- *Corresponding Author:
- Fen Liu
Department of Critical Care Medicine, The First Affiliated Hospital of Nanchang University, Jiangxi, Nanchang 330006, China
E-mail: iufen9934@163.com
| Date of Received | 15 March 2023 |
| Date of Revision | 27 Deember 2023 |
| Date of Acceptance | 08 May 2024 |
| Indian J Pharm Sci 2024;86(3):915-924 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Postoperative cognitive dysfunction is a common complication following anesthesia and surgery, and is associated with oxidative stress and apoptosis in the central nervous system. Resveratrol, an antioxidant, natural polyphenol, has been reported to have neuroprotective effect. The aim of this study was to examine whether resveratrol could attenuate the apoptosis and oxidative stress in a cell model of postoperative cognitive dysfunction. A cell model of postoperative cognitive dysfunction was constructed using human microglial clone 3 cells exposed to 1000 ng/ml lipopolysaccharide, 50 µM resveratrol, and their combination for 48 h. Then, the cell viability, proliferation, apoptosis, production of reactive oxygen species, and the expression of apoptosis-related genes and nuclear factor-E2-related factor 2 and its downstream antioxidant genes were examined. In the postoperative cognitive dysfunction cell model, lipopolysaccharide decreased the cell viability and proliferation, while it increased cell apoptosis and reactive oxygen species level. Lipopolysaccharide also upregulated the expression of pro-apoptotic genes (Bcl-2-associated X-protein and caspase-3) and genes involved in nuclear factor-E2-related factor 2-mediated antioxidant pathway (nuclear factor-E2-related factor 2, Kelch-like ECH-associated protein 1, heme oxygenase-1, and superoxide dismutase-2) in human microglial clone 3 cells. Resveratrol ameliorated the lipopolysaccharide-induced decrease of cell viability and proliferation, increase of cell apoptosis and reactive oxygen species level, and upregulation of the expression of pro-apoptotic genes. Additionally, resveratrol upregulated the expression of nuclear factor-E2-related factor 2 and its downstream antioxidant genes in lipopolysaccharide-treated human microglial clone 3 cells. Resveratrol protects human microglial clone 3 cells against lipopolysaccharide-induced injury by reducing oxidative stress through activating nuclear factor-E2-related factor 2-mediated antioxidant pathway.
Keywords
Lipopolysaccharide, nuclear factor-E2-related factor 2, postoperative cognitive dysfunction, reactive oxygen species, resveratrol
Postoperative Cognitive Dysfunction (POCD) is a neurological disorder that is characterized by impaired memory, concentration, and information processing[1,2]. Currently, most studies suggest that POCD is related to factors such as surgery, anesthesia, age, and psychology[3-5]. However, the specific mechanism and pathogenesis of POCD are still not completely understood. Although there are various pathological factors associated with POCD, experimental studies have shown that the key mechanisms underlying the development of POCD are neuroinflammation, oxidative stress, and apoptosis in the central nervous system, particularly in the hippocampus, which is involved in memory formation and learning[6-8]. Early detection and prevention are crucial for the clinical treatment of POCD, making it essential to clarify its pathogenesis and develop appropriate prevention strategies to reduce its incidence.
Microglial cells are a specialized type of immune cell that reside in the brain and are responsible for monitoring its activity. They play a critical role in protecting the brain from external damage and pathogen invasion, as well as monitoring the micro-environment of the central nervous system and maintaining neuro-homeostasis under normal physiological conditions[9,10]. In a state of normal physiology, microglia remains in a resting state. However, in the presence of inflammatory responses in the brain, microglia are stimulated and become activated[11]. Research has shown that over activation of microglia can contribute to neurodegenerative processes and play a pivotal role in the neuroinflammatory pathogenesis of Alzheimer's disease[12,13]. Human Microglial Clone 3 (HMC3) cells have been widely utilized in the study of neuroinflammatory diseases[14,15].
Lipopolysaccharide (LPS) is a component of the outer membrane of gram-negative bacteria and is a potent activator of the immune system[16]. LPS induces activation of microglia and the production of oxygen free radicals, thereby promoting the onset and progression of neurodegenerative diseases. Animal studies and preclinical research have demonstrated that the use of anti-inflammatory medications can effectively inhibit the activation of microglia, reduce the level of inflammatory factors, and alleviate neuronal damage in the brain[17,18].
Resveratrol (trans-3,4',5-trihydroxystilbene, (RES)) is a natural polyphenol that can be found in grapes, peanuts, red wine, and other dietary sources. It possesses a variety of biological activities, including anti-inflammatory, antioxidant, cardiovascular protection, and anti-aging properties[19-22]. Moreover, numerous animal models and in vitro studies have demonstrated that RES can protect neural cells from damage induced by various stimuli, such as corticosterone and cobalt chloride[23,24]. However, it is currently unknown whether RES can ameliorate the damage induced by LPS in HMC3 cells.
In this study, we aimed to establish a cell model of POCD using HMC3 cells induced by LPS and investigated the ameliorative effects of RES on LPS-induced damage to HMC3 cells. To elucidate the underlying mechanism, we examined the Nuclear factor erythroid 2-Related Factor 2 (NRF2)-mediated antioxidant signaling pathway. The results of this study will provide experimental evidence for the potential of RES in treating POCD.
Materials and Methods
Drugs and reagents:
LPS (L5293) was obtained from Sigma-Aldrich. RES (B20044) was purchased from Shanghai Yuanye Technology. The NRF2 antibody (AF0639) was acquired from Affinity Biosciences, and the Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) antibody (TA802519) was obtained from OriGene. Additionally, phosphorylated NRF2 antibody (ET1608-28) was purchased from HUABIO, while Heme Oxygenase-1 (HO-1) antibody (43966S), Kelch-like ECH Associated Protein 1 (KEAP1) antibody (8047S), Superoxide Dismutase 2 (SOD2) antibody (13141T), B-CELL LYMPHOMA-2 (BCL-2) antibody (3498T), BCL-2 Associated X (BAX) antibody (41162S), and caspase-3 (9668T) were purchased from cell signaling technology.
Cell culture and treatment:
The HMC3 cells (CL-0620) was obtained from Wuhan Procell Biotechnology Co., Ltd. The cells were cultured in Eagle’s Minimum Essential Medium (EMEM) (Corning) supplemented with 10 % (Fetal Bovine Serum (FBS), Gibco) and 1 % antibiotic mixture, and incubated at 37° with 5 % Carbon dioxide (CO2)[25]. The cells were seeded in 6-well plates or 96-well plates at a density of 2×105 cells/well or 5×103 cells/well. A stock solution of RES and LPS was prepared by dissolving in dimethyl sulfoxide (HY-Y0320, MedChemExpress). The cells were incubated with 50 μM RES, 1000 ng/ml LPS, or their combination. After 48 h, cells were harvested for subsequent experiments. In a separate set of plates, cells were incubated for 48 h before conducting the Cell Counting Kit-8 (CCK-8) assay to evaluate cell viability and the 5-Ethynyl-2′-deoxyuridine (EdU) assay to evaluate cell proliferation.
CCK-8 assay:
The CCK-8 assay (GlpBio) was utilized to assess cell viability. HMC3 cells were cultured and exposed to LPS and RES. The cells were treated with LPS (10, 100, 1000 ng/ml) in a 96-well plate at a density of 5×103 cells/well for 24, 48, and 72 h to determine the drug's cytotoxic effect. The 1000 ng/ml LPS and 48 h were selected for subsequent analysis. RES (50 µM) was co-administered with LPS to assess its protective effect against LPS. The CCK-8 solution (10 μl) was added to each well 4 h prior to the end of the incubation for 48 h. The difference in absorbance values obtained at 450 nm was used to evaluate the potential cytotoxic effects of LPS and/or RES. For each group, the average of replicates was utilized, and the outcomes were presented as a percentage of cell viability in comparison to untreated cells. The data was reported as mean±Standard Deviation (SD)[26].
EdU assay:
The EdU cell proliferation detection kit (CX003, Shanghai Epizyme) was used to determine the level of cell proliferation. The EdU reagent was added to the cells after treatment, and the cells were incubated at 37° for 2 h. To remove unbound EdU dye, the cells were washed with serum-free medium three times. The level of cell proliferation was determined by counting the number of fluorescence-positive cells, which were visualized using a fluorescence microscope (OLYMPUS IX73).
Assessment of Reactive Oxygen Species (ROS):
Intracellular levels of ROS were measured using the ROS detection kit (R6033, US Everbright). Specifically, 10 μM of 2,7-Dichlorodihydrofluorescein Diacetate (DCFH-DA) was added to HMC3 cells and incubated at 37° for 30 min. The cells were then washed three times with serum-free medium to remove any unbound ROS dye. Fluorescence experiments were conducted using a microplate reader (PerkinElmer EnSpire) and the data was analyzed quantitatively using GraphPrism 5.
Detection of apoptosis:
The apoptosis was detected using Annexin-V staining (Y6026M, US Everbright) according to the user manual. Briefly, cells were incubated with Annexin V dye and Annexin V binding buffer at a ratio of 1:100 at room temperature for 15 min in the absence of light. Subsequently, the cells were washed three times with serum-free medium to eliminate unbound Annexin-V dye. The level of cell apoptosis was determined by counting the number of red fluorescence-positive cells under an adherent state using a fluorescence microscope (OLYMPUS IX73).
Real-Time quantitative Polymerase Chain Reaction (RT-qPCR):
Total Ribonucleic Acid (RNA) was extracted from HMC3 cells using Trizol LS reagent (10296010CN, Invitrogen) for RT-qPCR. 1 µg of total RNA was reverse transcribed using a Superscript VILO kit (11754050, Invitrogen) in a final volume of 20 μl. The resulting complementary Deoxyribonucleic Acid (cDNA) (1 μl) was added to the SYBR green RT-PCR super mix (MF013-1, Mei5 Bioservices Co., Ltd.,) to achieve a final volume of 20 μl per well. Samples from each group were run in duplicate, and GAPDH was used as an endogenous control. Results were calculated using the 2−ΔΔCT method and expressed as an n-fold increase in gene expression using the control group as a calibrator[27]. The primers used for the target and reference genes are listed as follows; GAPDH, forward 5’-GGAGCGAGATCCCTCCAAAAT-3’ and reverse 5’-GGCTGTTGTCATACTTCTCATGG-3’; BAX, forward 5’-CCCGAGAGGTCTTTTTCCGAG-3’ and reverse 5’-CCAGCCCATGATGGTTCTGAT-3’; BCL-2, forward 5’-GGTGGGGTCATGTGTGTGG-3’ and reverse 5’-CGGTTCAGGTACTCAGTCATCC-3’.
Western blot:
Total proteins of HMC3 cells were prepared using the radio immunoprecipitation assay buffer (R0020, Solarbio) containing 1× protease inhibitor cocktail (HY-K0010, MedChemExpress) and 1× phosphatase inhibitor cocktail (HY-K0021, MedChemExpress). The concentration of the proteins was examined by disodium bicinchoninate protein assay kit (P0012, Beyotime Biotechnology). Total of 20 μg proteins were separated by electrophoresis on sodium dodecyl sulfate-polyacrylamide gels. After electrophoresis, the samples were transferred onto a polyvinylidene fluoride membrane (Amersham, Little Chalfont, United Kingdom (UK)) using a transfer buffer at 280 mA for 1.5 h. The obtained membranes were incubated with 5 % non-fat dry milk in Tris-Buffered Saline with Tween® 20 (TBST) for 1 h at room temperature, and then washed three times in TBST to remove the milk. The membranes were incubated with primary antibodies of caspase-3 (1:2000 dilution), BAX (1:2000 dilution), BCL-2 (1:2000 dilution), NRF2 (1:2000 dilution), phosphorylated NRF2 (1:2000 dilution), KEAP1 (1:1000 dilution), HO-1 (1:2000 dilution), and SOD2 (1:2000 dilution) overnight at 4°. The next day, the membranes were washed three times with TBST and then incubated with peroxidase-conjugated goat anti-mouse or anti-rabbit antibodies (1:10 000 dilution) for 1 h at room temperature. After washing, the membranes were analyzed using the enhanced chemiluminescence system (LumiGlo reserve; Seracare, Milford, Massachusetts, United States of America (USA)).
Statistical analysis:
The data are presented as mean±SD, and the reported values are the outcome of at least three experiments. To ensure reproducibility, all assays were repeated three times. The different groups were compared and analyzed using one-way analysis of variance with Tukey's post-test for comparison between the groups. A p<0.05 was considered statistically significant. The graphs were generated and organized using GraphPad Prism (Version 5.0, USA).
Results and Discussion
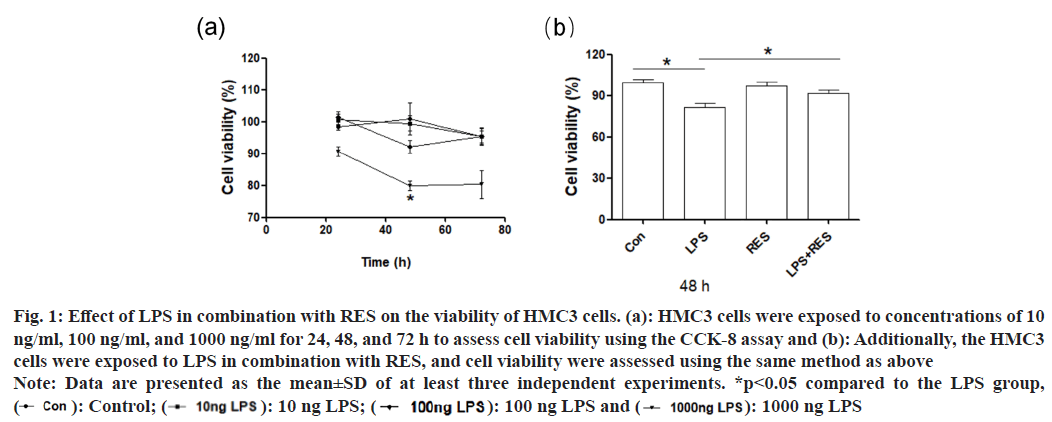
The result of CCK-8 assay showed that LPS of 1000 ng/ml decreased the viability of HMC3 cells at 48 h and 72 h, while LPS of 10 and 100 ng/ ml had no significant effect on cell viability (fig. 1a). Therefore, the concentration of 1000 ng/ml and duration of 48 h were selected for subsequent experiments. In addition, RES significantly ameliorated the LPS induced reduction in cell viability compared to the LPS group (fig. 1b).
Fig. 1: Effect of LPS in combination with RES on the viability of HMC3 cells. (a): HMC3 cells were exposed to concentrations of 10
ng/ml, 100 ng/ml, and 1000 ng/ml for 24, 48, and 72 h to assess cell viability using the CCK-8 assay and (b): Additionally, the HMC3
cells were exposed to LPS in combination with RES, and cell viability were assessed using the same method as above
Note: Data are presented as the mean±SD of at least three independent experiments. *p<0.05 compared to the LPS group,  100 ng LPS and
100 ng LPS and  1000 ng LPS
1000 ng LPS
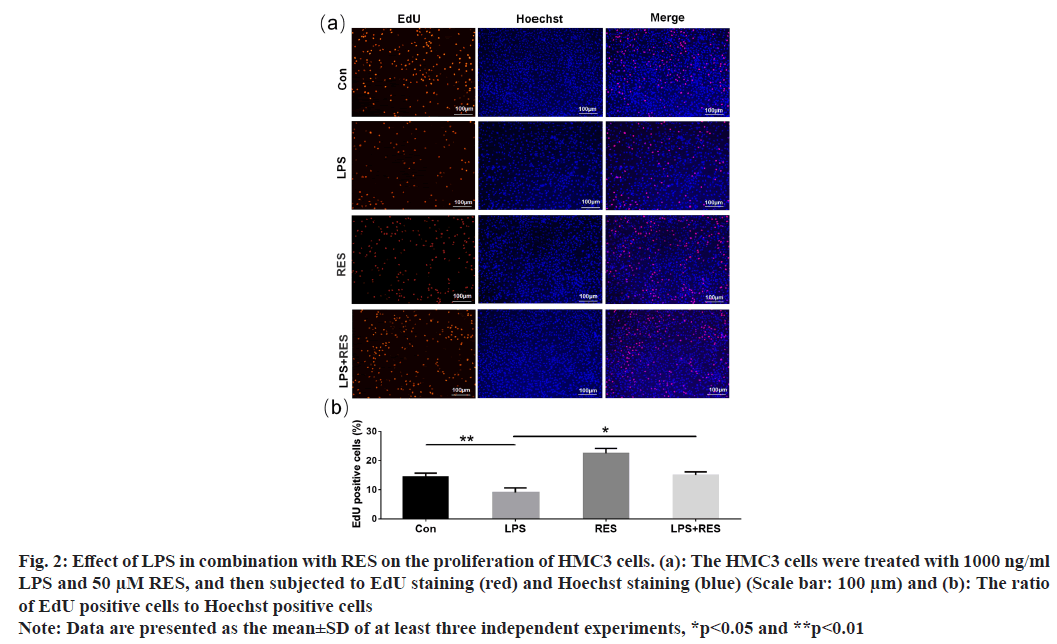
The EdU-positive cells (shown in red) indicated the proliferation of HMC3 cells (fig. 2a). Compared to the control group, the LPS group exhibited a decrease in the positive rate of HMC3 cells (fig. 2b). In addition, RES was able to restore the decreased positive rate induced by LPS. The findings indicate that RES can protect HMC3 cells from the decrease in proliferation induced by LPS.
Fig. 2: Effect of LPS in combination with RES on the proliferation of HMC3 cells. (a): The HMC3 cells were treated with 1000 ng/ml LPS and 50 μM RES, and then subjected to EdU staining (red) and Hoechst staining (blue) (Scale bar: 100 μm) and (b): The ratio of EdU positive cells to Hoechst positive cells
Note: Data are presented as the mean±SD of at least three independent experiments, *p<0.05 and **p<0.01
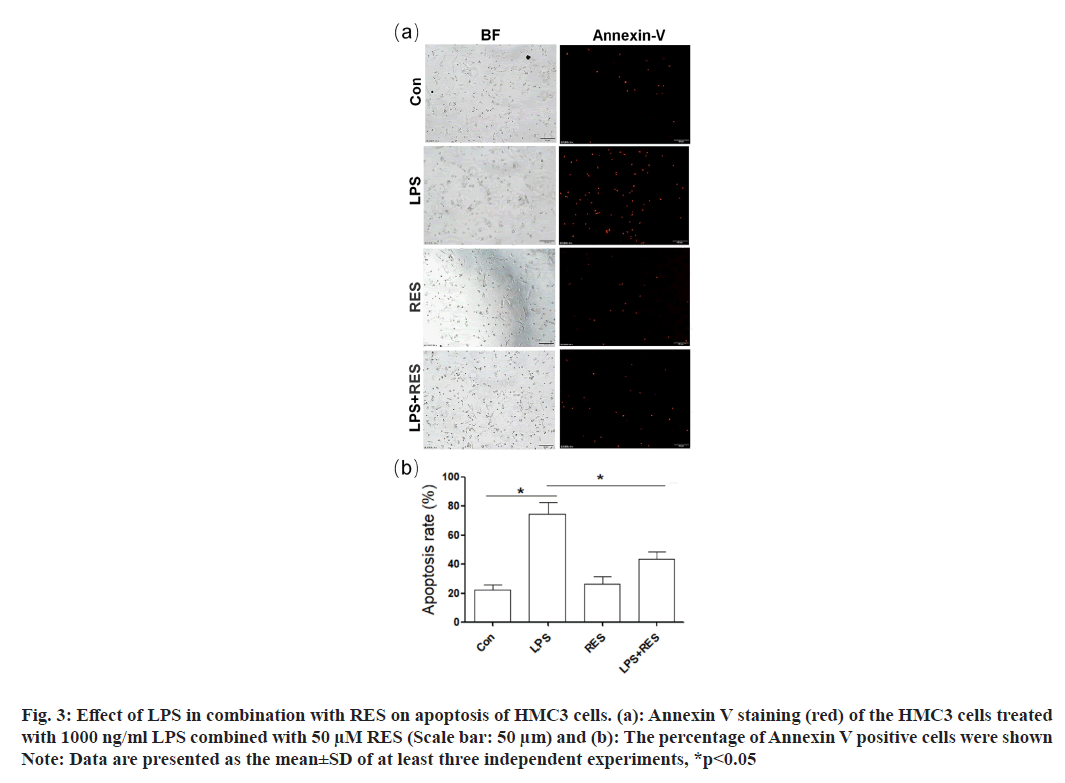
Annexin V-positive cells (red) were used to indicate the apoptosis of HMC3 cells (fig. 3a). In comparison to the control group, the LPS group exhibited an increase number of positive HMC3 cells (fig. 3b). In addition, RES was able to restore the increased number of positive HMC3 cells induced by LPS.
Fig. 3: Effect of LPS in combination with RES on apoptosis of HMC3 cells. (a): Annexin V staining (red) of the HMC3 cells treated
with 1000 ng/ml LPS combined with 50 μM RES (Scale bar: 50 μm) and (b): The percentage of Annexin V positive cells were shown
Note: Data are presented as the mean±SD of at least three independent experiments, *p<0.05
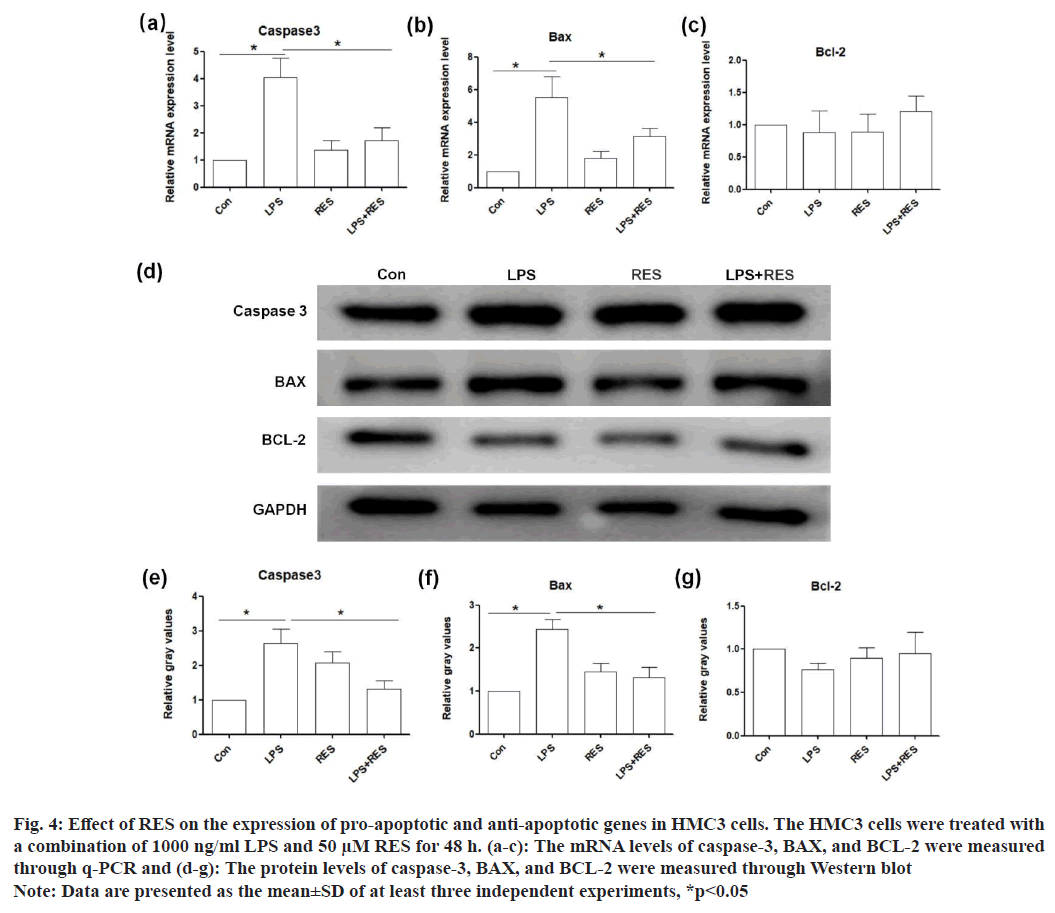
The messenger RNA (mRNA) levels of pro-apoptotic genes (caspase-3 and BAX) were upregulated in the LPS group compared to the control, while their expression in the LPS+RES group was lower than those in the LPS group (fig. 4a and fig. 4b). However, the mRNA level of anti-apoptotic gene (BCL-2) did not show significant difference between the groups (fig. 4c). In addition, the results of Western blot confirmed the changes of these genes at protein level consistent with those at the mRNA level (fig. 4d-fig. 4g).
Fig. 4: Effect of RES on the expression of pro-apoptotic and anti-apoptotic genes in HMC3 cells. The HMC3 cells were treated with
a combination of 1000 ng/ml LPS and 50 μM RES for 48 h. (a-c): The mRNA levels of caspase-3, BAX, and BCL-2 were measured
through q-PCR and (d-g): The protein levels of caspase-3, BAX, and BCL-2 were measured through Western blot
Note: Data are presented as the mean±SD of at least three independent experiments, *p<0.05
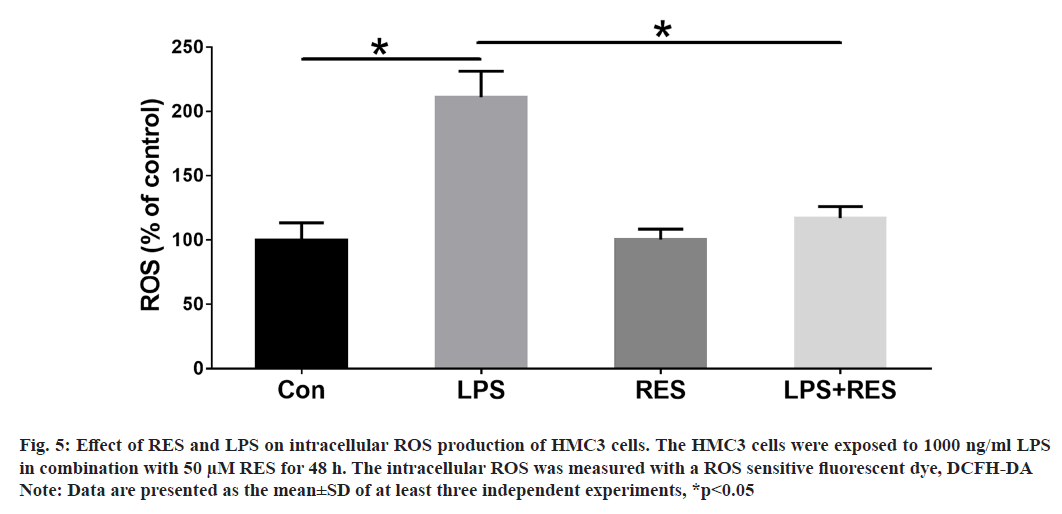
In this study, we wanted to understand whether RES could protect against ROS production induced by LPS in HMC3 cells. The ROS levels were evaluated in HMC3 cells treated with LPS and RES for 48 h. Our findings demonstrate that LPS exposure resulted in a significant increase in ROS production compared to the control group. However, treatment with RES significantly reduced the levels of ROS induced by LPS (fig. 5).
Fig. 5: Effect of RES and LPS on intracellular ROS production of HMC3 cells. The HMC3 cells were exposed to 1000 ng/ml LPS
in combination with 50 μM RES for 48 h. The intracellular ROS was measured with a ROS sensitive fluorescent dye, DCFH-DA
Note: Data are presented as the mean±SD of at least three independent experiments, *p<0.05
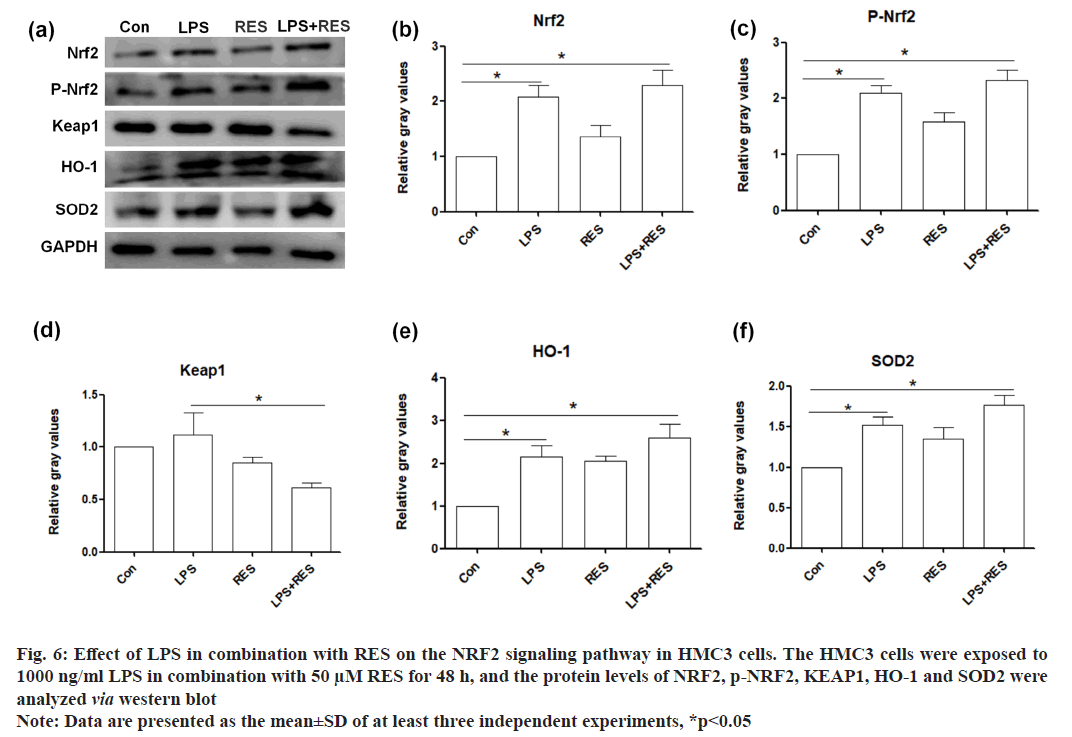
In this study, we examined whether the NRF2-mediated antioxidative signaling pathway was involved in the RES protective effect of RES against LPS induced toxicity in HMC3 cells. Therefore, the protein levels of several key components in the NRF2-mediated antioxidative signaling pathway (NRF2, KEAP1, HO-1, and SOD2) were examined. The results showed that LPS upregulated the protein level of NRF2, phosphorylated NRF2, KEAP1, HO-1, and SOD2 in HMC3 cells. Additionally, RES further elevated the protein levels of NRF2, phosphorylated NRF2, HO-1, and SOD2, while RES inhibited the increased protein level of KEAP1 in LPS-treated HMC3 cells (fig. 6).
Fig. 6: Effect of LPS in combination with RES on the NRF2 signaling pathway in HMC3 cells. The HMC3 cells were exposed to
1000 ng/ml LPS in combination with 50 μM RES for 48 h, and the protein levels of NRF2, p-NRF2, KEAP1, HO-1 and SOD2 were
analyzed via western blot
Note: Data are presented as the mean±SD of at least three independent experiments, *p<0.05
With the aging of China's population, there are a growing number of elderly individuals undergoing various surgical procedures and anesthesia, resulting in an increased incidence of POCD. Due to the potential (POCD) to impede patient rehabilitation, increase complications, raise mortality rates, and elevate medical expenses, it has become a current research focus[28-30]. However, the exact pathogenesis of POCD is not fully understood. Previous studies have shown that central inflammation, apoptosis, and oxidative stress also have significant impacts on the progression of POCD[31-33]. The cell models of POCD in vitro were constructed by using LPS induced microglia to simulate neuroinflammation[34,35]. It has been reported that neural inflammation models were constructed using BV2 and HMC3 microglia cells, whereas HMC3 microglia cells are used in this study. The phenotype of microglial cells can be classified into two types; M1 (pro-inflammatory) and M2 (anti-inflammatory). Upon exposure to LPS, microglial cells are stimulated to transform into the M1 phenotype, resulting in the generation of inflammatory response and exacerbates neuronal damage, ultimately resulting in neural dysfunction[36-38].
Previous research has demonstrated that activating Sirtuin-1 (SIRT1) has universally beneficial effect on neurological protection. RES, a SIRT1 agonist, has been widely utilized as a neuroprotective agent in vitro[39]. It has been shown that RES ameliorate damage to osteoblasts, cardiomyocytes and acute lung cells induced by LPS[40-42]. Additionally, RES alleviate short-term learning and memory impairment in older mice after local anesthesia[43]. Therefore, RES was selected as one of candidates of protection of LPS. In this study, the mRNA and protein expression levels of BAX, caspase-3, and ROS production were increased in the HMC3 cells induced by LPS, while the application of RES could restore LPS-induced increased ROS and apoptosis. Although the mRNA and protein expression level of BCL-2 in HMC3 cell induced by LPS had not reduced significantly, the ratio of BAX to BCL-2 was higher in LPS group. Therefore, it is speculated that RES supplement may become a protective drug during nervous system apoptosis and injury.
NRF2 is the main regulatory factor of the antioxidant cell defense system and is expressed in most tissues, including the brain. Under physiological conditions, NRF2 binds to KEAP1 in the cytoplasm to inactive. After exposure to ROS, NRF2 separated from KEAP1 is phosphorylated and then transfers to the nucleus, regulating its downstream antioxidant genes HO-1, SOD and CAT and so on[44,45]. In this study, the expression levels of NRF2, p-NRF2 and its downstream antioxidant genes HO-1, SOD2, and its partner KEAP1 were increased in HMC3 cells induced by LPS, while RES supplement could further increase LPS-induced NRF2 antioxidant pathway. It is suggested that the modified expression of NRF2 antioxidant pathway may be involved in the injury of HMC3 cells induced by LPS and a potential target for neural protection by RES. The decreased expression level of NRF2 should be regulated by siRNA to further prove the protective effect of RES on HMC3 cells induced by LPS.
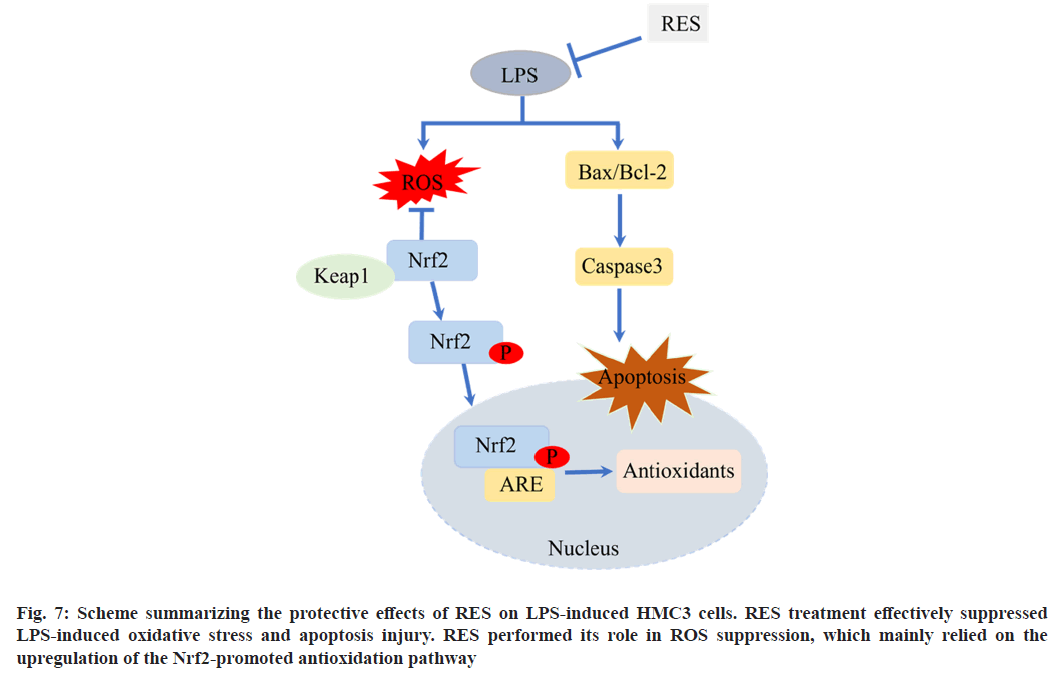
In conclusion, the present study demonstrates that the upregulation of NRF2 in HMC3 cells activate the NRF2-regulated antioxidant defense pathway, which contributes to POCD cell model-induced by LPS in combination with RES (fig. 7). Our findings also suggest that NRF2 activation in the brain may be a novel strategy for protective effect of RES on LPS to prevent the cognitive decline in POCD.
Funding:
This study was supported by the Traditional Chinese Medicine Project of Jiangxi Provincial Health Commission (No: 2020A0364).
Author contributions’:
Yousu Shen and MD have done conceptualization, data curation, formal analysis, investigation, methodology, validation, writing-original draft, and writing-review and editing; Xiaobing Liu, Xianwei Jin, Xia Jin and Chunhui Qin worked on data curation, investigation, methodology, and validation; Fen Liu, MD and Mingsheng Zhang worked on conceptualization and reviewing.
Conflict of interests:
The authors declared no conflict of interests.
References
- Rudolph JL, Schreiber KA, Culley DJ, McGlinchey RE, Crosby G, Levitsky S, et al. Measurement of post-operative cognitive dysfunction after cardiac surgery: A systematic review. Acta Anaesthesiol Scand 2010;54(6):663-77.
[Crossref] [Google Scholar] [PubMed]
- Skvarc DR, Berk M, Byrne LK, Dean OM, Dodd S, Lewis M, et al. Post-operative cognitive dysfunction: An exploration of the inflammatory hypothesis and novel therapies. Neurosci Biobehav Rev 2018;84:116-33.
[Crossref] [Google Scholar] [PubMed]
- Rundshagen I. Postoperative cognitive dysfunction. Deutsch Arztebl Int 2014;111(8):119.
- Evered LA, Silbert BS. Postoperative cognitive dysfunction and noncardiac surgery. Anesth Analg 2018;127(2):496-505.
[Crossref] [Google Scholar] [PubMed]
- O’Gara BP, Mueller A, Gasangwa DV, Patxot M, Shaefi S, Khabbaz K, et al. Prevention of early postoperative decline: A randomized, controlled feasibility trial of perioperative cognitive training. Anesth Analg 2020;130(3):586.
[Crossref] [Google Scholar] [PubMed]
- Ma Z, Ma Y, Cao X, Zhang Y, Song T. Avenanthramide-C activates Nrf2/ARE pathway and inhibiting ferroptosis pathway to improve cognitive dysfunction in aging rats. Neurochem Res 2023;48(2):393-403.
[Crossref] [Google Scholar] [PubMed]
- Xie X, Shen Z, Hu C, Zhang K, Guo M, Wang F, et al. Dexmedetomidine ameliorates postoperative cognitive dysfunction in aged mice. Neurochem Res 2021;46:2415-26.
[Crossref] [Google Scholar] [PubMed]
- Yin L, Gao S, Li C. Exogenous hydrogen sulfide alleviates surgery-induced neuroinflammatory cognitive impairment in adult mice by inhibiting NO signaling. BMC Anesthesiol 2020;20:1-9.
- Colonna M, Butovsky O. Microglia function in the central nervous system during health and neurodegeneration. Ann Rev Immunol 2017;35:441-68.
[Crossref] [Google Scholar] [PubMed]
- Stolero N, Frenkel D. The dialog between neurons and microglia in Alzheimer's disease: The neurotransmitters view. J Neurochem 2021;158(6):1412-24.
[Crossref] [Google Scholar] [PubMed]
- Walker D, Lue LF. Anti-inflammatory and immune therapy for Alzheimer's disease: Current status and future directions. Curr Neuropharmacol 2007;5(4):232-43.
[Crossref] [Google Scholar] [PubMed]
- Su F, Bai F, Zhang Z. Inflammatory cytokines and Alzheimer’s disease: A review from the perspective of genetic polymorphisms. Neurosci Bull 2016;32:469-80.
[Crossref] [Google Scholar] [PubMed]
- An Y, Zhang H, Huang S, Pei G. PL201, a reported rhamnoside against Alzheimer's disease pathology, alleviates neuroinflammation and stimulates Nrf2 signaling. Front Immunol 2020;11:162.
[Crossref] [Google Scholar] [PubMed]
- Lu Z, Liu S, Lopes-Virella MF, Wang Z. LPS and palmitic acid co-upregulate microglia activation and neuroinflammatory response. Comp Psychoneuroendocrinol 2021;6:100048.
[Crossref] [Google Scholar] [PubMed]
- Chiu YJ, Lin CH, Lin CY, Yang PN, Lo YS, Chen YC, et al. Investigating therapeutic effects of indole derivatives targeting inflammation and oxidative stress in neurotoxin-induced cell and mouse models of Parkinson’s disease. Int J Mol Sci 2023;24(3):2642.
[Crossref] [Google Scholar] [PubMed]
- Maldonado RF, Sá-Correia I, Valvano MA. Lipopolysaccharide modification in gram-negative bacteria during chronic infection. FEMS Microbiol Rev 2016;40(4):480-93.
[Crossref] [Google Scholar] [PubMed]
- Klegeris A, McGeer PL. Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) and other anti-inflammatory agents in the treatment of neurodegenerative disease. Curr Alzheimer Res 2005;2(3):355-65.
[Crossref] [Google Scholar] [PubMed]
- Chen X, Chen C, Fan S, Wu S, Yang F, Fang Z, et al. Omega-3 polyunsaturated fatty acid attenuates the inflammatory response by modulating microglia polarization through SIRT1-mediated deacetylation of the HMGB1/NF-κB pathway following experimental traumatic brain injury. J Neuroinflammation 2018;15(1):1-5.
[Crossref] [Google Scholar] [PubMed]
- de La Lastra CA, Villegas I. Resveratrol as an antioxidant and pro-oxidant agent: Mechanisms and clinical implications. Biochem Soc Trans 2007;35(5):1156-60.
[Crossref] [Google Scholar] [PubMed]
- Bonnefont-Rousselot D. Resveratrol and cardiovascular diseases. Nutrients 2016;8(5):250.
- Meng T, Xiao D, Muhammed A, Deng J, Chen L, He J. Anti-inflammatory action and mechanisms of resveratrol. Molecules 2021;26(1):229.
[Crossref] [Google Scholar] [PubMed]
- Zhou DD, Luo M, Huang SY, Saimaiti A, Shang A, Gan RY, et al. Effects and mechanisms of resveratrol on aging and age-related diseases. Oxid Med Cell Longev 2021;2021:9932218.
[Crossref] [Google Scholar] [PubMed]
- Wang P, Du B, Yin W, Wang X, Zhu W. Resveratrol attenuates CoCl2-induced cochlear hair cell damage through upregulation of sirtuin1 and NF-κB deacetylation. PLoS One 2013;8(11):e80854.
[Crossref] [Google Scholar] [PubMed]
- Girbovan C, Kent P, Merali Z, Plamondon H. Dose-related effects of chronic resveratrol administration on neurogenesis, angiogenesis, and corticosterone secretion are associated with improved spatial memory retention following global cerebral ischemia. Nutr Neurosci 2016;19(8):352-68.
[Crossref] [Google Scholar] [PubMed]
- Akhter R, Shao Y, Formica S, Khrestian M, Bekris LM. TREM2 alters the phagocytic, apoptotic and inflammatory response to Aβ42 in HMC3 cells. Mol Immunol 2021;131:171-9.
[Crossref] [Google Scholar] [PubMed]
- Liu H, Sun S, Liu B. Smurf2 exerts neuroprotective effects on cerebral ischemic injury. J Biol Chem 2021;297(2):100537.
[Crossref] [Google Scholar] [PubMed]
- Rao X, Huang X, Zhou Z, Lin X. An improvement of the 2ˆ (–delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat Bioinforma Biomath 2013;3(3):71.
[Google Scholar] [PubMed]
- Lin X, Chen Y, Zhang P, Chen G, Zhou Y, Yu X. The potential mechanism of postoperative cognitive dysfunction in older people. Exp Gerontol 2020;130:110791.
[Crossref] [Google Scholar] [PubMed]
- Butz M, Gerriets T, Sammer G, El-Shazly J, Tschernatsch M, Huttner HB, et al. Effects of postoperative cognitive training on neurocognitive decline after heart surgery: A randomized clinical trial. Eur J Cardiothorac Sur 2022;62(5):251.
[Crossref] [Google Scholar] [PubMed]
- Yang KL, Detroyer E, van Grootven B, Tuand K, Zhao DN, Rex S, et al. Association between preoperative anxiety and postoperative delirium in older patients: A systematic review and meta-analysis. BMC Geriatr 2023;23(1):198.
[Crossref] [Google Scholar] [PubMed]
- Zhao WX, Zhang JH, Cao JB, Wang W, Wang DX, Zhang XY, et al. Acetaminophen attenuates lipopolysaccharide-induced cognitive impairment through antioxidant activity. J Neuroinflammation 2017;14(1):17.
[Crossref] [Google Scholar] [PubMed]
- Zhao S, Chen F, Wang D, Han W, Zhang Y, Yin Q. NLRP3 inflammasomes are involved in the progression of postoperative cognitive dysfunction: From mechanism to treatment. Neurosurg Rev 2021;44(4):1815-31.
[Crossref] [Google Scholar] [PubMed]
- Yang YS, He SL, Chen WC, Wang CM, Huang QM, Shi YC, et al. Recent progress on the role of non-coding RNA in postoperative cognitive dysfunction. Front Cell Neurosci 2022;16:1024475.
[Crossref] [Google Scholar] [PubMed]
- Han X, Cheng X, Xu J, Liu Y, Zhou J, Jiang L, et al. Activation of TREM2 attenuates neuroinflammation via PI3K/Akt signaling pathway to improve postoperative cognitive dysfunction in mice. Neuropharmacology 2022;219:109231.
[Crossref] [Google Scholar] [PubMed]
- Wang XY, Liu WG, Hou AS, Song YX, Ma YL, Wu XD, et al. Dysfunction of EAAT3 aggravates LPS-induced post-operative cognitive dysfunction. Membranes 2022;12(3):317.
[Crossref] [Google Scholar] [PubMed]
- Orihuela R, McPherson CA, Harry GJ. Microglial M1/M2 polarization and metabolic states. Br J Pharmacol 2016;173(4):649-65.
[Crossref] [Google Scholar] [PubMed]
- Wen X, Xiao L, Zhong Z, Wang L, Li Z, Pan X, et al. Astaxanthin acts via LRP-1 to inhibit inflammation and reverse lipopolysaccharide-induced M1/M2 polarization of microglial cells. Oncotarget 2017;8(41):69370.
[Crossref] [Google Scholar] [PubMed]
- Yan A, Liu Z, Song L, Wang X, Zhang Y, Wu N, et al. Idebenone alleviates neuroinflammation and modulates microglial polarization in LPS-stimulated BV2 cells and MPTP-induced Parkinson’s disease mice. Front Cell Neurosci 2019;12:529.
[Crossref] [Google Scholar] [PubMed]
- Bai L, Liu R, Wang R, Xin Y, Wu Z, Ba Y, et al. Attenuation of Pb-induced Aβ generation and autophagic dysfunction via activation of SIRT1: Neuroprotective properties of resveratrol. Ecotoxicol Environ Safety 2021;222:112511.
[Crossref] [Google Scholar] [PubMed]
- Bai T, Hu X, Zheng Y, Wang S, Kong J, Cai L. Resveratrol protects against lipopolysaccharide-induced cardiac dysfunction by enhancing SERCA2a activity through promoting the phospholamban oligomerization. Am J Physiol Heart Circ Physiol 2016;311(4):H1051-62.
[Crossref] [Google Scholar] [PubMed]
- Ma J, Wang Z, Zhao J, Miao W, Ye T, Chen A. Resveratrol attenuates Lipopolysaccharides (LPS)-induced inhibition of osteoblast differentiation in MC3T3-E1 cells. Med Sci Monit 2018;24:2045.
[Crossref] [Google Scholar] [PubMed]
- Guo B, Peng Y, Gu Y, Zhong Y, Su C, Liu L, et al. Resveratrol pretreatment mitigates LPS-induced acute lung injury by regulating conventional dendritic cells’ maturation and function. Open Life Sci 2021;16(1):1064-81.
[Crossref] [Google Scholar] [PubMed]
- Abraham J, Johnson RW. Consuming a diet supplemented with resveratrol reduced infection-related neuroinflammation and deficits in working memory in aged mice. Rejuvenation Res 2009;12(6):445-53.
[Crossref] [Google Scholar] [PubMed]
- Hu LY, Cui JB, Xu XM, Huang ZH, Jiao HT. Expression of Nrf2-Keap1-ARE signal pathway in traumatic lung injury and functional study. Eur Rev Med Pharmacol Sci 2018;22(5):1402-8.
[Crossref] [Google Scholar] [PubMed]
- Wang H, Pan L, Si L, Miao J. The role of Nrf2-Keap1 signaling pathway in the antioxidant defense response induced by PAHs in the calm Ruditapes philippinarum. Fish Shellfish Immunol 2018;80:325-34.
[Crossref] [Google Scholar] [PubMed]