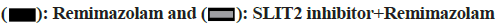- *Corresponding Author:
- Jijun Xiong
Department of Anesthesiology, Guilin People’s Hospital, Guilin, Guangxi 541002, China
E-mail: xiongjijun_edu@hotmail.com
| This article was originally published in a special issue, “Modern Applications in Biomedical Research and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2022:84(3) Spl Issue “125-130” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To investigate the effect of remimazolam on neuronal apoptosis in rat brain through slit guidance ligand 2/roundabout 4 signaling pathway. Primary cortical neurons were obtained from specific pathogen free 3 w old male Wistar rats and sub cultured in cell culture dishes. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay detected cell viability; Hochest/propidium iodide double staining and flow cytometry detected cell apoptosis. Western blot detected apoptosis-related gene expression and slit guidance ligand 2/roundabout 4 signaling pathway-related protein expression. In addition, after slit guidance ligand 2 inhibitor administration, Western blot detected the changes in expression levels of key genes in cells. Relative to control group, cell viability in each remimazolam group presented elevation and apoptosis presented depletion (p<0.05) in a dose-dependent manner. With the increase of remimazolam treatment time, Bcl-2- associated X protein, cleaved caspase-3 and cleaved poly adenosine diphosphate-ribose polymerase protein levels showed a gradual decrease and anti-apoptotic protein B-cell lymphoma 2 level showed a gradual increase relative to 0 h (p<0.05). At the same time, with the increase of remimazolam treatment time (0, 3, 6, 12 and 24 h), slit guidance ligand 2 and roundabout 4 protein levels increased continuously relative to 0 h. Additionally, relative to remimazolam group, Bcl-2-associated X protein, cleaved caspase-3 and cleaved poly adenosine diphosphate-ribose polymerase showed a gradual up regulation and B-cell lymphoma 2 showed a gradual down regulation in slit guidance ligand 2 inhibitor and remimazolam co-treatment group. Remimazolam pretreatment may inhibit rat cortical neuronal apoptosis through slit guidance ligand 2/ roundabout 4 signaling pathway and providing new ideas for clinical brain protection.
Keywords
Remimazolam, slit guidance ligand 2/roundabout 4 signaling pathway, neuron, apoptosis
Remimazolam is a new type of ultra-short acting benzodiazepine, which is widely used in general anesthesia and sedation of intensive care patients[1-3]. Remimazolam can affect synaptic transmission, induce neuronal apoptosis in a dose-dependent manner and lead to impairment of long-term learning and memory, resulting in anesthetic effects. The regenerative effect of Slit Guidance Ligand 2 (SLIT2) on blood vessels and the sprouting effect of neurons may be related to nerve regeneration. Trans membrane receptor Roundabout 4 (ROBO4), as a vascular specific receptor of SLIT, can also regulate proliferation and neuronal apoptosis[4]. SLIT2 suppresses the regeneration of blood vessels by combining with ROBO4, repressing tissue damage and edema formation, thereby exerting a protective role in the nervous tissue system[5,6]. The long-term use of remimazolam can facilitate glial cell apoptosis and reduce the Gamma (γ)-Aminobutyric Acid (GABA) neurons of nerve cells[7]. SLIT/ROBO4 signaling pathway has been proved to be an extracellular protein, which has the functions of controlling neuronal migration and repulsion, guiding the direction of neuron axons, etc[8]. This research investigated the changes of SLIT/ROBO4 signaling pathway in rats under remimazolam treatment and aimed to clarify the effect of remimazolam on neurons in the rat brain and its related effects.
Materials and Methods
Animals and drugs:
A total of 16 Specific Pathogen Free (SPF) 3 w old male Wistar rats (200-250 g) were purchased from Kaixue Biotechnology (Shanghai) Co., Ltd. and raised in the laboratory animal center of our hospital.
Breeding conditions: Change bedding twice a week, free food (standard basal feed) and ordinary drinking water, 4 per cage, well ventilated, temperature (20°±4°), humidity (60 %±20 %), indoor clean and quiet. Rats were acclimated for 1 w before the experiments.
This study was approved by the laboratory animal ethics committee. Remimazolam tosilate for injection was purchased from Jiangsu Hengrui Medicine Co., Ltd. The channel water maze was made of black plexiglass (developed by the Institute of Materia Medica, Chinese Academy of Medical Sciences).
Primary rat cortical neuron culture:
The rats were sacrificed, the brains were taken and the brain tissue was separated and then placed in D-Hank’s solution. The cerebral cortex was separated and cut into tissue fragments of 1 mm×1 mm×1 mm and then placed in a 15 ml centrifuge tube. An equal amount of 0.125 % trypsin, final concentration of 0.0625 %, was digested in a Carbon Dioxide (CO2) incubator for 12 min and shaken once at 6 min. After 12 min, an equal amount of inoculum (Dulbecco’s Modified Eagle Medium (DMEM), 10 % Fetal Bovine Serum (FBS) and 10 % Human Serum (HS)) was added to stop the trypsin digestion reaction. The supernatant was discarded after centrifugation. 2-3 ml of inoculation solution was added and gently pipetted 2-3 times and filtered through a 200 mesh filter. The obtained cell suspension was collected. The density of cells was counted and calculates with a hemocytometer under an inverted microscope. The cell density was adjusted to 1×106/ml with the inoculation solution and then inoculated into a 6 well plate (2 ml/well) pretreated with polylysine. The 6 well plates were cultured in an incubator with 5 % CO2 at 37°. After 24 h, the impurities were washed with sterile Phosphate-Buffered Saline (PBS) buffer and replaced with a neuron-specific medium (96 % neurobasal+2 % B27+2 % glutamine). The neuronspecific medium was used every 2-3 d and the medium was changed once every 2-3 d. The primary rat cortical neurons that were cultured for 7 d were identified by immunohistochemical staining using the neuronspecific protein Neuron-Specific Enolase (NSE) and the positive expression rate of more than 90 % could be used for following experiments. The successfully cultured primary rat cortical neurons were divided into control group and remimazolam group by random number table method.
Establishment of apoptosis model:
After the remimazolam anesthesia machine was tested, the remimazolam reagent was added to the vaporizer. Then the cells in remimazolam group were placed in a sterile special airtight container, the air outlet was connected to the analyzer and the air inlet (5 % CO2) was connected to the anesthesia machine. The container was placed in a constant-temperature water bath at 37° and the vaporizer was rotated to the specified scale according to the experiment. When the concentration reached the experimental requirements, the vaporizer was closed, the container was sealed. Each group was treated for 6 h. After treatment, cells were taken out from the airtight container and put into the incubator to continue culturing.
3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyl Tetrazolium Bromide (MTT):
Primary rat cortical neurons were seeded in 96-well plates (at a density of 1×104 cells) and incubated for 24 h. After cells adhered, Roswell Park Memorial Institute (RPMI)-1640 medium containing 1 % FBS was serum-starved for 2 h and then 1 μl of remimazolam at different concentrations (2, 4 and 8 μmol/l) was added to each well and incubated for 24 h (eight duplicate wells were set at each concentration range and 1 μl Dimethyl Sulfoxide (DMSO) was added to the solvent control group). 15 μl of MTT was added to each well for another 2-4 h of incubation. The culture medium was discarded, 100 μl of DMSO was added and the shaker was protected from light and mixed for 15 min.
Then, the Absorbance (A490) value was measured by Enzyme-Linked Immunosorbent Assay (ELISA) and the survival rate of primary cortical neurons in each group was calculated. The experiment was repeated 3 times in each group. Cell survival rate=(A490 value in remimazolam group-A490 value in zero adjustment group)/(A490 value in control group-A490 value in zero adjustment group)×100 %.
Flow cytometry analysis:
Primary rat cortical neurons were seeded in 6 well plates (1×105 cells per well). After the cells were treated with remimazolam at a final concentration of 8 μmol/l for different time periods (0, 3, 6, 12 and 24 h), the cells were collected in centrifuge tubes. Primary rat cortical neurons were stained according to the instructions of Annexin V-Fluorescein Isothiocyanate (V-FITC) cell apoptosis detection kit (Beyotime). After incubation in the dark at room temperature for 20 min, the cells were suspended in 500 μl of PBS and transferred to a flow tube. The early apoptosis and late apoptosis of cells were detected by flow cytometry. CytExpert 1.2 software was used to count the apoptosis of cells in each time period.
Western blot for apoptosis-related proteins:
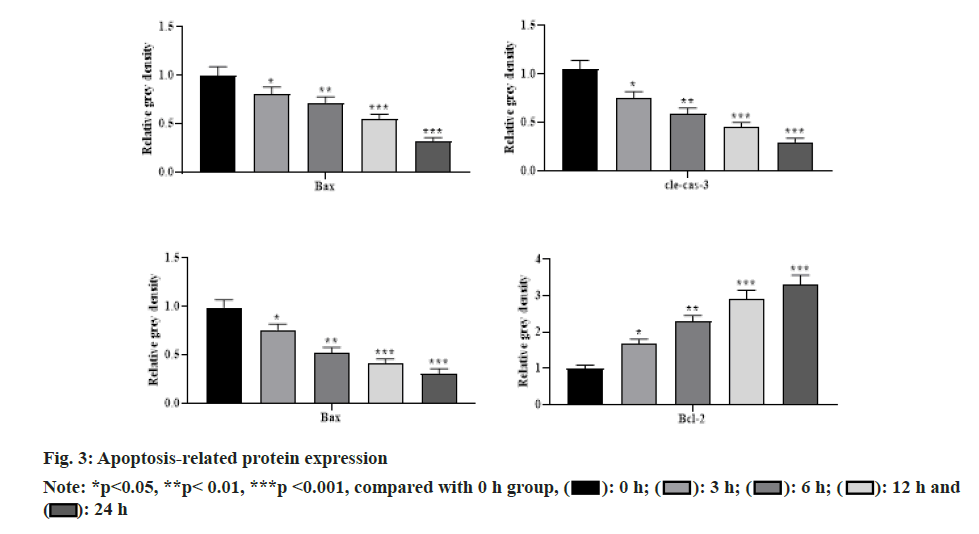
Primary rat cortical neurons treated with remimazolam for different time (0, 3, 6, 12 and 24 h) were collected in centrifuge tubes. Protein was extracted and 30 μg was loaded after denaturation. Proteins were separated by 10 %-12 % Sodium Dodecyl Sulfate- Polyacrylamide Gel Electrophoresis (SDS-PAGE) and transferred to Nitrocellulose (NC) membrane semi-dry. The membrane was blocked with 5 % skim milk for 1.5 h at room temperature and washed 5 times with Tween® 20 Detergent (TBST) for 5 min each. Primary antibodies such as Bcl-2-Associated X Protein (BAX), B-cell lymphoma 2 (Bcl-2), cleaved-caspase-3 (clecas- 3), cleaved-Poly Adenosine Diphosphate-Ribose Polymerase (cle-PARP) and Beta (β)-actin (dilution ratio of 1:2500) were added and incubated overnight on a shaker (4°). The corresponding primary antibody was withdrawn, washed 5 times with TBST and the secondary antibodies Horseradish Peroxidase (HRP) labeled goat anti-rabbit and anti-mouse Immunoglobulin G (IgG) were added to incubate for 2 h at room temperature on a shaker. β-actin was used as the internal reference. ImageJ 1.42q software was used for grayscale analysis.
Western blot for SLIT2 and ROBO4 expression:
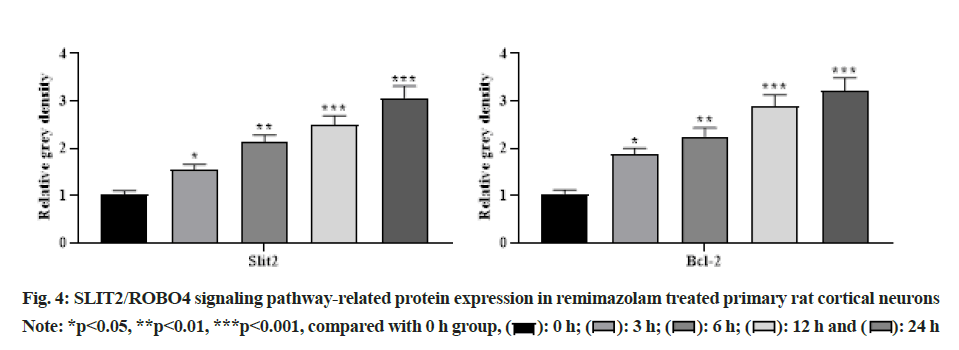
Cells or tissues were collected and lysed with lysis buffer Radioimmunoprecipitation Assay (RIPA). Total protein was extracted with TRIZOL (Total Ribonucleic Acid (RNA) isolation) reagent by boiling method and protein was quantified with a Bicinchoninic Acid (BCA) kit. Total proteins were separated by 10 % SDS-PAGE, transferred to Polyvinylidene Fluoride (PVDF) membrane, blocked with 5 % Bovine Serum Albumin (BSA) for 45 min and incubated with primary antibody overnight at 4°. Then the secondary antibody was added, shaken for 45 min and developed by Electrochemiluminescence (ECL). Both SLIT2 and ROBO4 antibodies were purchased from Abcam United States of America (USA) and the dilution ratio was 1:1000.
Statistical analysis:
Statistical Package for Social Sciences (SPSS) 20.0 software was used for statistical analysis. Measurement data were expressed as mean±standard deviation (x±s). The t-test was used for comparison between groups, with test level of Alpha (α)=0.05. p<0.05 indicated that the difference was statistically significant.
Results and Discussion
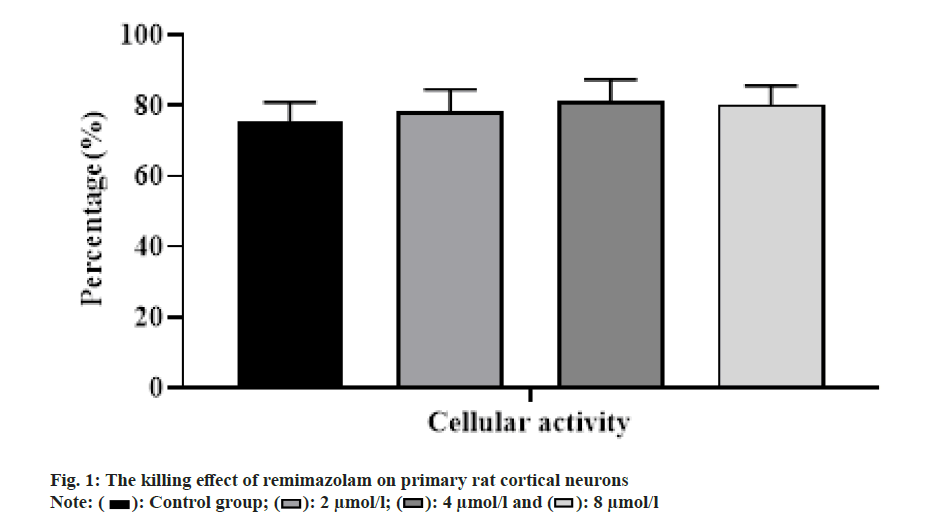
With the increasing concentration of remimazolam (2, 4 and 8 μmol/l), cell activity in each remimazolam concentration group presented elevation. The Half-maximal inhibitory concentration (IC50) of remimazolam was 8 μmol/l. There was no significant difference relative to control group (p>0.05) as shown in fig. 1. Relative to control group, apoptosis rate in each remimazolam concentration group showed a marked decline in a dose-dependent manner (p<0.05). Moreover, flow cytometry detected cell apoptosis after different treatment time (0, 3, 6, 12 and 24 h). With prolongation of remimazolam treatment time, the number of early-apoptotic and late-apoptotic primary rat cortical neurons showed a gradual decline in a certain time-dependent manner as shown in fig. 2. Additionally, Western blot detected apoptosis-related protein levels at the molecular level. With increase of remimazolam treatment time, BAX, cle-cas-3 and cle-PARP protein levels showed a gradual decline and Bcl-2 level showed a gradual elevation relative to 0 h as shown in fig. 3. To clarify molecular mechanism underlying remimazolam-triggered primary rat cortical neuronal apoptosis, Western blot detected SLIT2/ ROBO4 signaling pathway-related protein expression. With the increase of remimazolam treatment time, SLIT2 and ROBO4 protein expression increased continuously relative to 0 h as shown in fig. 4.
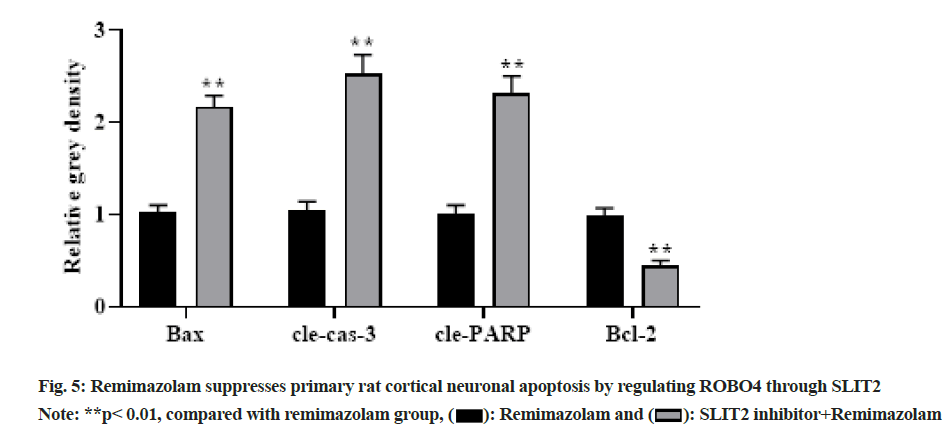
To further clarify relationship of SLIT2 and ROBO4, primary rat cortical neurons were administrated with remimazolam at a final concentration of 8 μmol/l for 24 h under SLIT2 inhibitor pretreatment and Western blot measured related protein levels. With prolongation of remimazolam treatment time, BAX, cle-cas-3 and cle-PARP showed a gradual up regulation and Bcl-2 showed a gradual down regulation in SLIT2 inhibitor and remimazolam co-treatment group relative to remimazolam group as shown in fig. 5, indicating that remimazolam represses primary rat cortical neuronal apoptosis via regulation of ROBO4 through SLIT2. Remimazolam, a short-acting intravenous anesthetic commonly used in clinical practice in various departments, has the advantages of quick recovery, less adverse reactions, rapid onset of action, no accumulation in continuous infusion, etc.[9,10]. Nevertheless, its mechanism of action has not yet been elucidated. Remimazolam is developed on the basis of midazolam, which has better efficacy and safety[11], better anesthesia effect[12] and remimazolam can also act on GABA receptors to reduce neuronal excitation. There is no accumulation in the body and the retention time is shorter than that of midazolam, which can induce less movement of rats, thereby exerting a better sedative role[13,14].
Herein, MTT colorimetry demonstrated that remimazolam effectively suppressed primary rat cortical neuronal apoptosis and enhance viability in a dose-dependent manner. The generation and transmission of neural excitation have a strong association with neuronal cell viability and apoptosis, and apoptosis exists in most neurons. Herein, MTT flow cytometry and Western blot demonstrated that remimazolam repressed primary cortical neuronal apoptosis in rats. SLIT2 signaling exerts a crucial role in modulating various cellular biological behaviors such as cell growth, proliferation, apoptosis, etc. In addition, ROBO4 signaling can transfer extracellular signals into nucleus and induce target gene activation. Previously, both SLIT2 and ROBO4 reduced neuronal and glial cell death via suppressing anti-inflammatory role of leukocytes or even directly protecting neurons/ glial cells, but the joint action of the two and its mechanism remain elusive[15,16]. Herein, to clarify molecular mechanism of remimazolam suppressing primary rat cortical neuronal apoptosis, Western blot measured SLIT2/ROBO4 signaling pathway-related protein expression and SLIT2 inhibitor was further administrated to clarify SLIT2 and ROBO4 signaling pathway. As a result, remimazolam suppressed primary rat cortical neuronal apoptosis through the SLIT2/ ROBO4 signaling pathway and SLIT2 exerts regulation on ROBO4 signaling pathway.
In conclusion, remimazolam pretreatment may suppress cortical neuronal apoptosis in rats through the SLIT2/ ROBO4 signaling pathway, providing new ideas for clinical brain protection.
Acknowledgments:
The work was supported by the Guilin City Technology Application and Promotion Plan (No. 2020011207- 10), Beijing Medical Award Foundation (No. YXJL- 2021-0307-0445); Xiao Ling Li and Dong Qin have contributed equally to this work.
Conflict of interests:
The authors declare that they have no competing interests.
References
- Thanavel M, Bankole PO, Selvam R, Govindwar SP, Sadasivam SK. Synergistic effect of biological and advanced oxidation process treatment in the biodegradation of Remazol yellow RR dye. Sci Rep 2020;10(1):1-9.
[Crossref] [Google Scholar] [Pub Med]
- Wang S, Fu G, Li J, Wei X, Fang H, Huang D, et al. High-efficiency secretion and directed evolution of chitinase bcchia1 in Bacillus subtilis for the conversion of chitinaceous wastes into chitooligosaccharides. Front Bioeng Biotechnol 2020;8:432.
[Crossref] [Google Scholar] [Pub Med]
- Jankowska K, Zdarta J, Grzywaczyk A, Kijeńska-Gawrońska E, Biadasz A, Jesionowski T. Electrospun poly (methyl methacrylate)/polyaniline fibres as a support for laccase immobilisation and use in dye decolourisation. Environ Res 2020;184:109332.
[Crossref] [Google Scholar] [Pub Med]
- Aryanti N, Nafiunisa A, Kusworo TD, Wardhani DH. Micellar-enhanced ultrafiltration using a plant-derived surfactant for dye separation in wastewater treatment. Membranes 2020;10(9):220.
[Crossref] [Google Scholar] [Pub Med]
- Góralczyk-Bińkowska A, Jasińska A, Długoński A, Płociński P, Długoński J. Correction: Laccase activity of the ascomycete fungus Nectriella pironii and innovative strategies for its production on leaf litter of an urban park. Plos One 2020;15(5):e0233553.
[Crossref] [Google Scholar] [Pub Med]
- Weng J, Zhou X, Xie H, Gao Y, Wang Z, Gong Y. Slit2/Robo4 signaling pathway modulates endothelial hyper-permeability in a two-event in vitro model of transfusion-related acute lung injury. Blood Cells Mol Dis 2019;76:7-12.
[Crossref] [Google Scholar] [Pub Med]
- Yu J, Zhang X, Kuzontkoski PM, Jiang S, Zhu W, Li DY, et al. Slit2N and Robo4 regulate lymphangiogenesis through the VEGF-C/VEGFR-3 pathway. Cell Commun Signal 2014;12(1):1-5.
- Bekes I, Haunerdinger V, Sauter R, Holzheu I, Janni W, Woeckel A, et al. Slit2/Robo4 signaling: Potential role of a VEGF-antagonist pathway to regulate luteal permeability. Geburtshilfe Frauenheilkd 2017;77(1):73-80.
[Crossref] [Google Scholar] [Pub Med]
- Lee A, Shirley M. Remimazolam: A review in procedural sedation. Drugs 2021;81(10):1193-201.
[Crossref] [Google Scholar] [Pub Med]
- Noor N, Legendre R, Cloutet A, Chitneni A, Varrassi G, Kaye AD. A comprehensive review of remimazolam for sedation. Health Psychol Res 2021;9(1):24514.
[Crossref] [Google Scholar] [Pub Med]
- Wang F, Zhou Q, Shen M, Quan J, Chen J, Shi J, et al. Efficacy and safety of remimazolam in procedural sedation and analgesia: A protocol for systematic review and meta-analysis. Medicine 2020;99(27):e20765.
[Crossref] [Google Scholar] [Pub Med]
- Chen W, Chen S, Huang Y. Induction and maintenance of procedural sedation in adults: Focus on remimazolam injection. Expert Rev Clin Pharmacol 2021;14(4):411-26.
[Crossref] [Google Scholar] [Pub Med]
- Zhu X, Wang H, Yuan S, Li Y, Jia Y, Zhang Z, et al. Efficacy and safety of remimazolam in endoscopic sedation-A systematic review and meta-analysis. Front Med 2021;8:655042.
[Crossref] [Google Scholar] [Pub Med]
- Kim KM. Remimazolam: Pharmacological characteristics and clinical applications in anesthesiology. Anesth Pain Med 2022;17(1):1-11.
[Crossref] [Google Scholar] [Pub Med]
- Jiang Z, Liang G, Xiao Y, Qin T, Chen X, Wu E, et al. Targeting the SLIT/ROBO pathway in tumor progression: Molecular mechanisms and therapeutic perspectives. Ther Adv Med Oncol 2019;11:1-16.
[Crossref] [Google Scholar] [Pub Med]
- Dai C, Gong Q, Cheng Y, Su G. Regulatory mechanisms of Robo4 and their effects on angiogenesis. Biosci Rep 2019;39(7):BSR20190513.
[Crossref] [Google Scholar] [Pub Med]

 ): Control group; (
): Control group; ( ): 2 μmol/l; (
): 2 μmol/l; ( ):
4 μmol/l and (
):
4 μmol/l and ( ): 8 μmol/l
): 8 μmol/l