- Corresponding Author:
- X. J. Meng Department of Neurosurgery, School of Medicine, First Affiliated Hospital of Xi’an Jiaotong University, Xi’an 710061, China E−mail: mengxijun99@163.com
| Date of Submission | 02 May 2013 |
| Date of Revision | 19 January 2014 |
| Date of Acceptance | 25 January 2014 |
| Indian J Pharm Sci 2014;76(2):125-131 |
Abstract
S100B protein in serum and cerebral spinal fluid is increasingly used as a biochemical marker in early examinations after seizure to assess brain damage. Resveratrol, a nonflavonoid polyphenol, has been identified as a potent antiepileptic agent. However, a potential association between epilepsy with S100B protein in the cerebral spinal fluid and the sera of animal models lacks investigation. In this study, we evaluated the effects of resveratrol on behaviour and S100B protein levels in cerebral spinal fluid and serum in a rat model of chronic epilepsy induced via pentylenetetrazole kindling. By Morris water maze experiment analysis, we found that recovery of cognitive function in the resveratrol group (15 mg/kg/day), was significantly better than that of either the untreated or the vehicle groups. Further Nissl staining revealed that resveratrol significantly reduced pentylenetetrazole-induced death of neurons in the CA1 and CA3 regions of the hippocampus. Moreover, S100B protein levels in the cerebral spinal fluid and serum of rats treated with resveratrol were significantly reduced compared with the untreated and vehicle groups. These novel findings suggest an important mechanism of resveratrol and contribute to the treatment of epilepsy.
Keywords
Epilepsy, pentylenetetrazole, resveratrol, cognition, neuroprotective
Epilepsy is a group of syndromic seizure disorders of the nervous system that affects about 50 million people worldwide[1]. Epileptic seizures can impair the psychological and cognitive functions of patients, and greatly affects their quality of life. Epilepsy is a worldwide major public health concern [WHO, http:// www.who.int/mediacentre/factsheets/fs999/en/], and its prevalence in China is 3.6−7%[2].
Previous studies of epilepsy have shown that the phytoalexin, resveratrol (3,5,4′−trihydroxy−trans−stilbene) may have antiepileptic effects[3], although no definitive conclusion has been made[4]. Resveratrol is a natural phenol produced by several plant species. Its cardiovascular and other health benefits are proven, and it acts primarily as an antioxidant, antiinflammatory and vasorelaxant[5].
Among foods, resveratrol is most concentrated in red wine[6]. An epidemiologic study confirmed that appropriate intake of wine reduced the risk of dementia[7], and it appears that resveratrol contributed to this protective effect[8]. Furthermore, a large number of recent studies, conducted in vitro and in vivo, have pointed towards a neuroprotective role for resveratrol in neurodegenerative diseases or traumatic brain injury[9,10]. Applied to the normal brain, resveratrol was associated with the maintenance of mitochondrial function and neuronal activity[9]. Resveratrol administered by peripheral route (gastrointestinal) can permeate through the blood–brain barrier, and its bioactivity was retained in the brain for up to 4 h[11]. Altogether, these studies indicate that further research into the possible antiepileptic pharmacological aspects of resveratrol is warranted.
Astrocytes are involved in the pathogenesis of epileptic seizure onset, development and termination[12]. S100B (or S100 calcium binding protein B) is a calcium−binding protein that is primarily expressed by astrocytes[13], and is useful as a marker for astrocyte damage[14] under normal physiological conditions, serum S100B protein levels are very low and relatively stable, but with structural or functional deficiency the blood–brain barrier becomes permeable and serum levels increase[15,16]. Research and clinical data have also indicated that the elevation of S100B protein levels in both serum and cerebrospinal fluid (CSF) is associated with seizures[17], and S100B protein in serum and CSF can be used as a biochemical marker in early examinations after seizure to assess brain damage. Thus, we hypothesised that levels of S100B in CSF and sera could be utilised to assess the efficacy of resveratrol in a pentylenetetrazole (PTZ)−kindling model of epilepsy.
The PTZ−kindling model of epilepsy in rats is the most reliable and widely used[18]. Favourable features of the model include ease of induction and spontaneous seizures[19]. It is easy to determine if the model has been successfully established, and epileptic behaviour is controllable and reproducible. Therefore, we chose this model for our study.
Materials and Methods
PTZ and resveratrol were obtained from Sigma, St. Louis, USA. The rat CSF and serum S100B protein ELISA kits were from Beijing Yonghui Biotechnology (Beijing, China). The SLY−WMS water maze system was purchased from Beijing Shuo Lin Yuan Technology (Beijing, China).
Experimental animals and model establishment
The present study was approved by the local ethical committee (No. XJTULAC201-016). Forty−eight healthy adult male Sprague Dawley rats, aged 6−7 weeks and weighing 220±20 g, were purchased from the Animal Center of Xi’an Jiaotong University School of Medicine. The rats were divided into a normal control group (n=16) and a PTZ model group (n=48) using a random number table. Treatments (saline, vehicle or PTZ) were administered by intraperitoneal (i.p.) injection daily for 28 days between 8:00 and 10:00 AM. Specifically, rats in the normal control group were given 0.9% saline (2 ml/kg/day) and those in the PTZ model group were given 1% PTZ at a subconvulsive dose of 35 mg/kg/day. The rats were weighed daily and the dosage adjusted according to body weight.
Behavioural changes in the rats were monitored for 2 h postinjection and general behaviour manifestations, seizure severity scores, latencies and durations were recorded. Racine’s classification[20] was used to score the severity of seizures; 0, no change in behaviour; (I) blink, ear and facial twitching, isolated myoclonic jerks; (II) gazing, nodding; (III) forelimb−limited seizures, twitching, scratching; (IV) major seizures (generalised without the tonic phase), double forelimb lifting; and (V) widespread muscle spasms, clonus of all four limbs, and generalised tonic–clonic seizures. Kindling was defined as ≥4 consecutive level−II seizures, or two seizures that were level IV or higher.
The model of chronic epilepsy induced by PTZ kindling was successfully established in the 48 rats of the PTZ group. These rats were randomly divided into three experimental groups (n=16, each). Resveratrol was dissolved in 10% dimethyl sulfoxide (DMSO) at a concentration of 10 mg/ml. Rats in the resveratrol group were given resveratrol via oral route (gavage), 15 mg/kg/day, between 8:00 and 10:00 AM for 10 consecutive days. Rats in the DMSO control group were treated identically, except that they were given 1.5 ml/kg/day of 10% DMSO only. The epilepsy model group was left untreated. The rats were weighed daily and the dosage was adjusted according to body weight. On day 10, a subconvulsive dose of PTZ (35 mg/kg/day) was given to induce seizure half an hour after PTZ or DMSO administration. Behavioural changes in the rats were monitored and recorded as above.
Morris water maze
The Morris water maze consisted of a circular pool with a diameter of 120 cm and a height of 50 cm. The pool was divided into four quadrants. A round platform with a diameter of 11 cm and a height of 25 cm was placed in one quadrant, 1 cm below the surface. The location of the platform remained unchanged and the water temperature was maintained at 22.5±1°. A camera was installed on the top of the central pool and was connected to a computer to synchronously capture and record the trajectories of the rats in the water maze. Reference items around the pool remained in the same locations during the experiment. The data was acquired using SLY−WMS Morris Water Maze system Version 2.1 software and the experimenters were blind to the drug intervention and grouping design. The water maze experiment consisted of a place navigation test and a spatial probe test. Six rats were randomly selected from each of the three experimental groups for the Morris water maze experiments.
Place navigation test
The place navigation test was used to test spatial learning and memory ability. The experiment lasted for 5 days and each day was divided into two time periods (morning and afternoon) with four training sessions in each time period. In each of the four trainings, the rats entered the water maze from different locations. Rats were trained to find and climb onto the platform with a staying time longer than 5 s. Once a rat found the platform, the software system would automatically record the time from entering the water to finding the platform (i.e. the escape latency period). If the rat failed to find the platform within 120 s, the administrator of the test guided the rat manually onto the platform and it was allowed to stay there for 5 s; a latency of 120 s was recorded. The interval between two consecutive training sessions was 60 s. The escape latency intervals for each rat in each training session during the five days of testing were observed and recorded.
Spatial probe test
The spatial probe test was administered after the rats had learned how to locate the platform, and was used to test location memory ability. The platform was removed after the fifth training on day 5. An entry site into the water maze was randomly selected (the SW quadrant marker was randomly chosen as the entry site into the water maze for these experiments). The rats were placed into the water facing the wall of the maze. The time that the rat stayed in the original quadrant, and the number of crossings the rat made across the original site of the platform within 120 s, were observed and recorded.
Specimen collection and detection
Rat CSF was collected by direct puncture of the foramen magnum[21]. Rats were anaesthetised and the skin from the neck puncture area was prepared. A #5.5 scalp needle was used. The needle sleeve was cut to expose the tip by ~7.0 mm. The rat’s head was fixed in a stereotaxic apparatus with the spine in a ventrally flexed position, which was gently pulled to increase the width of the intervertebral space. The sunken space above the first cervical vertebra above was determined as the location of the foramen magnum. Needle aspiration was performed in the centre, the fascia of the ring pillow was pierced, and CSF outflow was collected by gently pumping the syringe. The needle was subsequently removed quickly to retain the CSF. At the same time, 2 ml of rat heart blood was collected and the serum was prepared after 20 min of centrifugation at 3000 rpm. The samples were labelled and stored at –20°. The levels of S100B protein in CSF and serum were measured with the S100B ELISA kit in accordance with the manufacturer’s protocol.
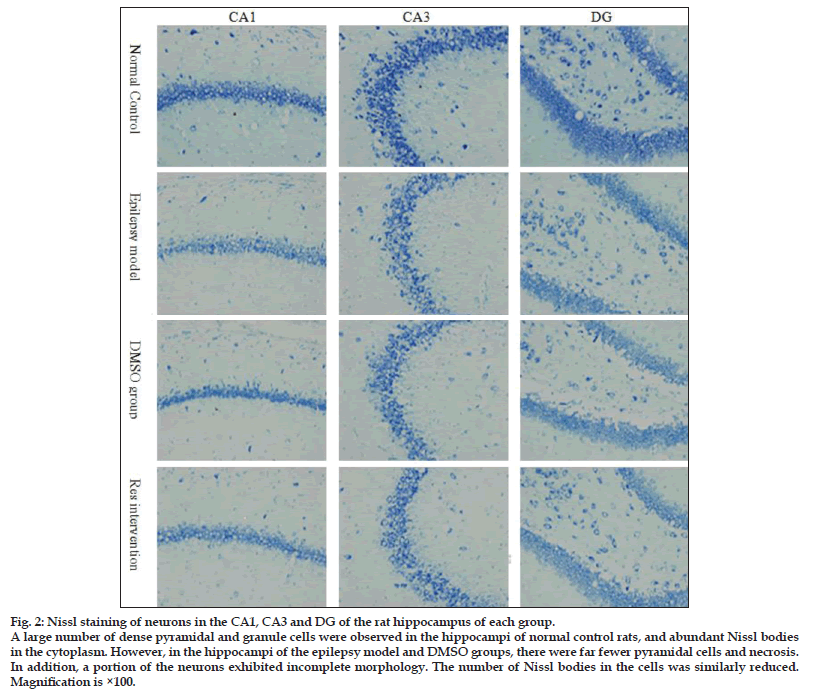
For tissue sectioning, paraffin−embedded brain specimens were placed in a Leica RM2125 rotary microtome and 5 μm−thick consecutive coronal slices were prepared until the hippocampal surface was reached. One slice was taken for every interval of five and a total of six slices were taken from each specimen. Two slices were randomly selected for Nissl staining and subjected to statistical analysis. Slices were observed under a microscope at ×40 magnification. Five non−overlapping fields were randomly chosen to calculate the numbers of the hippocampal CA1 and CA3 pyramidal cells and dentate gyrus (DG) granule cells. The mean was taken as the average number of each type of neuron in the hippocampus.
Statistical analyses
Data were analysed using SPSS 16.0 statistical software. The results are expressed as mean±standard deviation. One−way analysis of variance (ANOVA) and Tukey’s honestly significant differences (HSDs) were applied for the comparison between groups, with a significance level of 0.05. Results from the water maze test were analysed using the repeated measures ANOVA. The correlations between S100B protein in CSF and serum were analysed with Pearson’s correlation coefficients.
Results
Behavioural observations
Rats in the normal control group showed no abnormal behaviour after IP injection of saline; they had normal appetites and exhibited no seizures throughout the entire experimental process. Starting from 4 to 7 days after the initiation of IP injection of PTZ at a subconvulsive dose to establish the model, PTZ−treated rats exhibited epileptic symptoms including gazing, nodding, scratching, facial twitching and forelimb−limited seizures. These signs of seizures became daily more exacerbated. Twenty days after the start of PTZ administration, the rats displayed widespread muscle spasms, double forelimb lifting, and they eventually developed generalised tonic–clonic seizures. Seizures usually occurred 2−10 min after PTZ injection with a duration of 2−5 min per episode. The seizure frequency decreased after 30 min and was attenuated to an occasional seizure after 2 h. The seizures were scored in accordance with Racine’s classification method[20]. Kindling was considered as the occurrence of ≥4 consecutive level II seizures, or two seizures that were level IV or higher. After 28 days of daily dosing, 48 rats met Racine’s kindling standards.
The resveratrol treatment group, seizure latency was significantly longer (158.75±41.65 s) than for rats of the DMSO and untreated epilepsy model groups (124.38±31.98 s and 125.00±34.06 s, respectively). The differences between the means were statistically significant (P<0.05). In addition, seizure duration in the resveratrol group (49.00±8.94 s) was significantly shorter than that of the DMSO and untreated epilepsy model groups (74.44±17.93 s and 71.00±15.78 s) and the differences were statistically significant (P<0.05), while no significant difference was observed between the DMSO and epilepsy model groups. Moreover, the seizure score of the resveratrol group was lower than that of the epilepsy model group (fig. 1).
Place navigation test
In the place navigation test, the escape latencies for rats in all the groups were measured (Table 1). The number of days previous to a particular test, in which the rats became practiced, was a statistically significant factor in their performance (F=25.771, n=1.595, P<0.001), and the escape latency tended to shorten over time. That is, repeated measurements of the escape latencies showed a trend towards reduction in all the groups. However, the interaction between the number of days previous to the test and the experimental group (day×group) was not statistically significant (F=0.149, n=4.785, P=0.977), indicating that the role of this time factor did not vary among the groups. On days 1 and 2 of the test, the escape latencies of all four groups were not significantly different. There was no difference in the escape latencies of the DMSO and untreated model groups on days 3, 4 and 5. However, the escape latencies were significantly longer compared with the normal (nonmodel) control group (P<0.05). Moreover, the escape latency of the resveratrol group was significantly shorter than either the DMSO or untreated model groups (P<0.05), and the latency times of the resveratrol and normal control groups were not significantly different.
| Group | Treatment | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 |
|---|---|---|---|---|---|---|
| Normal (nonmodel) control | Untreated | 33.17±31.66 | 14.12±16.41 | 7.26±4.50 | 4.97±3.38 | 4.13±2.54 |
| Chronic epilepsy model | Resveratrol | 30.27±25.81 | 15.34±26.93 | 7.32±4.54 | 6.71±2.93 | 5.48±4.26 |
| DMSO vehicle | 39.29±40.24 | 25.96±22.66 | 15.01±7.17* | 13.31±6.98* | 10.29±3.73* | |
| Untreated | 42.28±35.49 | 26.59±23.21 | 13.45±8.05* | 12.65±6.88* | 8.82±5.15* |
Table 1: Escape Latencies During The Place Navigation Test.
Spatial probe test
During the spatial probe test the platform was removed. The number of times the rats crossed the original site of the platform is considered a direct indication of their spatial memory. There was no significant difference between the DMSO and nontreated model groups in the number of crossings through the original platform site (Table 2). However, in both these groups the numbers of crossings were significantly fewer than for those in the normal (nonmodel) control group (P<0.05) or the resveratrol group (P<0.05).
Similarly, there was no significant difference in the amount of time (i.e. length of stay) spent in the quadrant of the original site by the rats of the DMSO and untreated model groups, but this time was significantly reduced compared with rats of the normal (nonmodel) control group (P<0.05) and resveratrol group (P<0.05).
Histology
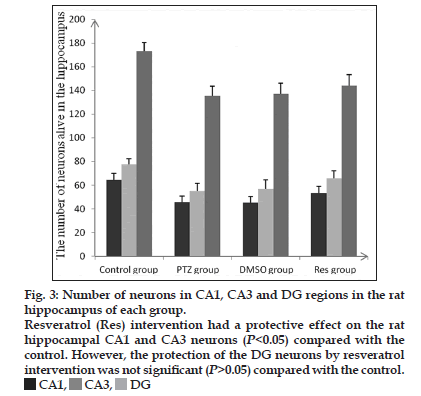
As observed from the Nissl body staining of neurons (fig. 2), a large number of dense pyramidal cells and granule cells were seen in the hippocampal CA1, CA3 and DG regions of normal (nonmodel) control rats; cells were intact with an orderly arrangement, and Nissl bodies were abundant in the cytoplasm. However, in the DMSO and nontreated model groups, there was a huge loss of pyramidal cells and necrosis was seen in CA1 and CA3. In addition, a portion of the neurons was incomplete in morphology with a vague outline. The intercellular space was increased, and the number of Nissl bodies in the cells was reduced. The DG granule cells were loosely arranged and some were missing. Resveratrol had a protective effect in rat hippocampal CA1 and CA3 neurons compared with the model groups that did not receive resveratrol (P<0.05), but no obvious effect was observed in the DG (P>0.05; fig. 3).
ELISA of S100B protein in CSF and serum
The S100B protein standard curve and the correlation coefficient were determined by ELISA (r=0.9998). Rats in the PTZ−kindling models of epilepsy who were not treated with resveratrol had significantly higher concentrations of S100B protein in CSF and serum than the normal nonmodel controls (P<0.05 and P<0.05 for the DMSO and nontreated models, respectively; Table 3). Compared with these model groups, the rats who received resveratrol intervention had significantly reduced S100B content in the CSF (P<0.05). Moreover, the level of S100B protein in the serum was also decreased significantly in the resveratrol group compared with the DMSO and nontreated epilepsy model groups (P<0.05), but remained significantly higher compared with the normal (nonmodel) control group (P<0.05; Table 3). The S100B protein content levels in serum and CSF were positively correlated (Pearson’s correlation coefficient r=0.389, P<0.05).
| Group | Treatment | Crossing | Duration of |
|---|---|---|---|
| times | stay(s) | ||
| Normal (nonmodel) control | Untreated | 6.88±1.55 | 63.61±5.51 |
| Chronic epilepsy model | Resveratrol | 6.00±1.31 | 58.65±4.89 |
| DMSO vehicle | 3.63±1.92* | 49.24±5.94* | |
| Untreated | 3.50±1.69* | 50.27±6.59* |
Table 2: Time In Target Quadrant And The Number Of Crossing Times During The Spatial Probe Test.
Discussion
In this study, we established a rat model of chronic epilepsy using a subconvulsive dose of PTZ (35 mg/kg/day). Our results indicate that resveratrol is an antiepileptic agent that can protect against seizure−induced brain damage. To explore the underlying mechanism of the neuroprotective effects of resveratrol, we used the rat model to investigate whether an association exists between resveratrol treatment and S100B levels in CSF and sera. Our study found that S100B protein levels in the CSF and serum of rats treated with resveratrol were significantly reduced compared with the untreated and DMSO−vehicle groups.
Figure 3:Number of neurons in CA1, CA3 and DG regions in the rat
hippocampus of each group.
Resveratrol (Res) intervention had a protective effect on the rat
hippocampal CA1 and CA3 neurons (P<0.05) compared with the
control. However, the protection of the DG neurons by resveratrol
intervention was not significant (P>0.05) compared with the control.
 CA1,
CA1,  CA3,
CA3,  DG
DG
| Groups | Treatment | CSF | Serum |
|---|---|---|---|
| (pg/ml) | (pg/ml) | ||
| Normal (nonmodel) control | Untreated | 12.20±0.51 | 7.85±0.40 |
| Chronic epilepsy model | Resveratrol | 12.02±0.62 | 8.47±0.33* |
| DMSO vehicle | 13.20±0.69* | 9.19±0.53* | |
| Untreated | 13.36±0.72* | 9.11±0.50* |
Table 3: s100b protein levels in rat csf and Serum
Many experimental research and clinical investigations have confirmed that a combination of factors lead to cognitive dysfunction after seizures. The results of the Morris water maze place navigation test in the present study indicate that PTZ−induced seizures led to impairment in spatial learning ability and reference memory in the model rats. It has been shown that the hippocampus is closely involved in learning and memory, and especially spatial cognitive function. Hippocampal long−term potentiation facilitates synaptic activity and is an important molecular mechanism of synaptic plasticity. Changes in the synapses have a direct impact on the performance of rats in Morris water maze learning and memory tests[22]. Our study found that resveratrol ameliorated the spatial memory impairment in PTZ−induced model rats. The beneficial effects of resveratrol on spatial learning found in this study may be explained by the ability of resveratrol to induce phosphorylation of mitogen−activated protein kinases (MAPKs), as shown in previous studies[9]. MAPKs in turn induce a variety of signal transduction, including phosphorylation and activation of extracellular−signal−regulated kinase 1/2 (ERK1/2), while activated pERK2 is involved in the process of synaptic changes in learning and memory.
We established a chronic epilepsy model in rats via PTZ kindling and in these animals S100B protein levels of CSF and sera increased. This suggests that in the rats with chronic epilepsy, blood–brain barrier damage occurred and astrocyte function was impaired. Interestingly, there were significant reductions in S100B protein in the CSF and sera of model rats that were treated with resveratrol, compared with rats in the untreated epilepsy model group. Moreover, a reduction in S100B was associated with reduced seizures, indicating an antiepileptic role for resveratrol. This is in accord with previous studies that have suggested that resveratrol has a variety of neurological effects that could contribute to its antiepileptic role: protection of hippocampal CA1 and CA3a neurons, inhibition of mossy fibre sprouting[3], neuronal activity in the CA1 region[23], excitatory neurotransmitter release[24] and reduction of brain malondialdehyde levels via antioxidation[25]. The present study showed that the application of resveratrol in chronic epileptic rats reduced S100B protein levels in the CSF and serum. We suggest that resveratrol may have the ability to mitigate brain damage incurred by seizures, possibly through the regulation of the secretion of S100B protein in astrocytes or through the regulation of blood–brain barrier permeability. Thus, S100B reduction could potentially contribute to the treatment of epilepsy.
In summary, our study provides new insights into the mechanisms of the antiepileptic effects of resveratrol. We propose that resveratrol has a protective role in PTZ−induced epilepsy through a combination of multiple biological effects, and could potentially be used in antiepileptic treatment.
Acknowledgements
This work was sponsored by a research grant from the National Natural Science Foundation of China (81271431 to Xi Jun Meng). The authors thank Medjaden Bioscience (Hong Kong, China) for assisting in the preparation of this manuscript.
References
- Scorze FA, Arida RM, Naffah-Mazzacoratti MG, Scerni DA, Calderazzo L, Cavalheiro EA. The pilocarpine model of epilepsy: What have we learned An Acad Bras Cienc 2009;81:345-65.
- Zhou Y, Liu M, Liang W. Progress in the epidemiology study of epilepsy. Chin J Epidemiol 2007;28:92-4
- Xu Q, Wu Z, Zhang L. Study on antiepileptic effect and mechanism of resveratrol. ActaUniv Med Anhui 2009;44:57-61.
- Friedman LK, Goldstein B, Rafiuddin A, Roblejo P, Friedman S. Lack of resveratrol neuroprotection in developing rats treated with kainic acid. Neuroscience 2013;230:39-49.
- Zhang F, Liu J, Shi JS. Anti-inflammatory activities of resveratrol in the brain: Role of resveratrol in microglial activation. Eur J Pharmcol 2010;636:1-7.
- Gu X, Creasy L, Kester A, Zeece M. Capillary electrophoretic determination of resveratrol in wines. J Agric Food Chem 1999;47:3223-7.
- Mehling K, Skoog I, Guo X, Schütze M, Gustafson D, Waern M, et al. Alcoholic beverages and incidence of dementia: 34-year follow-up of the prospective population study of women in Goteborg. Am J Epidemiol 2008;167:684-91.
- German JB, Walzem RL. The health benefits of wine. Annu Rev Nutr 2000;20:561-93.
- Shetty AK. Promise of resveratrol for easing status epilepticus and epilepsy.PharmacolTher 2011;131:269-86.
- Singleton RH, Yan HQ, Fellows-Mayle W, Dixon CE. Resveratrol attenuates behavioral impairments and reduces cortical and hippocampal loss in a rat controlled cortical impact model of traumatic brain injury. J Neurotrauma 2010;27:1091-9.
- Wang Q, Xu J, Rottinghaus GE, Simonyi A, Lubahn D, Sun GY, et al. Resveratrol protects against global cerebral ischemic injury in gerbils. Brain Res 2002;958:439-47.
- Clasadonte J, Donq J, Hines DJ, Haydon PG. Astrocyte control of synaptic NMDA receptors contributes to the progressive development of temporal lobe epilepsy. ProcNatlAcadSci USA 2013;110:17540-5.
- Shashoua VE, Hesse GW, Moore BW. Proteins of the brain extracellular fluid: Evidence for release of S-100 protein. J Neurochem 1984;42:1536-41.
- Naeimi ZS, Weinhofer A, Sarahrudi K. Predictive value of S-100B protein and neuron specific-enolase as markers of traumatic brain damage in clinical use. Brain Inj 2006;20:463-8.
- Steiner J, Bogerts B, Schroeter ML, Bernstein HG. S100B protein in neurodegenerative disorders.ClinChem Lab Med 2011;49:409-24.
- Koh SX, Lee JK. S100B as a marker for brain damage and blood-brain barrier disruption following exercise. Sports Med 2014;44:369-85.
- Lu C, Li J, Sun W, Feng L, Li L, Liu A, et al. Elevated plasma S100B concentration is associated with mesial temporal lobe epilepsy in Han Chinese: A case-control study. NeurosciLett 2010;484:139-42.
- Albright PS, Burnham WM. Development of a new pharmacological seizure model: Effects of anticonvulsants on cortical- and amygdala-kindled seizures in the rat. Epilepsia 1980;21:681-9.
- Xiang HX, Zhang G, Wang D. Research and application progress of the kindling effect. Hebei Med 2010;16:892-5.
- Racine RJ. Modification of seizure activity by electrical stimulation. I. After-discharge threshold. ElectroencephalogrClinNeurophysiol 1972;32:269-79.
- Nirogi R, Kandikere V, Mudigonda K, Bhyrapuneni G, Muddana N, Saralaya R, et al. A simple and rapid method to collect the cerebrospinal fluid of rats and its application for the assessment of drug penetration into the central nervous system. J Neurosci Methods 2009;178:116-9.
- Malone JI, Hanna S, Saporta S, Mervis RF, Park CR, Chong L, et al. Hyperglycemia not hypoglycemia alters neuronal dendrites and impairs spatial memory. Pediatr Diabetes 2008;9:531-9.
- Li M, Wang QS, Chen Y. Resveratrol inhibits neuronal discharges in rat hippocampal CA1 area. Sheng Li XueBao 2005;57:355-60.
- Gao ZB, Hu GY. Trans-resveratrol, a red wine ingredient, inhibits voltage-activated potassium currents in rat hippocampal neurons. Brain Res 2005;1056:68-75.
- Gupta YK, Briyal S, Chaudhary G. Protective effect of trans-resveratrol against kainic acid induced seizures and oxidative stress in rats. PharmacolBiochemBehav 2002;71:245-9.
 Res group,
Res group,  PTZ group,
PTZ group,  DMSO group.
DMSO group.