- *Corresponding Author:
- Qinghong Cheng
Department of Cardiology, Shihezi University School of Medicine, Shihezi, Xinjiang Uyghur Autonomous Region 832000, China
E-mail: Xunfeicheng@aliyun.com
| This article was originally published in a special issue, “Clinical Advancements in Life Sciences and Pharmaceutical Research” |
| Indian J Pharm Sci 2024:86(5) Spl Issue “196-207” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
A key factor in septic patient’s deaths is cardiac dysfunction brought on by sepsis. The molecular causes of septic cardiomyopathy and effective preventative treatments, however, remain unknown. Resveratrol is a naturally occurring antitoxin with several biological effects, including anticancer, a protective impact on the cardiovascular system, antiapoptotic, antioxidant, anti-free radical, antibacterial, antiviral, anti-inflammatory, and immunomodulatory. Whether resveratrol mediates bioactive resistance in septic myocardial damage is unknown, though. The c-Jun NH2-terminal kinase/Bcl-2-associated X protein/cytochrome C signaling pathway is involved in mitochondrial apoptosis. Activated c-Jun NH2-terminal kinase, or phospho-c-Jun NH2-terminal kinase, causes Bcl-2-associated X protein to move to the outer mitochondrial membrane, increasing its permeability, and later releases cytochrome C into the cytoplasm, initiating apoptosis. To decrease cardiac damage in sepsis, it was determined in this study if resveratrol mediates the apoptotic process of cardiomyocytes via the c-Jun NH2-terminal kinases/Bcl-2-associated X protein/cytochrome C signaling pathway. Rats with sepsis underwent cecum ligation puncture to cause myocardial damage, which was then treated with resveratrol. The outcomes demonstrated that resveratrol substantially reduced myocardial apoptosis, slowed reactive oxygen species generation, and ameliorated sepsis-induced cardiac dysfunction. The c-Jun NH2-terminal kinases/Bcl-2-associated X protein/cytochrome C pathway was shown to be much more agonistic in the H9c2 rat cardiomyocyte sepsis model than in the control group, although this was significantly decreased following treatment with resveratrol. We further demonstrated whether resveratrol mediates the c-Jun NH2-terminal kinase/Bcl-2-associated X protein/cytochrome C pathway by agonizing the c-Jun NH2-terminal kinase signaling pathway in vitro with a c-Jun NH2-terminal kinase-activator to increase sepsis-induced cardiac injury, while using resveratrol to verify its protective effect on myocardial injury in sepsis. All things considered, it has been established that resveratrol lessens myocardial cell death by controlling the c-Jun NH2-terminal kinase/Bcl-2-associated X protein/cytochrome C signaling pathway, thereby reducing the myocardial harm brought on by sepsis.
Keywords
Resveratrol, in vitro, apoptosis, myocardial injury, immunomodulatory, sepsis
Sepsis can result in life-threatening organ failure and is brought on by an imbalance in the body's reaction to infection[1]. The systolic and/or diastolic dysfunction of the heart caused by sepsis is known as Sepsis-Induced Myocardial Dysfunction (SIMD), also referred to as Sepsis-Induced Cardiomyopathy (SIC), and it is characterized by an early onset and high incidence. It significantly raises the mortality rate of sepsis patients[2]. Apoptosis, an inflammatory response, and oxidative stress are all significant causes of SIC, although the exact mechanisms underlying these phenomena are complex and yet poorly understood[3].
The normal organism undergoes apoptosis, which is a genetically scheduled death. The intrinsic pathway, often known as the mitochondrial pathway, is one of several apoptotic processes. The B-Cell Lymphoma-2 (Bcl-2) protein family’s members regulate mitochondrial apoptosis, which begins with the depolarization of the mitochondria. Cytochrome-C (Cyt-C) is a protein that mitochondria release into the cytoplasmic matrix after releasing apoptotic signals. Cyt-C mixes with other compounds to form the apoptosome, which activates caspase-3 and starts apoptosis[4]. A key node in the control of apoptosis is the JNK signaling pathway, is crucial to the process of apoptosis[5]. c-Jun NH2-Terminal Kinase (JNK) also plays a regulatory role in modulating oxidative stress, among other things.
With anti-apoptotic, antioxidant, anti-inflammatory, and anti-cancer properties, resveratrol is a polyphenolic plant antitoxin that is commonly found in berries, peanuts, and wine[6]. An in-depth analysis of its protective effect on septic cardiomyopathy and comprehension of its mechanism of action can give a theoretical basis for the prevention and treatment of septic cardiomyopathy due to the safer and less residual features of the plant's functional components.
In the current investigation, we used CLP to induce a rat model of SIC, and lipopolysaccharide to induce a septic cell model by acting on H9c2 cardiomyocytes. This study’s objective was to observe how resveratrol protects against septic myocardial injury and to learn more about how it has anti-apoptotic effects.
Materials and Methods
Materials and reagents:
The following is how these experimental reagents were utilized in this investigation, resveratrol (501-36-0, Beyotime, China); Lipopolysaccharide (LPS; L2880-10 mg, Biotopped, China); Terminal Deoxynucleotidyl Transferase dUTP Nick end Labeling (TUNEL) assay kit (1215692910, Roche, China); Cell Counting Kit-8 (CCK-8) (CK04, Dojindo, Japan); Interleukin (IL)-6 (CK-E30219), IL-10 (CK-E30194), Tumor Necrosis Factor Alpha (TNF-α) (CK-E31063); Enzyme-Linked Immunosorbent Assay (ELISA) kits (ELISA, Shanghai Yuchun Biotechnology, China); Malondialdehyde assay kit (MDA, BC0025), Glutathione Peroxidase Assay kit (GPX, BC1195) and Superoxide Dismutase (SOD assay kit, BC0175) (Beijing Solarbio Science & Technology Co., Ltd., China); Reactive Oxygen Species (ROS) assay kit (C1300-1, Applygen, China); primary antibodies JNK (bs-2592R), p-JNK (bsm-52452R), Bcl-2–associated X protein (BAX) (bs-0127R), Bcl-2 (bs-4563R), caspase 3 (bsm-52289R) (Bioss, China), cleaved caspase-3 (WL01992), Cyt-C (WL02410) (Wanleibio, China) and JNK-activator (Anisomycin) (SC1032, Beyotime, China).
Animals and modeling:
The National Institutes of Health’s 1996 ethical guidelines for the use of laboratory animals were followed by the research, which received clearance from the Animal Protection and Use Committee of Shihezi University (Shihezi, China). A2018-018-01 is the lot number. Male Sprague-Dawley (SD) rats (6-8) w old, (180-220) g were acquired from the Animal Experiment Center of Xinjiang Medical University under animal permit number SYXK (new) 2011-010101. Before the trial, the rats were domesticated for 1 w. In a nutshell, a 12 h preoperative fast was carried out, followed by 350 mg/kg of 10 % pentobarbital anesthesia and supine immobilization. After cleaning and preparing the skin, at the center of the abdomen, a 2 cm cut was created. The cecum was then removed, the distal 2/3 of the cecum was tied off with a 4-gauge ligature, the appendix was punctured using two 16-gauge needles with some digestive material poking out, the cecum was then retracted into the abdomen, and the abdomen was stitched shut[7]. 50 ml/kg of sodium lactate immediately following surgery, all rats received a subcutaneous injection of ringer's solution and nutritional assistance through a vein. The identical abdominal incision technique was used on the rat that underwent sham surgery, but there was no cecum ligation or puncture.
Sample collection:
Rats were put to sleep with pentobarbital (350 mg/kg), blood was drawn from the abdominal aorta, and rat heart tissue was preserved. This was done 12 h after CLP. The supernatant from centrifuged blood samples was collected and kept at -80° after being spun at 3500 rpm for 10 min. For pathological examination, the hearts were partially stored at -80° and submerged in 4 % paraformaldehyde. According to the procedure described in the literature[8], the preserved heart tissues were sectioned at a thickness of 5 μm, immersed in paraffin wax, baked at 60° for 30 min to remove the wax, and then rinsed with water, stained with hematoxylin-eosin, dehydrated in gradient ethanol, made transparent, sealed with coverslips after adding the appropriate amount of neutral gum dropwise, and allowed to dry in sections for observation under a light microscope and photographic preservation.
Biochemical indicators:
After centrifuging abdominal aortic blood, the supernatant was collected, and Lactate Dehydrogenase (LDH) and Creatine Kinase-Myocardial Band (CK-MB) were determined following the guidelines. Excised cardiac tissue was homogenized in Phosphate-Buffered Saline (PBS). Centrifugation was used to separate the supernatant for 10 min at 8000 g at 4°. According to the guidelines, the inflammatory markers IL-10, IL-6 and TNF-α were detected in cardiac tissue.
Echocardiography:
Rats were given pentobarbital anesthesia and transthoracic echocardiography to evaluate heart function 12 h after CLP. A 10 MHz transducer equipped M-mode echocardiography machine (KR-S80) was used to take measurements. Afterward, using Vevo Lab 3.1.0 software, cardiac function metrics such as Left Ventricular Ejection Fraction (LVEF) and Left Ventricular Shortening Fraction (LVFS) was calculated.
TUNEL detection:
TUNEL labeling was used to determine the degree of apoptosis in each set of cardiomyocytes under the TUNEL assay kit's instructions. Using an Olympus FV1000 laser confocal microscope, pictures were taken (Olympus, Tokyo, Japan). Using ImageJ software, apoptotic fluorescence was evaluated, and the proportion from each group's fluorescence intensity in relation to the control condition was used to indicate the degree of apoptosis.
Transmission electron microscopy:
12 h after CLP, the rats' myocardial tissues were removed and kept at -80°. To observe mitochondrial changes in rat myocardial tissue, ultrathin section machine sections were fixed in copper mesh after being double stained with uranyl acetate and lead citrate, sealed, and photographed. This was done after raising the sections in an ethanol series and embedding the sections in epoxy resin for dehydration.
Cell culture and processing:
H9c2 cells generated from rat embryonic cardiomyocytes were incubated in 10 % fetal bovine serum and 1 % penicillin/streptomycin-containing Dulbecco’s Modified Eagle (DMEM) medium (BL304A, Biosharp, China) at 37° and 5 % Carbon dioxide (CO2). H9c2 cardiomyocytes were treated with LPS (5 μg/ml) to simulate sepsis injury conditions in vitro. In the first experiment, H9c2 cells were divided into the following groups, control group; LPS-treated group (control+LPS); resveratrol-treated group (con+Res); resveratrol-treated LPS group (LPS+resveratrol) and Dimethyl Sulfoxide (DMSO)-treated LPS group (LPS+DMSO). Subsequently, to explore the mechanisms through which the protective effect of resveratrol on the myocardial model of sepsis occurs, the experiments were divided into the following groups, control group; LPS group; resveratrol group (control+resveratrol); resveratrol-treated LPS group (LPS+resveratrol); JNK-activator group (control+JNK-activator); JNK-activator-treated LPS group (LPS+JNK-activator); JNK-activator and resveratrol co-treated LPS group (LPS+JNK-activator+resveratrol).
Cell viability:
According to the CCK-8 test kit’s instructions, the CCK-8 assay was used to measure the viability of the cells. In a nutshell, H9c2 cells were seeded in 96-well plastic plates with around 3000 cells per well and were then treated with various doses of resveratrol (with or without LPS); treated independently according to the previous grouping. Following the 24 h treatment period, each well received 10 μl of CCK-8 solution and was incubated for 3 h at 37° with 5 % CO2. Subsequently, absorbance at 450 nm was measured using an enzyme marker. SpectraMax M5 spectrophotometer (Molecular Devices, California, United States of America (USA)) was used to detect changes in the viability of H9c2 cardiomyocytes in each group.
Cellular inflammatory cytokines and other biochemical indicators:
ELISA kits were used to detect the pro-inflammatory factors IL-6 and TNF-α, and the anti-inflammatory factor IL-10 in each group of cardiomyocytes. Redox-related substances assay, including MDA, GPX, and SOD.
Measurement of ROS:
Following the directions on the ROS assay kit, DCFH-DA were used to identify mitochondrial ROS generation in H9c2 cells. Using an Olympus FV1000 laser confocal microscope, pictures were taken (Olympus, Tokyo, Japan). Using ImageJ software, the intensity of the ethidium fluorescence reflecting ROS levels was calculated.
Western blotting:
Total proteins in H9c2 cardiomyocytes were extracted using Radio-Immunoprecipitation Assay (RIPA) lysate (R0010, Solarbio, China), while the concentration of total proteins in them was determined later. Separation of total cellular proteins was performed using 10 %-12 % Sodium Dodecyl-Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE), after which moved to Polyvinylidene Difluoride (PVDF) membranes based on PVDF. The membranes were sealed for 15 min at room temperature using rapid seal solution (P1626, APPLYGEN, China). JNK (1:1000), p-JNK (1:1000), Bcl-2 (1:1000), BAX (1:1000), Cyt-C (1:1000), caspase-3 (1:1000), cleaved caspase-3 (1:1000) were incubated overnight in the refrigerator at 4° in the appropriate ratio. The PVDF membranes were washed with Tris-Buffered Saline with 0.1% Tween® 20 detergent (TBST) for 10 min, 3 times. After that, the PVDF membrane was contacted with the corresponding enzyme-labeled coupled secondary antibody (1:10 000) for 1 h at room temperature and washed again with TBST for 3 times (10 min, 3 times). Finally, the bands were observed and exposed using a Tanon-5200 (Tanon, Shanghai, China) imaging system and Enhanced Chemiluminescence (ECL) reagent (7E501K1, Vazyme Biotech Co., Ltd., Nanjing, China). Finally, the density of the target protein was detected using ImageJ software.
Statistical analysis:
Statistical analyses in this study were performed using GraphPad Prism software version 9.0 (GraphPad Software, Inc., San Diego, California, USA), and all values were expressed using the mean and standard deviation, and data were compared between groups using one-way Analysis of Variance (ANOVA), with differences considered statistically significant when p<0.05.
Results and Discussion
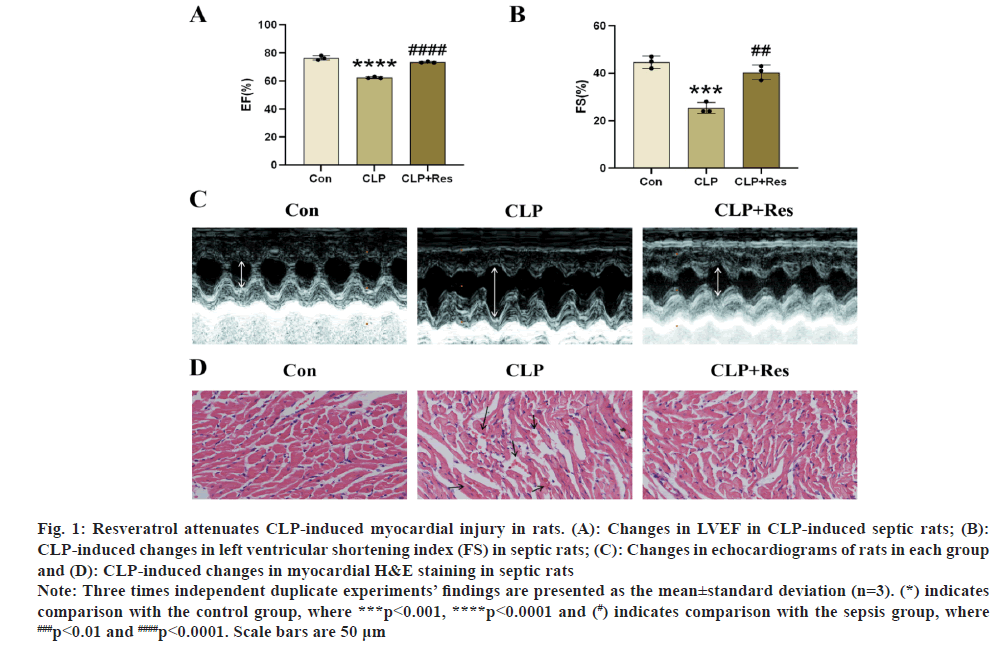
The rats had CLP surgery first. In fig. 1A-fig. 1C, m-mode echocardiographic pictures are displayed. In this study, sepsis caused cardiac systolic dysfunction, which was seen in the rats. The rat’s LVEF and LVEF were both dramatically decreased (LVFS). Rats with septic shock who received resveratrol had much better heart health. Similar to how myocardial cell morphology was more erratic in the sepsis group compared to the control group, myocardial fibers were also more disordered. Tissue structure improved in the resveratrol-treated group (fig. 1D).
Fig. 1: Resveratrol attenuates CLP-induced myocardial injury in rats. (A): Changes in LVEF in CLP-induced septic rats; (B):
CLP-induced changes in left ventricular shortening index (FS) in septic rats; (C): Changes in echocardiograms of rats in each group
and (D): CLP-induced changes in myocardial H&E staining in septic rats.
Note: Three times independent duplicate experiments’ findings are presented as the mean±standard deviation (n=3). (*) indicates
comparison with the control group, where ***p<0.001, ****p<0.0001 and (#) indicates comparison with the sepsis group, where ###p<0.01 and ####p<0.0001. Scale bars are 50 μm.
According to echocardiography revealed significantly worsened cardiac function following CLP treatment in comparison to controls, which was supported by lower levels of LVEF and LVFS. By raising LVEF and LVFS levels, resveratrol administration dramatically reduced heart dysfunction in septic rats. The control group displayed intact cardiomyocyte shape and precisely aligned cardiac fibers. The sepsis group, however, displayed structural problems. Once the resveratrol therapy was given, these traits were reduced. Our findings show that the sepsis rat model substantially impairs cardiac function and that the administration of resveratrol somewhat restored cardiac function in these rats, indicating a cardio protective effect of resveratrol.
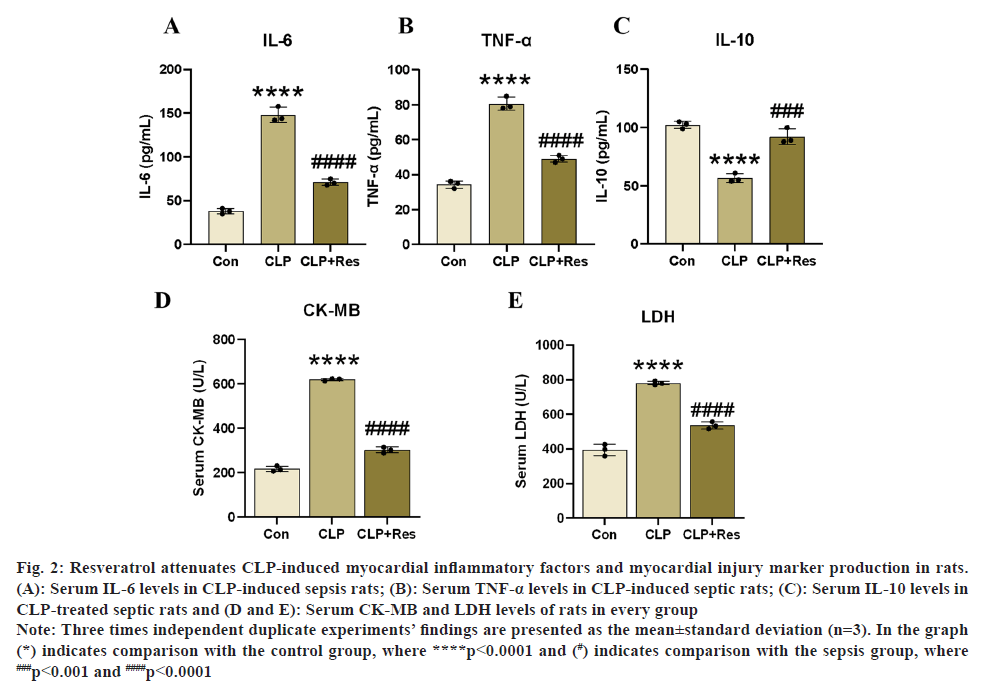
One of the most harmful pathophysiological processes in sepsis-induced heart damage is severe inflammatory myocardial injury. We measured inflammatory factor levels in rat serum using a relevant ELISA kit, as shown in fig. 2A-fig. 2C, to investigate the effect of resveratrol on the production of inflammatory factors in a rat model of myocardial injury in sepsis. Significant increase in serum levels of pro-inflammatory factors IL-6 and TNF-α after CLP surgery in experimental rats, causing a severe myocardial inflammatory response, according to ELISA findings. Furthermore, the resveratrol therapy significantly reduced the effects of CLP on LDH and CK-MB in rats (p<0.05, fig. 2D and fig. 2E), and the resveratrol treatment elevated IL-10 levels while decreasing IL-6 and TNF-α levels. According to the aforementioned findings, resveratrol may reduce the inflammatory response of CLP-induced SIC and attenuate myocardial injury.
Fig. 2: Resveratrol attenuates CLP-induced myocardial inflammatory factors and myocardial injury marker production in rats.
(A): Serum IL-6 levels in CLP-induced sepsis rats; (B): Serum TNF-α levels in CLP-induced septic rats; (C): Serum IL-10 levels in
CLP-treated septic rats and (D and E): Serum CK-MB and LDH levels of rats in every group.
Note: Three times independent duplicate experiments’ findings are presented as the mean±standard deviation (n=3). In the graph
(*) indicates comparison with the control group, where ****p<0.0001 and (#) indicates comparison with the sepsis group, where ###p<0.001 and ####p<0.0001.
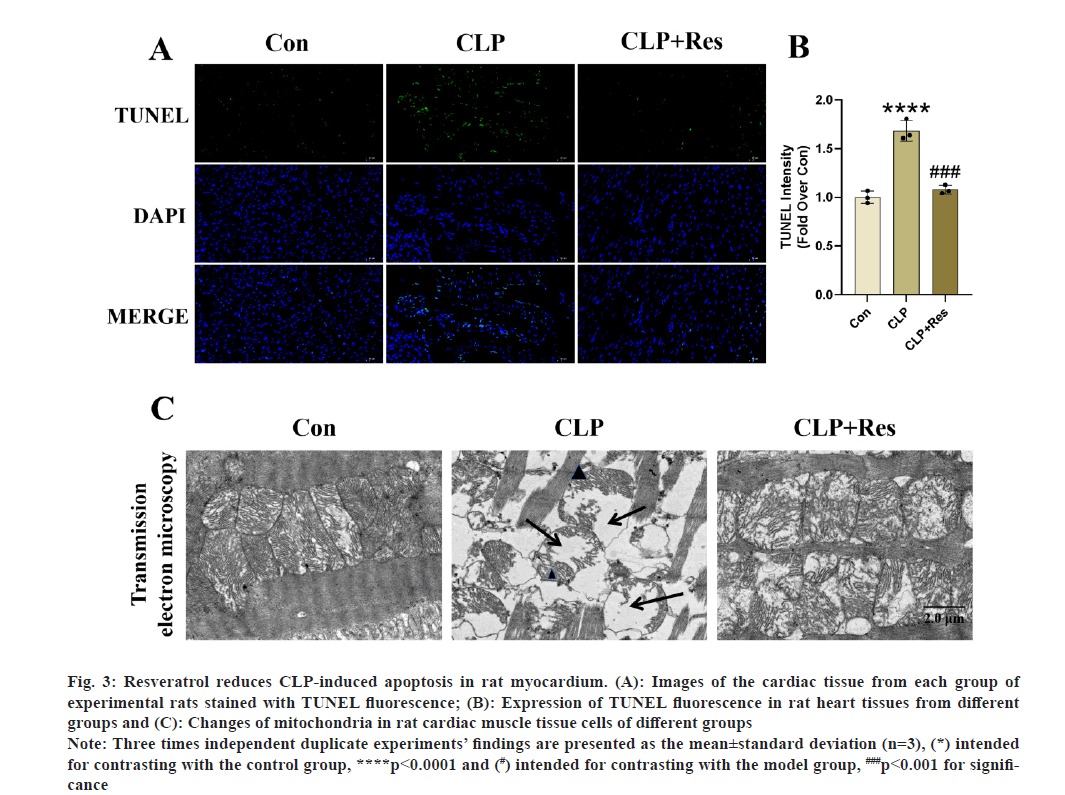
After resveratrol treatment, we were able to see a significant reduction in TUNEL fluorescence density in the CLP+resveratrol group compared to the CLP group (fig. 3A-fig. 3C). The mitochondria of rat cardiomyocytes in the CLP group were significantly swollen and deformed, with a disorganized arrangement, significantly reduced internal ridges, and significant mitochondrial damage compared to the control group, based on the analysis of mitochondrial morphology and structure of cardiomyocytes in the control group of rats. These findings suggest that Res can prevent apoptosis in cardiomyocytes, while being able to protect cellular mitochondria from CLP-induced cardiac injury. Thus, a link between the two is considered.
Fig. 3: Resveratrol reduces CLP-induced apoptosis in rat myocardium. (A): Images of the cardiac tissue from each group of
experimental rats stained with TUNEL fluorescence; (B): Expression of TUNEL fluorescence in rat heart tissues from different
groups and (C): Changes of mitochondria in rat cardiac muscle tissue cells of different groups.
Note: Three times independent duplicate experiments’ findings are presented as the mean±standard deviation (n=3), (*) intended
for contrasting with the control group, ****p<0.0001 and (#) intended for contrasting with the model group, ###p<0.001 for significance.
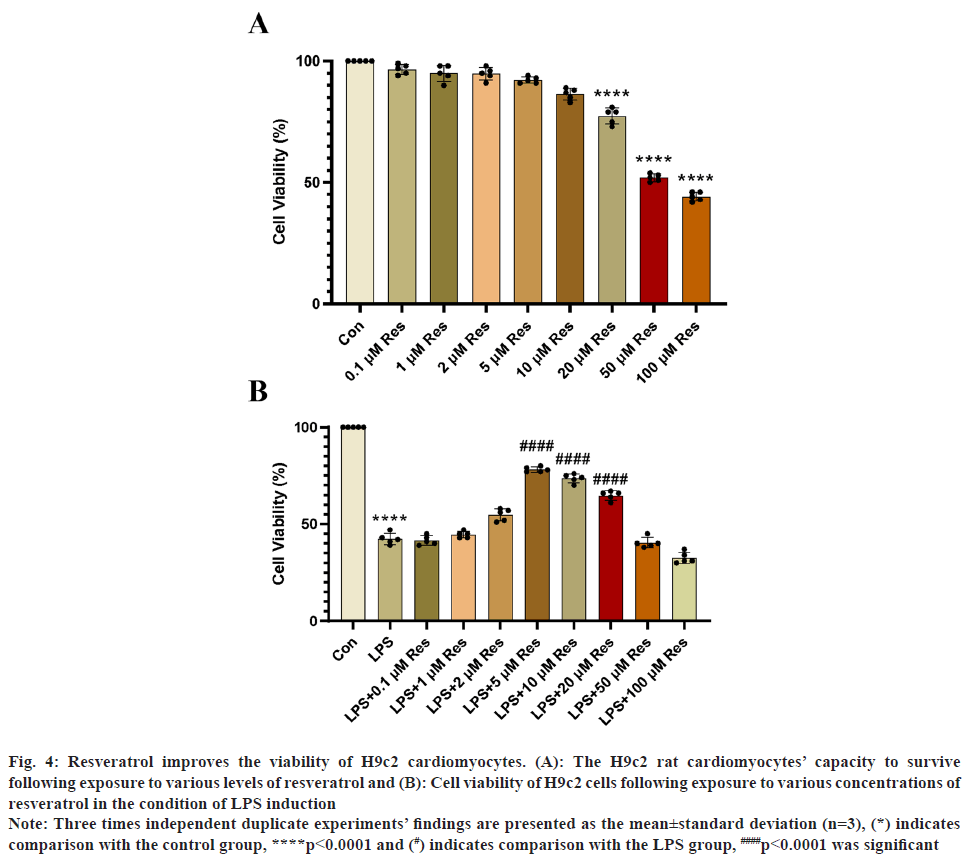
In order to investigate the toxic effect on H9c2 cardiomyocytes when the concentration of Res is high, we used different concentrations of resveratrol on H9c2 cardiomyocytes. Fig. 4A, the results obtained showed that resveratrol concentrations <20 μM were not toxic to H9c2 cells. To simulate sepsis in vivo, we stimulated H9c2 cells in vitro with LPS and exposed them to appropriate concentrations of resveratrol. Using the CCK-8 kit, the activity of H9c2 cardiomyocytes exposed to various resveratrol concentrations was measured. In the experiment, resveratrol had the greatest effect on LPS-induced H9c2 cells at a concentration of 5 μM (fig. 4B).
Fig. 4: Resveratrol improves the viability of H9c2 cardiomyocytes. (A): The H9c2 rat cardiomyocytes’ capacity to survive
following exposure to various levels of resveratrol and (B): Cell viability of H9c2 cells following exposure to various concentrations of
resveratrol in the condition of LPS induction.
Note: Three times independent duplicate experiments’ findings are presented as the mean±standard deviation (n=3), (*) indicates
comparison with the control group, ****p<0.0001 and (#) indicates comparison with the LPS group, ####p<0.0001 was significant.
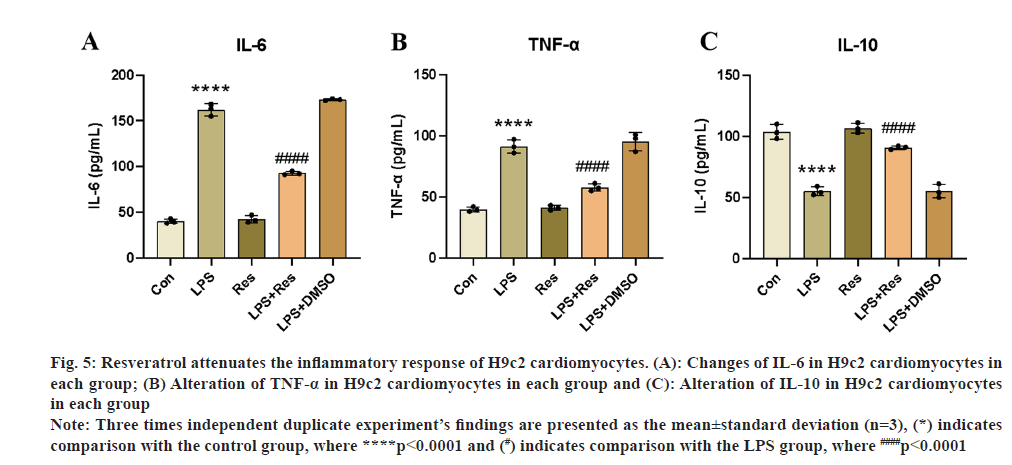
To see if resveratrol could have comparable anti-inflammatory effects on LPS-stimulated H9c2 cells, we measured the levels of their pro-inflammatory cytokines IL-6 and TNF-α using ELISA. The outcomes matched those of the in vivo tests. Inflammatory cytokine levels could be suppressed by resveratrol treatment, as shown in fig. 5A-fig. 5C, and inflammatory markers were markedly elevated in the LPS group. Resveratrol treatment in in vitro cellular assays successfully decreased the LPS-induced inflammatory reaction in H9c2 cardiomyocytes and stimulated the production of the anti-inflammatory cytokine IL-10, which is in line with the findings of in vivo investigations (fig. 5A-fig. 5C).
Fig. 5: Resveratrol attenuates the inflammatory response of H9c2 cardiomyocytes. (A): Changes of IL-6 in H9c2 cardiomyocytes in
each group; (B) Alteration of TNF-α in H9c2 cardiomyocytes in each group and (C): Alteration of IL-10 in H9c2 cardiomyocytes
in each group.
Note: Three times independent duplicate experiment’s findings are presented as the mean±standard deviation (n=3), (*) indicates
comparison with the control group, where ****p<0.0001 and (#) indicates comparison with the LPS group, where ####p<0.0001.
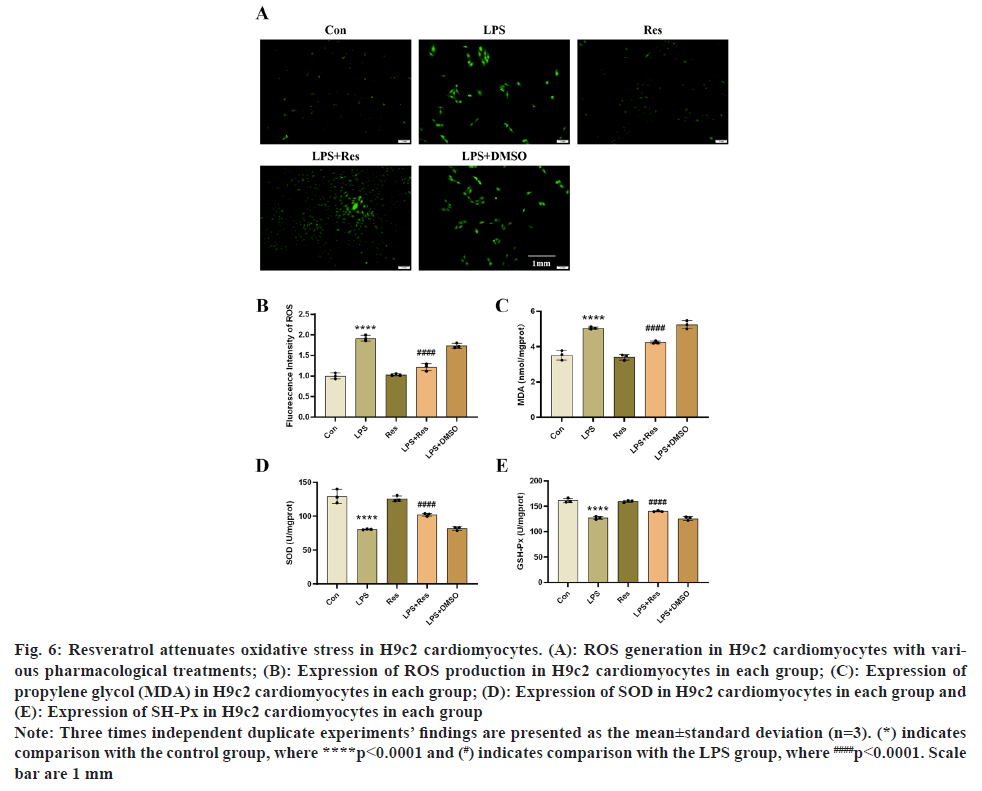
To examine resveratrol impact on oxidative stress in H9c2 cells that have been exposed to LPS. Using a fluorescence microscope, we used a ROS detection kit to monitor the generation of ROS in several groups of H9c2 cells. Using assay kits, we were also able to find the levels of MDA, SOD, and GPX in various groups. According to the outcomes of our in vitro investigation, resveratrol significantly reduced intracellular ROS and MDA production, while increasing SOD and GPX production (fig. 6A-fig. 6E).
Fig. 6: Resveratrol attenuates oxidative stress in H9c2 cardiomyocytes. (A): ROS generation in H9c2 cardiomyocytes with various
pharmacological treatments; (B): Expression of ROS production in H9c2 cardiomyocytes in each group; (C): Expression of
propylene glycol (MDA) in H9c2 cardiomyocytes in each group; (D): Expression of SOD in H9c2 cardiomyocytes in each group and
(E): Expression of SH-Px in H9c2 cardiomyocytes in each group.
Note: Three times independent duplicate experiments’ findings are presented as the mean±standard deviation (n=3). (*) indicates
comparison with the control group, where ****p<0.0001 and (#) indicates comparison with the LPS group, where ####p<0.0001. Scale
bar are 1 mm.
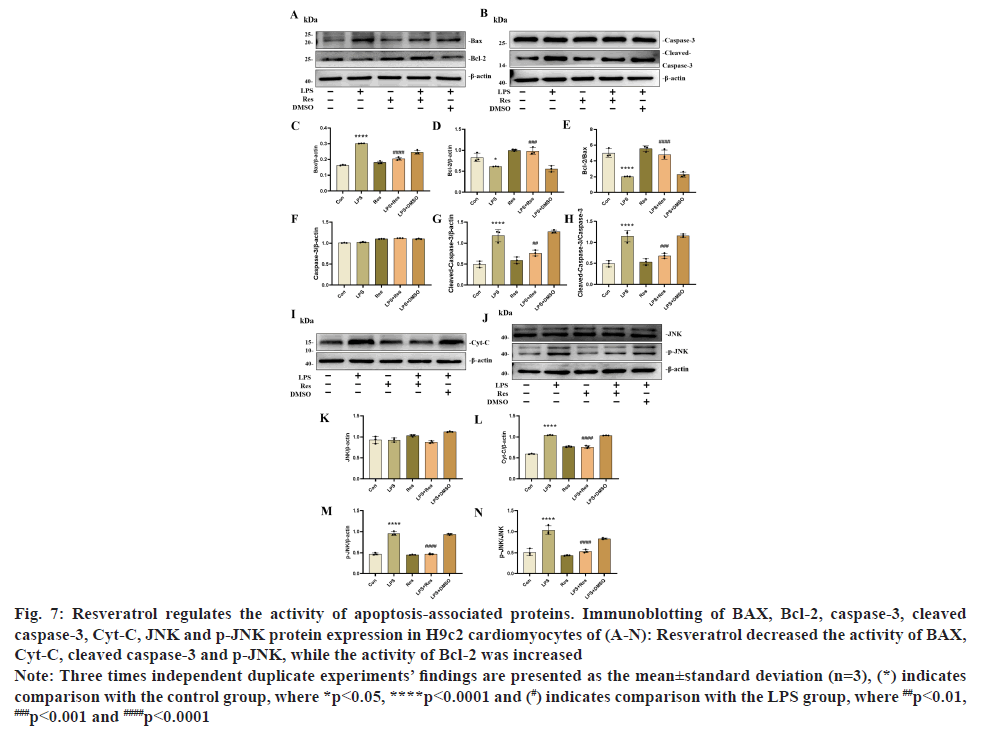
Our in vitro results showed that resveratrol dramatically increased the protein levels of the anti-apoptotic protein Bcl-2 while considerably decreasing the level of the intracellular pro-apoptotic proteins BAX and cleaved caspase-3, letting us conclude that the effect of resveratrol on apoptosis production in LPS-stimulated H9c2 cardiomyocytes (fig. 7A-fig. 7N). At the same time, LPS markedly increased the expression of the Cyt-C protein in H9c2 cells compared to the control group, while LPS+resveratrol significantly decreased the expression of the Cyt-C protein (fig. 7). p<0.05. It implies that LPS promotes Cyt-C transfer from mitochondria to cytoplasm and that resveratrol can prevent this from happening. In addition to this, p-JNK, the upstream protein of the above mentioned proteins, was also significantly increased by LPS, and this effect was decreased after resveratrol treatment. The control group and the other group did not have any different values for these factors. These values were the same for both the control group and the control+resveratrol group.
Fig. 7: Resveratrol regulates the activity of apoptosis-associated proteins. Immunoblotting of BAX, Bcl-2, caspase-3, cleaved
caspase-3, Cyt-C, JNK and p-JNK protein expression in H9c2 cardiomyocytes of (A-N): Resveratrol decreased the activity of BAX,
Cyt-C, cleaved caspase-3 and p-JNK, while the activity of Bcl-2 was increased.
Note: Three times independent duplicate experiments’ findings are presented as the mean±standard deviation (n=3), (*) indicates
comparison with the control group, where *p<0.05, ****p<0.0001 and (#) indicates comparison with the LPS group, where ##p<0.01, ###p<0.001 and ####p<0.0001.
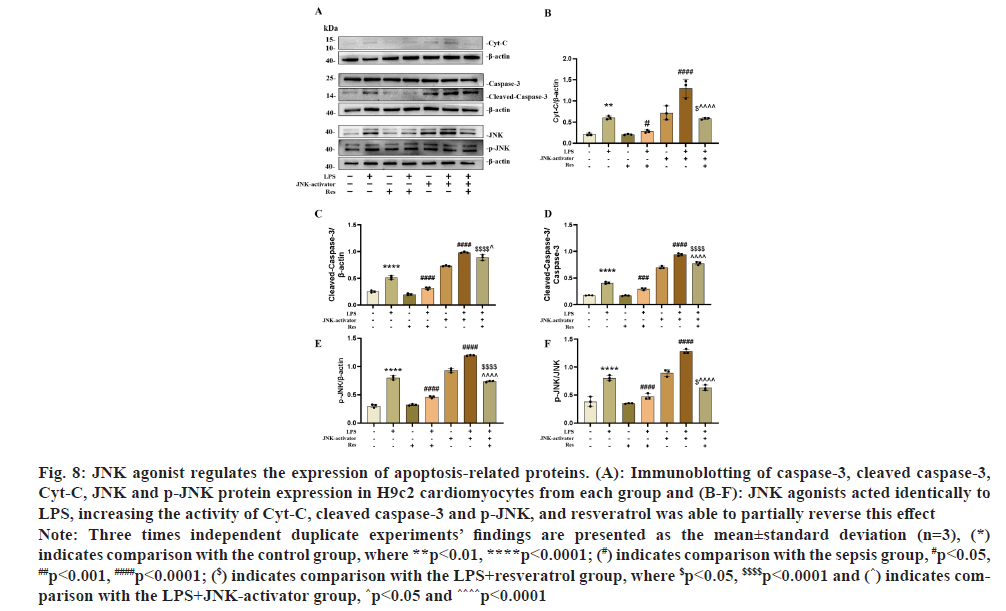
Resveratrol was applied to H9c2 cells at a specific dose (5 μmol/l) to test whether it has an anti-apoptotic impact on LPS-induced H9c2 cardiomyocytes via the JNK/BAX/Cyt-C signaling pathway. Western blotting results showed that anti-apoptotic and pro-apoptotic protein expression was upregulated in the LPS+resveratrol+JNK-activator group compared to the LPS+resveratrol group for Cyt-C, cleaved caspase-3 and p-JNK protein expression (fig. 8). When resveratrol was not present, the results were reversed. In addition, Cyt-C, cleaved caspase-3 and p-JNK protein expression levels were significantly increased in the LPS+JNK activator group compared to the LPS group. As shown in fig. 8A-fig. 8F. In conclusion, the protective effect of resveratrol against myocardial injury in sepsis may be achieved through the signaling pathway JNK/BAX/Cyt-C.
Fig. 8: JNK agonist regulates the expression of apoptosis-related proteins. (A): Immunoblotting of caspase-3, cleaved caspase-3,
Cyt-C, JNK and p-JNK protein expression in H9c2 cardiomyocytes from each group and (B-F): JNK agonists acted identically to
LPS, increasing the activity of Cyt-C, cleaved caspase-3 and p-JNK, and resveratrol was able to partially reverse this effect.
Note: Three times independent duplicate experiments’ findings are presented as the mean±standard deviation (n=3), (*)
indicates comparison with the control group, where **p<0.01, ****p<0.0001; (#) indicates comparison with the sepsis group, #p<0.05, ##p<0.001, ####p<0.0001; ($) indicates comparison with the LPS+resveratrol group, where $p<0.05, $$$$p<0.0001 and (^) indicates comparison
with the LPS+JNK-activator group, ^p<0.05 and ^^^^p<0.0001.
In septic cardiomyopathy, our study discovered that resveratrol reduces cardiomyocyte apoptosis via a JNK/BAX/Cyt-C-dependent pathway and also has an impact on reducing inflammation and oxidative stress. According to our in vivo findings, the protection of resveratrol against myocardial injury in sepsis is achieved by reducing the occurrence of inflammatory responses, decreasing the formation of reactive oxygen species, and inhibiting cardiomyocyte apoptosis. Further in vitro investigations revealed that resveratrol primarily controls the JNK/BAX/Cyt-C signaling pathway to reduce apoptosis in cardiomyocytes. The mitochondrial apoptotic pathway can initiate apoptosis, an active form of cell death[9,10]. Overall, the present results show that resveratrol is able to treat myocardial injury in sepsis by inhibiting JNK/BAX/Cyt-C signaling pathway-mediated apoptosis.
Herbal therapy has been extensively researched in recent years and shown to be remarkably effective in treating septic cardiomyopathy. Resveratrol has recently been shown to be able to reduce the iron death impact on rat cardiac damage caused by CLP via the Sirt1/Nrf2 signaling pathway[11]. Being a significant plant antitoxin, resveratrol also has a number of beneficial health benefits, including anticancer, cardiovascular system protection, anti-apoptosis, antioxidant, anti-free radical, antibacterial, antiviral, anti-inflammatory, and immunomodulatory properties. It has received extensive and in-depth study and application in the sectors of nutraceuticals, food, medicine, and plant physiology both domestically and internationally in recent years[12]. In the beginning, our team preliminarily examined the impact of Res components on anti-iron mortality in septic cardiomyopathy and studied the impact of resveratrol on it[11-13]. Thus, we reasoned that resveratrol may via anti-apoptosis; also enhance heart function in sepsis patients. In this study, we examined the cardiac function of rats in each group using echocardiography and the myocardial cell morphology of rats in each group using Hematoxylin and Eosin (H&E) staining to test the hypothesis of this experiment. We observed that the intraperitoneal injection of resveratrol 0.5 h after CLP surgery significantly improved the cardiac function of rats with sepsis, and their cardiomyocyte morphology was more perfect than that of the septic group. Being a biologic with significant therapeutic potential and low toxicity, resveratrol will undoubtedly get significant interest. This study offers a fresh viewpoint on how to manage septic cardiomyopathy.
Oxidative stress[15], inflammation, cardiomyocyte apoptosis[14-16], calcium response[17], and coronary microvascular dysfunction[18] are the primary pathophysiological processes of septic cardiomyopathy. The inflammatory response is an important response in the acute infection phase of sepsis, during which the body will produce an inflammatory waterfall response with a massive increase in the pro-inflammatory factor IL-6 and tumor necrosis factor alpha, leading to an imbalance of endothelial and cardiomyocyte death. During this period, the body's ROS are produced in large quantities, while at the same time, the Reactive Nitrogen Species (RNS) level will increase. Excessive ROS generation can harm the heart, directly harm the structure of cardiomyocytes, and encourage apoptosis when there is an imbalance between oxidative and antioxidative processes[19]. Moreover, increased ROS production might decrease the endothelium-dependent vasodilatory response, exacerbate microcirculatory abnormalities, damage vascular endothelial cells during sepsis, and worsen cardiac inflammation.
We discovered that the LPS group had considerably more ROS accumulation than the normal group. Resveratrol, on the other hand, dramatically reversed these effects. In accordance with this observation, resveratrol treatment significantly enhanced the expression of Bcl-2 while decreasing BAX and cleaved caspase-3 protein levels in septic rats, as evidenced by a reduction in TUNEL-positive cell density. The results obtained from our in vitro assays were corroborated by those obtained from in vivo experiments. Furthermore, in order to assess the extent of cardiac inflammation in infected rats, we measured the levels of pro-inflammatory cytokines including IL-6 and TNF-α in the cardiac tissues and blood samples of each group. Our findings indicate that the septic group exhibited a marked increase in the concentration of IL-6 and TNF-α compared to the control group. Resveratrol therapy relieved these traits, and in vitro tests with the same outcomes as in vivo tests were able to validate this.
In conclusion, significant pathophysiological signs and symptoms of sepsis include oxidative stress, inflammation, and myocardial apoptosis. Our study suggests that resveratrol may reduce CLP- and LPS-induced myocardial oxidative stress, apoptosis and inflammatory damage.
Cyt-C is an important substance in mitochondrial apoptosis, which is released from the outer mitochondrial membrane into the cytoplasm and forms apoptotic vesicles with other substances, which then cause the apoptotic response[20]. Cysteinases are produced intracellularly as dormant zymogens before being cleaved by apoptotic signaling stimuli, and they represent a key group of drivers of apoptosis. Caspase-3, which is connected to the mitochondrial pathway, is activated when Cyt-C is released[21-25].Throughout the course of our research, we found that cleaved caspase-3, Cyt-C protein expression was significantly elevated in response to LPS. Therefore, LPS may lead to apoptosis by increasing the production of cleaved caspase-3 and Cyt-C.
The extrinsic pathway (death receptors) and the intrinsic pathway (mitochondria) are the two primary mechanisms of apoptosis[26,27]. The outer mitochondrial membrane's permeability is controlled by the mitochondrial apoptotic pathway, which also produces stomata in the membrane and releases apoptosis-related proteins, including Cyt-C[28]. The control of mitochondrial activity is directly influenced by ROS, a significant molecule[29-33]. Our research demonstrated that LPS enhanced the formation of ROS in the cardiomyocytes of infected rats and that resveratrol prevented this behavior, which is consistent with the findings above. Resveratrol may prevent Cyt-C from leaving the mitochondria and entering the cytoplasm by preventing ROS buildup, which would prevent cardiomyocyte death.
As previously indicated, sepsis can control functional impairment in different organs through a variety of routes, including inflammation, oxidative stress, apoptosis, and the regulation of immune responsiveness. One of the key mechanisms of disease pathogenesis is the apoptotic pathway, which is known to be induced or exacerbated by increased reactive oxygen species as well as inflammation. Specifically, activation of JNK phosphorylation leads to the upregulation of p-JNK, which further stimulates the production of BAX and Cyt-C. Thus, JNK is also an important protein in mitochondrial apoptosis. The present study demonstrates in both in vitro and in vivo experiments that sepsis-associated cardiomyocyte apoptosis is facilitated by the activation of the JNK/BAX/Cyt-C pathway, in response to CLP or LPS, resulting in elevated oxidative stress and inflammation levels. Resveratrol significantly reduced myocardial inflammatory injury and JNK/BAX/Cyt-C activation. We added JNK-activator in in vitro experiments to further demonstrate whether resveratrol prevents viral myocardial injury via the JNK/BAX/Cyt-C pathway. Our results showed that agonizing JNK greatly increased LPS-induced myocardial injury and inhibited the ability of resveratrol to protect against infectious myocardial injury. In conclusion, our findings demonstrate that resveratrol elicits cardio protective effects in sepsis by inhibiting the JNK/BAX/Cyt-C signaling pathway and attenuating oxidative stress, inflammation, and apoptosis in the myocardial tissue. These results introduce a fresh avenue for the pharmaceutical treatment of cardiac injury in individuals with sepsis.
Funding:
This research was funded by the National Natural Science Foundation of China (Grant No: 81860336) and the Xinjiang Production and Construction Corps Science and Technology Tackling and Achievement Transformation Project (Grant No: 2016AD003) in accordance with their respective guidelines and requirements.
Author’s contribution:
Liang Lin, Guodong Cao and Youcheng Zeng performed the experiments. Liang Lin analyzed the data. Youcheng Zeng participated in the design of the study. Liang Lin drafted the manuscript with the support of all authors.
Conflict of interests:
The authors declared no conflict of interests.
References
- Remick DG. Pathophysiology of sepsis. Am J Pathol 2007;170(5):1435-44.
[Crossref] [Google Scholar] [PubMed]
- Hanumanthu BK, Nair AS, Katamreddy A, Gilbert JS, You JY, Offor OL, et al. Sepsis-induced cardiomyopathy is associated with higher mortality rates in patients with sepsis. Acute Crit Care 2021;36(3):215-22.
[Crossref] [Google Scholar] [PubMed]
- Jiang LQ, Zhang LY, Yang JC, Shi H, Zhu HZ, Zhai ME, et al. 1-Deoxynojirimycin attenuates septic cardiomyopathy by regulating oxidative stress, apoptosis, and inflammation via the JAK2/STAT6 signaling pathwa. Biomed Pharmacother 2022;155.
- Estaquier J, Vallette F, Vayssiere J, Mignotte B. The mitochondrial pathways of apoptosis. Adv Exp Med Biol 2012;942:157-83.
- Gao GY, Ma J, Lu P, Jiang X, Chang C. Ophiopogonin B induces the autophagy and apoptosis of colon cancer cells by activating JNK/c-Jun signaling pathway. Biomed Pharmacother 2018;108:1208-15.
[Crossref] [Google Scholar] [PubMed]
- del Mar Blanquer-Rosselló M, Hernández-López R, Roca P, Oliver J, Valle A. Resveratrol induces mitochondrial respiration and apoptosis in SW620 colon cancer cells. Biochim Biophys Acta Gen Subj 2017;1861(2):431-40.
[Crossref] [Google Scholar] [PubMed]
- Tang Y, Li L Strategies for making animal models of sepsis and their applications. Chin J Exp Surg 2006(12):1433-4.
- Hong XF, Li L, Yang ZX, Yan J. Paeoniflorin improves myocardial injury via inhibition of Src/VE-cadherin pathway in septic rats. Zhonghua Nei Ke Za Zhi 2022;61(6):652-8.
[Crossref] [Google Scholar] [PubMed]
- Wen PY, Kesari S. Malignant gliomas in adults. New Engl J Med 2008;359(5):492-507.
- Xiong J, Zhou LI, Lim Y, Yang M, Zhu YH, Li ZW, et al. Mature brain-derived neurotrophic factor and its receptor TrkB are upregulated in human glioma tissues. Oncol Lett 2015;10(1):223-7.
[Crossref] [Google Scholar] [PubMed]
- Zeng Y, Cao G, Lin L, Zhang Y, Luo X, Ma X, et al. Resveratrol attenuates sepsis-induced cardiomyopathy in rats through anti-ferroptosis via the Sirt1/Nrf2 pathway. J Invest Surg 2023;36(1):2157521.
[Crossref] [Google Scholar] [PubMed]
- Li X, Li H, Li S, Song N, Hou X, Zhou W, et al. Advances in resveratrol research. Chin Tradit Herbal Drugs 2016;47(14):2568-78.
- Zeng YC, Zhou YL, Cao GD, Lin L, Guo LC, Zhao YH, et al. Effect and mechanism of resveratrol on iron death in septic cardiomyopathy in rats. Herald Med 2022;41(12):1740-6.
- Zhao L, Jin L, Luo Y, Wang L, Li Y, Xian S, et al. Shenfu injection attenuates cardiac dysfunction and inhibits apoptosis in septic mice. Ann Transl Med 2022;10(10):597.
[Crossref] [Google Scholar] [PubMed]
- Zhu H, Zhang L, Jia H, Xu L, Cao Y, Zhai M, et al. Tetrahydrocurcumin improves lipopolysaccharide-induced myocardial dysfunction by inhibiting oxidative stress and inflammation via JNK/ERK signaling pathway regulation. Phytomedicine 2022;104:154283.
[Crossref] [Google Scholar] [PubMed]
- Su LJ, Zhang JH, Gomez H, Murugan R, Hong X, Xu D, et al. Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy and ferroptosis. Oxid Med Cell Longev 2019;2019(1):5080843.
[Crossref] [Google Scholar] [PubMed]
- Zhang C, Mo M, Ding W, Liu W, Yan D, Deng J, et al. High-Mobility Group Box 1 (HMGB1) impaired cardiac excitation–contraction coupling by enhancing the Sarcoplasmic Reticulum (SR) Ca2+ leak through TLR4-ROS signaling in cardiomyocytes. J Mol Cell Cardiol 2014;74:260-73.
[Crossref] [Google Scholar] [PubMed]
- Vellinga NA, Boerma EC, Koopmans M, Donati A, Dubin A, Shapiro NI, et al. International study on microcirculatory shock occurrence in acutely ill patients. Crit Care Med 2015;43(1):48-56.
[Crossref] [Google Scholar] [PubMed]
- Joffre J, Hellman J. Oxidative stress and endothelial dysfunction in sepsis and acute inflammation. Antioxid Redox Signal 2021;35(15):1291-307.
[Crossref] [Google Scholar] [PubMed]
- Hüttemann M, Pecina P, Rainbolt M, Sanderson TH, Kagan VE, Samavati L, et al. The multiple functions of cytochrome c and their regulation in life and death decisions of the mammalian cell: From respiration to apoptosis. Mitochondrion 2011;11(3):369-81.
[Crossref] [Google Scholar] [PubMed]
- Ly JD, Grubb DR, Lawen A. The mitochondrial membrane potential (deltapsi (m)) in apoptosis: An update. Apoptosis 2003;8 (2):115-28.
[Crossref] [Google Scholar] [PubMed]
- Parrish AB, Freel CD, Kornbluth S. Cellular mechanisms controlling caspase activation and function. Cold Spring Harb Perspect Biol 2013;5(6):a008672.
[Crossref] [Google Scholar] [PubMed]
- Kothakota S, Azuma T, Reinhard C, Klippel A, Tang J, Chu K, et al. Caspase-3 generated fragment of gelsolin: Effector of morphological change in apoptosis. Science 1997;278(5336):294-8.
[Crossref] [Google Scholar] [PubMed]
- Launay S, Hermine O, Fontenay M, Kroemer G, Solary E, Garrido C. Vital functions for lethal caspases. Oncogene 2005;24(33):5137-48.
[Crossref] [Google Scholar] [PubMed]
- Suzuki N, Urano J, Tamanoi F. Farnesyltransferase inhibitors induce cytochrome-c release and caspase-3 activation preferentially in transformed cells. Proc Natl Acad Sci 1998;95(26):15356-61.
[Crossref] [Google Scholar] [PubMed]
- Ichim G, Tait SW. A fate worse than death: Apoptosis as an oncogenic process. Nat Rev Cancer 2016;16(8):539-48.
[Crossref] [Google Scholar] [PubMed]
- Mace PD, Riedl SJ, Salvesen GS. Caspase enzymology and activation mechanisms. Methods Enzymol 2014;544:161-78.
[Crossref] [Google Scholar] [PubMed]
- Cheng MH, Pan CY, Chen NF, Yang SN, Hsieh S, Wen ZH, et al. Piscidin-1 induces apoptosis via mitochondrial reactive oxygen species-regulated mitochondrial dysfunction in human osteosarcoma cells. Sci Rep 2020;10(1):5045.
- Ray PD, Huang BW, Tsuji Y. Reactive Oxygen Species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal 2012;24(5):981-90.
[Crossref] [Google Scholar] [PubMed]
- Chen HM, Chang FR, Hsieh YC, Cheng YJ, Hsieh KC, Tsai LM, et al. A novel synthetic protoapigenone analogue, WYC02-9, induces DNA damage and apoptosis in DU145 prostate cancer cells through generation of reactive oxygen species. Free Radic Biol Med 2011;50(9):1151-62.
[Crossref] [Google Scholar] [PubMed]
- Lv L, Zheng L, Dong D, Xu L, Yin L, Xu Y, et al. Dioscin, a natural steroid saponin, induces apoptosis and DNA damage through reactive oxygen species: A potential new drug for treatment of glioblastoma multiforme. Food Chem Toxicol 2013;59:657-69.
[Crossref] [Google Scholar] [PubMed]
- Morel I, Lescoat G, Cillard J, Pasdeloup N, Brissot P, Cillard P. Kinetic evaluation of free malondialdehyde and enzyme leakage as indices of iron damage in rat hepatocyte cultures: Involvement of free radicals. Biochem Pharmacol 1990;39(11):1647-55.
[Crossref] [Google Scholar] [PubMed]
- Wang J, Yi J. Cancer cell killing via ROS: To increase or decrease, that is the question. Cancer Biol Ther 2008;7(12):1875-84.
[Crossref] [Google Scholar] [PubMed]