 %-100.15 %. It was discovered that the approach was accurate, reliable and within quantification and detection bounds. According to the force degradation research, the base attack is what breaks down the maximal medicine, thus it should be shielded against that as well. More than published publications, the current research is superior in terms of asymmetry, analysis time, and insight for degrading studies for further research.
Conflict of interests:
The authors declared no conflict of interests.
References
- Hull C, McKeough M, Sebastian K, Kriesel J, Spruance S. Valacyclovir and topical clobetasol gel for the episodic treatment of herpes labialis: a patient?initiated, double?blind, placebo?controlled pilot trial. J Eur Acad Dermatol Venereol 2009;23(3):263-7.
[Crossref] [Google Scholar] [PubMed]
- Mostafa AA, Bebawy LI, Refaat HH. Spectrophotometric determination of clobetasol propionate, halobetasol propionate, quinagolide hydrochloride, through charge transfer complexation. J Pharm Biomed Anal 2002;27(6):889-99.
[Crossref] [Google Scholar] [PubMed]
- Nagar A, Deore S, Bendale A, Kakade R, Sonawane C. Analytical method development and validation of ramipril and candesartan cillexetil in synthetic mixture. Innov Pharm Pharmacother 2020;8(2):14-20.
- Bhuyian MH, Rashid PD, Islam AF, Tareque M. Development and validation of method for determination of clobetasol propionate and salicylic acid from pharmaceutical dosage form by HPLC. Br J Pharm Res 2015;7:375-85.
- Fontana MC, Bastos MO, Beck RC. Development and validation of a fast RP-HPLC method for the determination of clobetasol propionate in topical nanocapsule suspensions. J Chromatogr Sci 2010;48(8):637-40.
[Crossref] [Google Scholar] [PubMed]
- Bagal D, Nagar A, Joshi A, Chachare A, Shirkhedkar A, Khadse S. Development and validation of stability-indicating RP-HPLC method for estimation of dalfampridine in bulk drug and tablet dosage form. Futur J Pharm Sci 2021;7:1-7.
- Yang W, Yang X, Shi F, Liao Z, Liang Y, Yu L, et al. Qualitative and quantitative assessment of related substances in the compound ketoconazole and clobetasol propionate cream by HPLC-TOF-MS and HPLC. J Pharm Anal 2019;9(3):156-62.
[Crossref] [Google Scholar] [PubMed]
- Devi N, Kumar S, Rajan S, Gegoria J, Mahant S, Rao R. Development and validation of UV spectrophotometric method for quantitative estimation of clobetasol 17-propionate. Asian J Chem Pharm Sci 2016;1(1):36-40.
- European Pharmacopoeia (2012) Strasbourg (FR): Directorate for the Quality of Medicines and HealthCare of the Council of Europe (EDQM); 7th edition, 1574-7.
- Shinde SU, Bardiya RS, Jain PS, Shikhedkar AA, Nagar AA. Comparison of acid degradant product with metabolic pathway of remogliflozin etabonate in developed and validated RP-HPTLC method. Indian Drug 2022;59(11):46-53.
- Patel C, Jain P, Shinde S, Shirkhedkar A. Simultaneous estimation of pyridoxine HCl and FMOC-leucine using derivative and chromatographic approach in pharmaceutical dosage form. J Chem Metrol 2021;15(2):124-34.
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Harmonised Tripartite Guideline-Validation of Analytical Procedures. Text and Methodology Q2(R1). 2005.
- Guidance for industry. Q1A(R2) stability testing of new drug substances and products: US department of Health and Human Services, FDA. Centre for drug evaluation and Research and Centre for biologics evaluation and Research;2003:1-25.
- Marika K, Katherine F, Jianmin L, Mike CG, Debra F. A sensitive high-throughput HPLC assay for simultaneous determination of everolimus and clobetasol propionate. J Chromatogr Sci 2008;46(1):23-9.
[Crossref] [Google Scholar] [PubMed]
- Ahmed NR, Mohamad NS. Spectrophotometric determination of clobetasol propionate in pharmaceutical preparations and environmental samples. World J Pharm Pharm Sci 2018;7(10):167-73.
- Nagar A, Jain P, Bodhke S. Active enantiomeric form of pregabalin based on sole and couples chromatography methods. Pharm Methods 2022;13(1):14-27.
- Nagar A, Chugh NN. Simultaneous estimation method development as analytical method for flupentixol dihydrochloride and melitracen hydrochloride from their combine pharmaceutical dosage forms by RP-HPLC. Pharm Innov 2015;4(1, Part B):81-6.
- Bendale AR, Narkhede SP, Palande S, Nagar AA, Vidyasagar GL. Validated RP-HPLC method as a tool for estimation of lornoxicam in pharmaceutical dosage form. Indo Am J Pharm Res 2013;3(7):5491-8.
- Shah R, Nagar A, Bardiya R, Shirkhedkar A, Purohit D, Bendale A. Combined and comparative analytical studies with stability studies and validation for estimation of prenoxdiazine HCl in pharmaceutical dosage form. Futur J Pharma Sci 2023;9(1):53.