- *Corresponding Author:
- Z. H. Chu
Department of Oncology, Huashan Hospital, Fudan University, Shanghai 200040, China
E-mail: zhaohuichu5196@qq.com
| This article was originally published in a special issue, “Current Trends in Pharmaceutical and Biomedical Sciences” |
| Indian J Pharm Sci 2022:84(5) Spl Issue “125-132” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
To understand the efficacy and safety of preferred hepatectomy combined with postoperative systemic drug therapy for breast cancer liver metastases is the objective of the study. We retrospectively analyzed 3 patients with liver metastases included in the initial metastatic sites after breast cancer surgery that preferred hepatectomy and postoperative systemic drug therapy in our department between October 2016 and November 2019, combined with literature review to share our experience. All patients were estrogen receptor and/or human epidermal growth factor receptor-2 positive with good performance status and no obvious drug resistance but still had some unfavorable factors. Patient 1 was 73 y old; patient 2 developed 8 liver metastases after only 7 mo after breast surgery and patient 3 had the largest (6.3 cm) liver metastatic lesion and simultaneous extra-hepatic lymph nodes metastases. They all underwent radical (R0) resection of hepatectomy without complications, combined with systemic drug therapy including timely postoperative chemotherapy, sequential targeted therapy and endocrine therapy. During 28-64 mo of follow-up, they all have eventually achieved clinically complete remission of tumor in the whole body with good quality of life. The longest progression-free survival has been above 64 mo. Preferred hepatectomy combined with postoperative systemic drug therapy was safe and may benefit for estrogen receptor and/or human epidermal growth factor receptor-2 positive breast cancer patients with liver included in the initial metastatic sites who had good performance status, no drug resistance, potential R0 resection, limited and controllable extrahepatic metastasis.
Keywords
Breast cancer, liver metastases, hepatectomy, systemic drug therapy, targeted therapy
Breast cancer is the malignant tumor with the highest diagnosis rate among women all over the world. The liver is the second most common site of breast cancer in young women[1] and has been seen more often after breast cancer surgery than at initial diagnosis[2]. In the course of breast cancer, over 50 % of patients develop liver involvement eventually and only 2 % of patients have liver involvement alone[3]. With a poor prognosis, there are 2-3 y of the median Overall Survival (OS) among patients with Breast Cancer Liver Metastases (BCLM)[4].
When distant metastases including BCLM occur, Systemic Drug Therapy (SDT) is prioritized by current guidelines. Before that, the biopsy of metastatic lesions is recommended for pathological diagnosis to guide the choice of drugs. In the real world, the resection of liver metastases has occasionally been used for diagnosis and local therapy. A systematic review[1] delivered that hepatectomy which is the complete resection of liver metastases could improve the OS with the longest median OS (45 mo) and 5 y survival rate (41 %) in selected patients. However, few literatures have contributed data on the intervention timing of hepatectomy. Moreover, the suggestions of the 5th European Stroke Organisation (ESO)-European Society for Medical Oncology (ESMO) international consensus guidelines for Advanced Breast Cancer (ABC) were also included[5]. Since there are no randomized data on the effect of local therapy on survival, prospective Randomized Controlled Trials (RCTs) of local therapy for BCLM are urgently needed.
However, still there was no RCT data about hepatectomy improving the survival of BCLM due to the inherent complex progress of BCLM. With the development of SDT, it has been seen in some selected patients with BCLM that hepatectomy combined with SDT can improve survival and quality of life. In this study, we collected patients with BCLM who preferred hepatectomy combined with postoperative SDT treatment of liver included in the initial metastatic sites and retrospectively analyzed their clinicopathological characteristics, therapy, safety and the short-term and long-term outcomes combined with a literature review to share our experience.
Materials and Methods
Subjects:
We analyzed the patients with BCLM who had preferred hepatectomy combined with postoperative SDT of liver included in the initial metastatic sites after breast cancer surgery in our department between October 2016 and November 2019.
Methods:
Assessments before hepatectomy: The primary breast cancer was recorded with pathological classification and stage based on the American Joint Committee on Cancer, 7th edition[6]. The Immunohistochemistry (IHC) expression of Estrogen Receptor (ER), Progesterone Receptor (PR), Kiel 67 (Ki67) and Human Epidermal Growth Factor Receptor-2 (HER2) status were collected from the primary breast cancer tissues. IHC of HER2 was scored as 0, 1+, 2+, 3+, when 2+ needed to be confirmed by Fluorescence In Situ Hybridization (FISH) analysis. HER2-positive was defined as IHC 3+ or FISH-based HER2 gene amplification; otherwise, it was HER2-negative. Triple-negative breast cancer was defined as ER-negative, PR- negative and HER2-negative. Liver Metastasis-Free Interval (LMFI) was defined as the time from breast cancer surgery to the diagnosis of BCLM.
The careful assessment and full discussion should be performed by a multidisciplinary team consisting of oncologists, breast surgeons, hepatic surgeons, pathologists and radiologists before the operation of hepatectomy. Patients were considered of being suitable for hepatectomy when meeting the following criteria. Eastern Cooperative Oncology Group Performance Status (ECOG PS) was scored as 0~1; the primary breast cancer was ER-positive and/or HER2-positive, but not triple-negative breast cancer which was generally considered to be the poorest sub-type of breast cancer; liver metastases were among the first metastatic sites after breast cancer surgery; liver function is classified as Child- Pugh A; after estimation according to enhanced liver Magnetic Resonance Imaging (MRI), liver metastases were valued as potential liver radical (R0) resection and residual volume of the liver would be above 40 %; after estimation of the whole- body by Positron Emission Tomography (PET)/ Computed Tomography (CT), simultaneous extra- hepatic metastases were also permissible but limited to asymptomatic bone and soft tissue metastasis (for example lymph nodes); patients were willing to accept hepatectomy and SDT after hepatectomy. Between October 2016 and November 2019, there were 207 patients with BCLM after breast cancer surgery that was diagnosed in our department. Only 15 patients met the above conditions. Because the patients worried about the trauma of hepatectomy, wanted to receive SDT to control the metastases as soon as possible, only 3 patients finally preferred hepatectomy and postoperative SDT.
Treatments:
Hepatectomy with the aim of R0 resection used liver- sparing techniques. Laparoscope was first chosen to reduce surgical injury compared with laparotomy. Anatomical hepatectomy was preferred for multiple liver metastases. The major or minor hepatectomy was defined as ≥3 or <3 segments being removed. Liver metastases from breast cancer were confirmed pathologically by hepatectomy. The IHC expression of ER, PR, Ki67 and HER2 in liver tumor tissues were also collected, which would guide SDT after hepatectomy.
Follow-up:
After hepatectomy, postoperative complications such as bleeding, biliary fistula, intrahepatic abscess, etc. should be detected carefully. The recurrence of intra- hepatic, extra-hepatic metastases and the health- related quality of life were assessed within 1 mo, followed by every 3 mo in the first 2 y and every 6 mo above 2 y. The final follow-up was conducted in March 2022. The follow-up time was defined relative to the date of hepatectomy. The Intra-Hepatic Recurrence-Free Survival (IHRFS) was defined as the interval from hepatectomy to the reappearance of liver metastases. Progression-Free Survival (PFS) was defined as the interval between the reappearance of liver metastases and extra-hepatic metastases progression. OS was defined as the interval from hepatectomy to death due to any cause.
Results and Discussion
We collected clinicopathological characteristics and the outcomes of hepatectomy from three patients with BCLM in our department (Table 1).
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| The primary breast cancer surgery | Mastectomy and ALN dissection | Mastectomy and ALN dissection | Breast-conserving surgery |
| Age (years) | 68 | 49 | 42 |
| Tumor size (cm) | 2.5 | 2 | 2 |
| Number of ALN metastases | 1 | 0 | 0 |
| Pathology | |||
| Invasive ductal carcinoma | Yes | Yes | Yes |
| ER | Positive | Negative | Positive |
| PR | Negative | Negative | Positive |
| HER2 | Positive | Positive | Negative |
| Ki67 positive | 30 % | 15 % | 20 % |
| Pathological Tumor-Node-Metastasis (pTNM) stage | pT2N1M0 | pT1cN0M0 | pT1cN0M0 |
| Adjuvant chemotherapy | TC×4 | EC×4 | No |
| Adjuvant radiotherapy | No | No | 50Gy/25fx |
| Adjuvant endocrine therapy | Anastrozole | No | Tamoxifen |
| 5 y | 3.5 y | ||
| Liver metastases | |||
| Age | 73 | 50 | 47 |
| ECOG PS | 0 | 0 | 0 |
| LMFI (months) | 63 | 7 | 69 |
| Number | 1 | 8 | 6 |
| Maximum diameter (cm) | 3.4 | 3 | 6.3 |
| Site (liver lobe) | Right | Left and right | Left and right |
| Extra-hepatic metastases simultaneous | No | No | Yesa |
| Major/minor hepatectomy | Minor | Major | Major |
| Laparoscopic hepatectomy | Yes | No | No |
| Anatomical hepatectomy | No | Yes | Yes |
| Microscopic negative margins | Yes | Yes | Yes |
| Operative complication | No | No | No |
| Pathology of liver metastases | |||
| ER | Positive | Negative | Positive |
| PR | Negative | Negative | Positive |
| HER2 | Positive | Positive | Negative |
| Ki67 positive | 40 % | 60 % | 30 % |
| SDT after hepatectomy | |||
| Chemotherapy | EC×4-TH×4 | THP×8 | Nab-paclitaxel×2 |
| Anti-HER2 target therapy | Trastuzumab | Yes | No |
| CDK4/6 inhibitor target therapy | No | No | Palbociclib |
| Endocrine therapy | Exemestane | No | Letrozole |
| IHRFS (months) | >64 | >44 | >28 |
| PFS (months) | >64 | >44 | >28 |
| OS (months) | >64 | >44 | >28 |
Note: Major hepatectomy, resection of ≥3 segments; Minor resection, resection of <3 segments. aindicates the left supraclavicular, axillary and retroperitoneal lymph nodes metastases; TC: Docetaxel 75 mg/m2 d1 q3w+cyclophosphamide 600 mg/m2 d1 q3w; EC: Epirubicin 100 mg/m2 d1 q3w+cyclophosphamide 600 mg/m2 d1 q3w; TH: Docetaxel 75 mg/m2 d1 q3w+trastuzumab 8 mg/kg first then 6 mg/kg q3w; THP: Docetaxel 75 mg/m2 d1 q3w+pertuzumab 840 mg first then 420 mg q3w+trastuzumab 8 mg/kg first then 6 mg/kg q3w; Nab-paclitaxel 260 mg/m2 d1 q3w
Table 1: The Prognosis of Hepatic Resection For BCLM And Their Clinicopathological Characteristics
Patient medical history and response to treatment was explained in detail. Patient no. 1 was a 68 y old woman. She underwent mastectomy and right Axillary Lymph Node (ALN) dissection due to a 2.5 cm in the right breast in June 2011. The pathology was invasive ductal carcinoma with one ALN positive and IHC expression showed ER-positive, PR-negative, 30 % of Ki67 positive and HER2 (3+) (HER2-positive). Adjuvant chemotherapy included 4 cycles of docetaxel (75 mg/m2 day 1 (d1) once every 3 w (q3w)) and cyclophosphamide (600 mg/m2 d1 q3w), which was followed by 5 y of anastrozole as adjuvant endocrine therapy without adjuvant radiotherapy. In September 2016, the whole- body PET-CT scan detected a solitary metastatic mass (the maximum diameter was 3.4 cm) in the right lobe of the liver. LMFI was 63 mo. Although the patient was 73 y old, her ECOG PS was very good, scored as 0. In October 2016, the patient underwent one segmental hepatectomy by laparoscopy. The pathology and IHC of BCLM showed the following results. The margins were negative and the tumor was ER-positive, PR- negative, 40 % of Ki67 positive and HER2 (3+) (HER2- positive). On the 4th w after hepatectomy, the patient recovered very well without obvious complications and was checked with no obvious metastatic lesions in the whole body. Then the chemotherapy combined with anti-HER2 targeted therapy were administered to this patient; 4 cycles of epirubicin (100 mg/m2 d1 q3w) and cyclophosphamide (600 mg/m2 d1 q3w), which was followed by 4 cycles of docetaxel (75 mg/m2 d1 q3w) and trastuzumab 8 mg/kg first then 6 mg/kg q3w. At the end of chemotherapy, the patient still had no obvious intra-hepatic recurrence and extra-hepatic metastases. The later maintenance treatment was given for 20 mo of exemestane 25 mg Once Daily (QD) plus trastuzumab and sequential 38 mo of exemestane monotherapy. Until March 2022, the IHRFS, PFS and OS of patient no.1 have been above 64 mo.
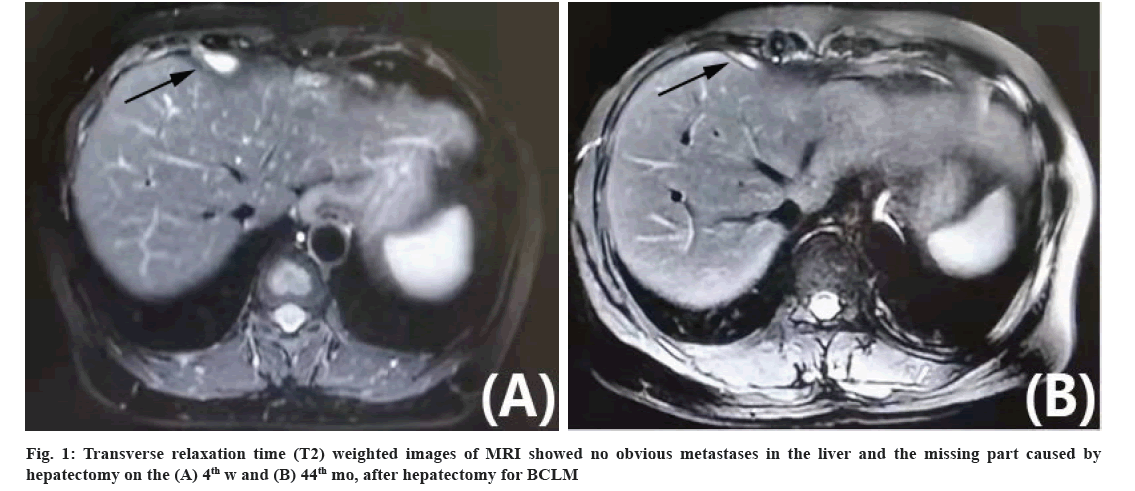
The patient no. 2 found a 2 cm lump in the right breast and received a mastectomy and right ALN dissection in November 2017 at the age of 49 y. The pathology confirmed invasive ductal carcinoma without ALN involved. The IHC expression showed ER-negative, PR-negative, 15 % of Ki67 positive and HER2 (3+) (HER2-positive). Adjuvant chemotherapy was given for 4 cycles (epirubicin 100 mg/m2 d1 q3w+cyclophosphamide 600 mg/m2 d1 q3w) in another hospital. Then she had side effects of serious vomiting and rejected more chemotherapy and adjuvant targeted therapy. In June 2018, the whole-body PET/CT detected 8 metastases (the maximum diameter was 3 cm) in the left and right liver lobe without extra-hepatic metastasis. This patient LMFI was only 7 mo. This patient required more active treatment. The patient was 50 y old and has good ECOG PS scored as 0. In July 2018, she underwent anatomical hepatectomy and major resection by laparotomy. The pathology showed a negative margin. IHC expression of BCLM showed as follows: ER-negative, PR-negative, 60 % of Ki67 positive and HER2 (3+) (HER2-positive). On the 4th w after hepatectomy, she had no complications and recovered well. Sequential SDT included the chemotherapy and anti-HER2 targeted therapy, docetaxel 75 mg/ m2 d1 q3w+pertuzumab 840 mg first then 420 mg q3w+trastuzumab 8 mg/kg first then 6 mg/kg q3w. After chemotherapy, the patient found no obvious metastases in the whole body detected by PET/CT scan. Then anti-HER2 targeted therapy of pertuzumab plus trastuzumab was given as maintenance treatment until now. There were no metastatic lesions found again in the liver from the 4th w to the 44th mo after hepatectomy and the missing part caused by hepatectomy was filled with regenerated hepatic tissue (fig. 1A and fig. 1B). Moreover, there were still no metastases in other sites. This patient IHRFS, PFS and OS have been above 44 mo until March 2022.
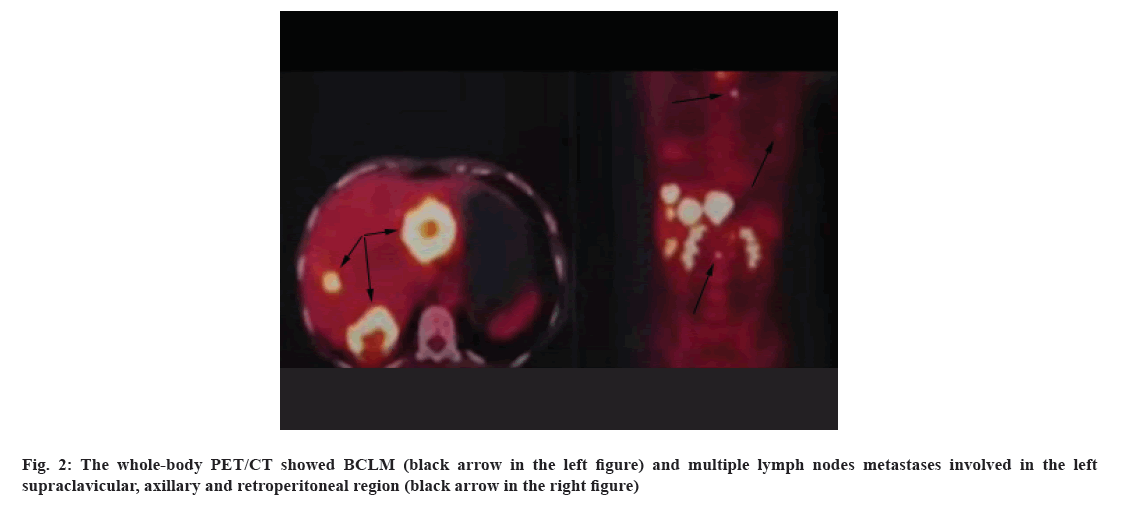
The patient no. 3 is a 42 y old woman, accepted breast- conserving surgery due to a 2 cm lump of the right breast without ALN abnormality found in ultrasound in January 2014. The pathology was invasive ductal carcinoma with ER-positive, PR-positive, 20 % of Ki67 positive and HER2 (1+) (HER2-negative). Adjuvant chemotherapy was not given to her. Adjuvant radiotherapy (50Gy/25fx) was performed timely after surgery. However, tamoxifen as a 5 y adjuvant endocrine therapy plan was terminated after 3.5 y by the patient herself. In October 2019, she developed 6 liver metastases (the maximum was 6.3 cm) located in the left and right liver lobe and multiple lymph nodes metastases involved in the left supraclavicular, axillary and retroperitoneal regions shown in PET/CT (fig. 2). BCLM counted the most of tumor burden. This patient LMFI was 69 mo.
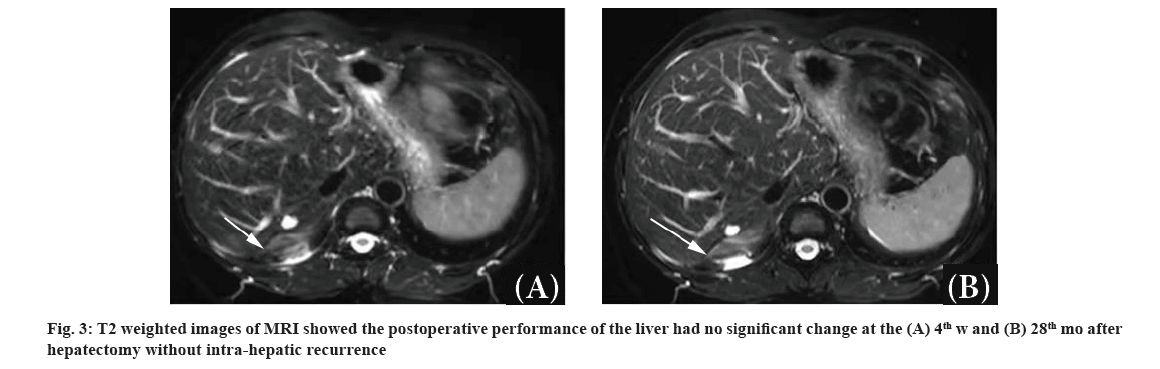
In November 2019, this patient was 47 y old with good ECOG PS (0). She underwent anatomical hepatectomy and major resection by laparotomy. The pathology was confirmed as a negative margin. BCLM expressed ER- positive, PR-positive, 30 % of Ki67 positive and HER- 2 (0) (HER2-negative). On the 4th w after hepatectomy, she recovered well and had no obvious complications. No metastases reappeared in the liver in a short time and the extra-hepatic metastases were valued as stable disease. Then the chemotherapy of nab-paclitaxel (260 mg/m2 d1 q3w) was given in two cycles. After chemotherapy, Cyclin-Dependent Kinase 4/6 (CDK4/6) inhibitor (palbociclib 75 mg d1-21 q4w) plus letrozole (2.5 mg QD) has been given to control the tumor from January 2020 to the present. During the follow-up of every 3 mo, intra-hepatic recurrence hasn’t still been found and the postoperative performance of the liver had no significant change in MRI (fig. 3A and fig. 3B). The extra-hepatic metastases before hepatectomy disappeared and could not be detected. The patient IHRFS, PFS and OS have been above 28 mo.
The longest follow-up time across all patients was 64 mo. No complications about hepatectomy have been found. All patient’s tolerance and compliance with SDT were very good. Their quality of life was like healthy people. Until March 2022, all patients were still alive and had no intra-hepatic recurrence and obvious extra-hepatic metastases, achieving almost complete remission of the tumor. The longest of IHRFS, PFS and OS have been more than 64 mo.
In this study, all patients were BCLM with the following common features-The ECOG PS was very good in all patients, when underwent hepatectomy whatever the age; liver metastases were listed in the first metastatic sites of breast cancer; they all accepted R0 resection of liver metastases as soon as the detection of BCLM with no postoperative complications; SDT after hepatectomy included chemotherapy beginning within 1 mo and sequential targeted therapy and/or endocrine therapy; they were ER-positive and/or HER2-positive, not triple- negative breast cancer, and all had effective targeted drugs and/or endocrine therapy to control tumor. In this study, all patients’ ECOG PS was scored as 0 even the oldest patient was 73 y old. Good ECOG PS is the basic one of all conditions for hepatectomy, which is underlined in ABC stage 5 (ABC 5)[5]. It considers the PS not the age is important for hepatectomy.
In this study, we selected patients with liver metastases in the first occurrence of breast cancer for hepatectomy because it has been reported that patients with first recurrence of BCLM only had longer OS than patients with subsequent recurrent BCLM (26 mo vs. 18 mo) [7]. We think BCLM occurring before drug resistance will be easier to treat when tumor burden reduces significantly by hepatectomy. All patients in this study had lived through, with no obvious complication after hepatectomy whether major or minor resection, whether by laparoscope or by laparotomy and whether anatomical hepatectomy or not. It was reported that the mortality of hepatectomy was consistently very low (from 0 % to 5.5 %) in an analysis of 19 studies[1]. However, the complication morbidity was 11 % when hepatectomy was performed within 1 y after the detection of BCLM and it was 38 % when hepatectomy was performed 1 y later[8]. So that we think if patients meet the conditions of hepatectomy, the earlier the operation, the safer it will be.
Although all patients had negative margins, we gave chemotherapy which is a quick effect for the tumor to them after hepatectomy to reduce the tumor outbreak risk in the perioperative period. All patients could tolerate chemotherapy. But considering the poor long- term tolerance, chemotherapy was used for a short time in this study. After chemotherapy, low-toxic targeted therapy and/or endocrine therapy were continuously given to all patients to get a high quality of life. The IHRFS, PFS and OS of all patients have been more than 28 mo and even 64 mo. It has been reported that intra- hepatic and extra-hepatic recurrence after hepatectomy occurred in 52 % of patients at 24 mo[9]. So we think effective and continuous SDT after hepatectomy is as important as hepatectomy for long-term survival. With more effective drugs applied in recent years, the value of hepatectomy will get smaller and smaller. A cost-utility analysis told us that hepatectomy proved to be cost-effective for BCLM when compared with SDT alone[10]. For example as follows, the median OS of patients with ER-positive BCLM undergoing hepatectomy plus letrozole was 58.3 mo, compared with 36.5 mo for patients receiving letrozole alone and 47.6 mo for patients receiving letrozole plus palbociclib. The median OS of patients with HER2-positive BCLM who received hepatectomy plus docetaxel plus trastuzumab was 63.8 vs. 38.5 mo for those receiving docetaxel plus trastuzumab and 54.6 mo for patients receiving docetaxel plus trastuzumab and pertuzumab. In this study, the OS of patient no. 3 using hepatectomy plus letrozole plus palbociclib has been above 28 mo, the OS of patient no. 1 using hepatectomy plus docetaxel plus trastuzumab has been above 64 mo and the OS of patient no. 2 using hepatectomy plus docetaxel plus trastuzumab plus pertuzumab has been above 44 mo. Therefore, we think that hepatectomy will still take a place.
All patients we selected for hepatectomy were ER- positive and/or HER2-positive because it has been reported that HER2-positive or ER-positive was identified as an independent good prognostic factor for BCLM patients[6]. With only SDT, owing to having the targeted therapy and endocrine therapy, the PFS and OS of HER2-positive or ER-positive patients with BCLM were better than those of triple-negative breast cancer patients[7]. In the Surveillance, Epidemiology and End Results (SEER) database, the median OS of patients with BCLM was 20 mo, there was a significant difference between ER-positive/HER2-positive and triple-negative breast cancer patients (38 mo vs. 9 mo) [11]. When hepatectomy was added to SDT, it yielded a significant OS benefit in ER-positive patients compared with ER-negative patients (50.5 mo vs. 26.9 mo)[12] and it yielded the median PFS of 60 mo for ER-negative/ HER2-positive patients with solitary BCLM which has the best outcome among all breast molecular subtypes[13]. Up to now, patient no. 1 with ER-positive/ HER2-positive has more than 64 mo of PFS.
Except for the common characteristics, there are still some differences among patients in this study. The LMFI of patient no. 2 was extremely short; liver metastasis in patient no. 1 was solitary and others were multiple; the maximum diameter of liver metastases varied broadly (from 3 cm to 6.3 cm); extra-hepatic metastases were simultaneously existent for patient no. 3.
The liver metastases of patient no. 2 occurred 7 mo after breast cancer surgery. It was poor prognostic factors of OS for hepatectomy that LMFI was shorter than 24 mo[14]. The earlier appearance of BCLM often means more drug resistance when drugs used continuously after breast cancer surgery. But we assessed the reason for liver metastasis of patient no. 2 was mainly the previous omission of adjuvant anti-HER2 targeted therapy, no drug resistance. Therefore we suggest preoperative evaluation before hepatectomy should be more thoughtful.
Patient no. 1 had above 64 mo of PFS after resection of solitary liver metastasis. The database of Sweden between 2009 and 2016 showed hepatectomy for 2-5 or 1 BCLM was all safe and might provide survival benefits[15]. Patient no. 2 had 8 (the most) liver lesions and her PFS is also long (above 44 mo). It has been reported there has no obvious difference in OS between solitary and multiple BCLM[14]. In this study, although the maximum diameter of liver lesion was 6.3 cm, patient no. 3 also got R0 resection without postoperative complications. An analysis of 131 patients with the median 3.0 (2.0-5.0) cm liver lesions showed that 90.8 % of patients got R0 resection safely and acquired a relatively long survival[9]. In the analysis of patients with mean 4.0 (1.0-11.0) cm liver metastases, the maximum diameter less than 5.0 cm and R0 resection were independent factors of longer OS[16]. Inspired by this study, we think that regardless of the number or maximum diameter of liver metastases, patients who may reach potential R0 resection by detailed preoperative imaging evaluation may benefit from liver resection.
Patient no. 3 with extra-hepatic metastases involving lymph nodes has lived more than 28 mo without progression after hepatectomy combined with short- term chemotherapy followed by long-term targeted drug (palbociclib) plus endocrine therapy (letrozole). A univariate retrospective analysis of 16 patients with extra-hepatic metastases showed their OS after hepatectomy was similar to 103 patients without extra- hepatic metastases[9]. We suggest that hepatectomy should not be excluded if the extra-hepatic tumor is assessed as limited and easy to control because patients with extra-hepatic metastases accepted SDT after hepatectomy more actively which was beneficial for long-term survival.
As a result, all the patients have eventually achieved clinical complete remission of the tumor during 28-64 mo of follow-up after hepatectomy in this study. Our findings showed that hepatectomy was safe and it may be applied first in ER-positive and/or HER2-positive breast cancer patients with liver included in the initial metastatic sites who had good PS, no drug resistance, potential R0 resection, limited and controllable extra- hepatic metastasis. With the development of drugs, SDT (timely postoperative chemotherapy followed by effective and low-toxic targeted therapy and/ or endocrine therapy) combined with hepatectomy will improve survival and it will also challenge the necessity of hepatectomy. Therefore, the selection for hepatectomy will be higher to pursue complete remission of the tumor in the whole body as far as possible.
Given some key limitations, the results of our analysis must be interpreted carefully. Firstly, this is a retrospective study full of heterogeneity. Secondly, no control group can be compared. Finally, due to the choice of hepatectomy before SDT and there are only few cases. We fully know the shortcomings of this study.
However, we still believe that our analysis is valuable for that hepatectomy combined with updated SDT to apply in patients whose prognosis was thought as poor in the traditional sense. Inspired by this study, we will prospectively explore the intervention timing and efficacy of hepatectomy combined with SDT to achieve complete remission of the tumor in patients with BCLM.
Author’s contributions:
The contribution of the first author, Zhaohui Chu is responsible for conception and ideas of this study, collecting the data, interpreting the data, writing paper for this study and also did the submission of this manuscript. Other author, Jianhua Wu is responsible for writing the surgery part.
Funding:
This study was funded by grants from Clinical Research Fund of Chinese Society of Clinical Oncology (Y-Young2021-0092).
Acknowledgements:
The authors thank Dr. Tao Liu and Jingwei Jiang for some help in treating the patients in this study.
Conflict of interests:
The authors have no conflicts of interest to disclose.
References
- Rivera K, Jeyarajah DR, Washington K. Hepatectomy, RFA and other liver directed therapies for treatment of breast cancer liver metastasis: A systematic review. Front Oncol 2021;312:1-11.
[Crossref] [Google scholar] [PubMed]
- Cummings MC, Simpson PT, Reid LE, Jayanthan J, Skerman J, Song S, et al. Metastatic progression of breast cancer: Insights from 50 y of autopsies. J Pathol 2014;232(1):23-31.
[Crossref] [Google scholar] [PubMed]
- Hoe AL, Royle GT, Taylor I. Breast liver metastases-incidence, diagnosis and outcome. J R Soc Med 1991;84(12):714-6.
[Crossref] [Google scholar] [PubMed]
- Rashid NS, Grible JM, Clevenger CV, Harrell JC. Breast cancer liver metastasis: Current and future treatment approaches. Clin Exp Metastasis 2021;38(3):263-77.
[Crossref] [Google scholar] [PubMed]
- Cardoso F, Paluch-Shimon S, Senkus E, Curigliano G, Aapro MS, André F, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol 2020;31(12):1623-49.
[Crossref] [Google scholar] [PubMed]
- Ji L, Fan L, Zhu X, Gao Y, Wang Z. A prognostic model for breast cancer with liver metastasis. Front Oncol 2020;10:1342.
[Crossref] [Google scholar] [PubMed]
- Tian C, Liu S, Wang Y, Song X. Prognosis and genomic landscape of liver metastasis in patients with breast cancer. Front Oncol 2021;11:588136.
[Crossref] [Google scholar] [PubMed]
- Lucidi V, Bohlok A, Liberale G, Bez M, Germanova D, Bouazza F, et al. Extended time interval between diagnosis and surgery does not improve the outcome in patients operated for resection or ablation of breast cancer liver metastases. Eur J Surg Oncol 2020;46(2):229-34.
[Crossref] [Google scholar] [PubMed]
- Margonis GA, Buettner S, Sasaki K, Kim Y, Ratti F, Russolillo N, et al. The role of liver-directed surgery in patients with hepatic metastasis from primary breast cancer: A multi-institutional analysis. HPB 2016;18(8):700-5.
[Crossref] [Google scholar] [PubMed]
- Spolverato G, Vitale A, Bagante F, Connolly R, Pawlik TM. Liver resection for breast cancer liver metastases. Ann Surg 2017;265(4):792-9.
[Crossref] [Google scholar] [PubMed]
- Ji L, Cheng L, Zhu X, Gao Y, Fan L, Wang Z. Risk and prognostic factors of breast cancer with liver metastases. BMC Cancer 2021;21(1):1-5.
[Crossref] [Google scholar] [PubMed]
- Feng Y, He XG, Zhou CM, Zhang QY, Huang SY, Li Z, et al. Comparison of hepatic resection and systemic treatment of breast cancer liver metastases: A propensity score matching study. Am J Surg 2020;220(4):945-51.
[Crossref] [Google scholar] [PubMed]
- Pocard M, Pouillart P, Asselain B, Salmon RJ. Hepatic resection in metastatic breast cancer: Results and prognostic factors. Eur J Surg Oncol 2000;26(2):155-9.
[Crossref] [Google scholar] [PubMed]
- He X, Zhang Q, Feng Y, Li Z, Pan Q, Zhao Y, et al. Resection of liver metastases from breast cancer: A multi-centre analysis. Clin Transl Oncol 2020;22(4):512-21.
[Crossref] [Google scholar] [PubMed]
- Sundén M, Hermansson C, Taflin H, Andersson A, Sund M, Hemmingsson O. Surgical treatment of breast cancer liver metastases-A nationwide registry-based case control study. Eur J Surg Oncol 2020;46(6):1006-12.
[Crossref] [Google scholar] [PubMed]
- Ercolani G, Zanello M, Serenari M, Cescon M, Cucchetti A, Ravaioli M, et al. Ten-year survival after liver resection for breast metastases: A single-center experience. Dig Surg 2018;35(4):372-80.
[Crossref] [Google scholar] [PubMed]