- *Corresponding Author:
- Rui Zhao
Department of Anesthesiology, Baoji Central Hospital, Baoji, Shaanxi Province 721008, China
E-mail: 18328326700@163.com
| Date of Received | 05 January 2023 |
| Date of Revision | 27 October 2023 |
| Date of Accepted | 15 May 2024 |
| Indian J Pharm Sci 2024;86(3):1069-1075 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Remifentanil has anti-tumor effects, but its effects on colorectal cancer are unclear. Therefore, the present study investigated whether remifentanil mediates colorectal cancer cell malignant behaviors through the modulation of chemokine ligand 2-C-C chemokine receptor 2 signaling. SW480 cells were divided into control, low remifentanil dose (0.5 ng/ml), medium remifentanil dose (5.0 ng/ml), high remifentanil dose (50.0 ng/ml), and high remifentanil dose+GW0742 (1.0 μM) groups. Detection of SW480 cell proliferation, apoptosis, and migration was performed by cell counting kit-8, flow cytometry, and wound-healing assays. Bcl-2-associated X protein, proliferating cell nuclear antigen, programmed death-ligand 1, B-cell lymphoma 2, chemokine ligand 2, and C-C chemokine receptor 2 protein levels were examined by Western blot. Remifentanil inhibited SW480 cell proliferation and migration, and facilitated SW480 cell apoptosis in a dose-dependent pattern, accompanied by a striking decrease in proliferating cell nuclear antigen, programmed death-ligand 1, and B-cell lymphoma 2 protein levels and an overt elevation in Bcl-2-associated X protein levels. Bindarit had similar effects as remifentanil, and GW0742 attenuated remifentanil-mediated effects on SW480 cell proliferation, migration, and apoptosis. Remifentanil and bindarit inhibited chemokine ligand 2 and C-C chemokine receptor 2 protein levels, but GW0742 attenuated remifentanil-mediated effects on chemokine ligand 2 and C-C chemokine receptor 2 protein levels. Remifentanil may inhibit colorectal cancer malignant behaviors by inhibiting the chemokine ligand 2/ C-C chemokine receptor 2 signaling.
Keywords
Colorectal cancer, remifentanil, C-C chemokine receptor 2, chemokine ligand 2, Western blot
Colorectal Cancer (CRC) is a common malignancy in the digestive system, including both colon and rectum cancers. The number of CRC incidents in 2020 exceeded 1.93 million cases within worldwide, accounting for 9.7 % of diagnosed malignancies[1]. It is predicted that new cases of CRC will reach 2.5 million worldwide by 2035[2]. As the clinical symptoms of CRC are insidious in the early stage, a large number of patients have already developed into intermediate and advanced stages with poor prognosis when they are first diagnosed[3,4]. The prevalence of CRC tends to be younger, with the cause of death closely related to tumor invasion and distant metastasis[4]. Although aberrant alterations in tumor-suppressive genes and oncogenes have been reported to drive CRC carcinogenesis and progression[5,6], the exact molecular mechanisms involved in CRC pathogenesis and metastasis are unclear. Therefore, searching for molecular mechanisms associated with CRC advancement is a hot topic of current research.
Remifentanil (Rem) is a commonly used anesthetic drug with rapid onset of action and perfect functional recovery[7]. In addition, Rem can be used as an analgesic[8]. Findings have shown that Rem can inhibit postoperative immunosuppression in tumor patients, enhance the body’s immune ability and inhibit tumor metastasis[9]. Some studies have shown that Rem has the anti-proliferative and apoptosis-promoting functions in lung cancer cells and hepatocellular carcinoma[10,11]. As a commonly used clinical drug, the action mechanism of Rem on CRC is still unclear.
Chemokine C-C Motif Ligand 2 (CCL-2) is involved in the regulation of the tumor[12]. CCL-2 works together with C-C Chemokine Receptor 2 (CCR2) to mediate the chemotaxis of some tumorassociated cells, thus promoting the progression of tumor cells[13]. Multiple reports have demonstrated that CCL2 and CCR2 are highly expressed in diverse malignant tumors, and CCL2 binds to CCR2 and activates tumor cells through the CCL2/CCR2 molecular axis, promoting malignant biological processes[14-16]. Moreover, the CCL2/CCR2 axis also recruits Tumor-Associated Macrophages (TAMs) into tumor tissues and promotes tumor progression[15,17]. Obstruction of the CCL2/CCR2 axis promises to be an innovative tactic for malignant tumors.
Therefore, the effect of Rem on CRC cell proliferation, apoptosis, and migration was explored based on the CCL2/CCR2 signaling axis.
Materials and Methods
Cell culture:
Human CRC cell line SW480 (Baililai Biotechnology Co., Ltd., Shanghai, China) was cultured in Roswell Park Memorial Institute (RPMI)-1640 medium (Cat No: FK-QV509, Joe feather Biotechnology Co., Ltd., Shanghai, China) containing 10 % fetal bovine serum at 37° with 5 % Carbon dioxide (CO2). When the cell growth density reached >70 %, the cells were collected and passaged for subsequent studies.
Reagents and instruments:
The Cell Counting Kit-8 (CCK-8) (Cat. No: BN15201-20x500T) from Bairui Biotechnology Co., Ltd., (Beijing, China); Rem (Cat. No: CNPq H20030199) from Yichang Humanwell (China); the annexinV-Fluorescein Isothiocyanate (FITC)/ Propidium Iodide (PI) apoptosis detection kit (Cat. No: FP2050M) from Gaochuang Chemical Technology Co., Ltd., (Shanghai, China); Bindarit (Cat. No. ab143292) from BIOCREATIVE (Beijing, China); total protein extraction kit (Cat. No. MBP0314-100T) from Bihe Biochemical Technology Co., Ltd (Shanghai, China); GW0742 (Cat. No. M2742-50 mg) from Kehao Biotechnology Co., Ltd (Wuhan, China); the Bicinchoninic Acid (BCA) kit (Cat. No. YT8173) from Ita Biotechnology Co., Ltd (Beijing, China); an antibody against BCL-2-Associated X Protein (BAX) (Cat. No. FS-1098) from Fusheng Industrial Co., Ltd (Shanghai, China); antibodies against Programmed Death-Ligand 1 (PD-L1) (Cat. No. PL0403171), B-Cell Lymphoma 2 (Bcl-2) (Cat. No: 251711), CCL2 (Cat. No: PL0304039), and CCR2 (Cat. No: 250580) from Otwo Biotech (Shenzhen, China); an antibody against Proliferating Cell Nuclear Antigen (PNCA) (Cat. No: orb824257) from Biorbyt (Wuhan, China); skimmed milk powder (Cat. No: BB-35122) from Bestbio (Shanghai, Cina); crystal purple (Cat. No: Js19004-25g) Yanjin Biotechnology Co., Ltd., (Shanghai, China); gel imager (Cat. No: EBOX CX5) from Hecheng Technology Co., Ltd., (Wuhan, China); the CO2 cell culture incubator (Thermo) from Fuze Trading Co., Ltd., (Shanghai, China); the flow cytometer (BD FACSCALIBUR) from Exsson (Beijing, China); the microplate reader (Cat. No: 51119080) from Shanghai Sunshine Biotechnology Co., Ltd., (Shanghai, China).
Cell grouping:
Normal cultured SW480 cells were used as the control group. SW480 cells cultured in a medium containing different concentrations of Rem were divided into the low-dose Rem group (0.5 ng/ml, L-Rem), the medium-dose Rem group (5.0 ng/ml, M-Rem), and the high-dose Rem group (50.0 ng/ ml, H-Rem), respectively. SW480 cells cultured with Bindarit (the CCL2/CCR2 signaling inhibitor) at a concentration of 300 μM were divided into the Bindarit group. SW480 cells cultured with Rem at a concentration of 50.0 ng/ml and GW0742 (the CCL2/CCR2 signaling activator) at a concentration of 1.0 μM were divided into the H-Rem+GW0742 group.
Detection of SW480 cell pr oliferation:
Trypsin digestion was executed when SW480 cells in each group grew to the logarithmic phase. Digested cells were cultured for 48 h. Following incubation with the CCK-8 solution, the absorbance values at 450 nm were detected on a microplate reader.
Measurement of SW480 cell apoptosis:
SW480 cells cultured for 48 h were digested with trypsin, followed by washing with pre-cooled Phosphate Buffer Solution (PBS). The precipitate obtained by centrifugation was supplemented with binding buffer, followed by the addition of AnnexinV-FITC and PI. The reaction was carried out under dark conditions for 10 min, and apoptosis was detected by flow cytometry.
Determination of the migratory ability of SW480 cells:
SW480 cells grown to log phase were inoculated in 6-well plates. 24 h later, the 6-well plates were lightly scratched with the tip of the pipette tip to create a wound. The cell migration was observed under an inverted microscope at 0 h and 48 h after scratching, respectively. The healing rate (%) of the scratches was also calculated.
Western blot:
The cultured SW480 cells from each group were collected, followed by the addition of a certain amount of protein lysate on ice for 30 min. The lysed cells were centrifuged, the supernatant was collected, and the total protein of the cells in each group was extracted with the total protein extraction kit, and their concentrations were determined according to the BCA kit instructions. The protein extracts were separated using Sodium dodecyl- Sulfate Polyacrylamide Gel Electrophoresis (SDSPAGE) gel electrophoresis, followed by transfer to Polyvinylidene Difluoride (PVDF) membrane. After sealing using 5 % skimmed milk powder, the membranes were incubated with primary antibodies against BAX, PNCA, PD-L1, Bcl-2, CCL2, CCR2, or Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH). After overnight incubation at 4°, the membranes were incubated with secondary antibodies. The membrane was visualized using a chemiluminescent detection system.
Western blot:
All experiments were performed in triplicate. Statistical analysis were performed using GraphPad Prism software (version 7.0, United States of America (USA)). All data are expressed as mean±standard deviation. The two groups were compared using the t-test or Mann-Whitney U-test, depending on normal distribution. Other experiments used ordinary one-way Analysis of Variance (ANOVA). p<0.05 was considered statistically significant.
Results and Discussion
A gradual decrease in the Optical Density (OD) values of SW480 cells was observed in the L-Rem, M-Rem, and H-Rem groups in comparison to the control group with an increase in the Rem dose (p<0.05). The inhibitor of CCL2/CCR2 signaling, bindarit, resulted in a conspicuous reduction in the OD values of SW480 cells relative to the control group (p<0.05). Notably, GW0742, an agonist of CCL2/CCR2 signaling, elevated the OD values of H-Rem treated SW480 cells significantly compared to the H-Rem group (p<0.05) (Table 1).
| Group | OD values |
|---|---|
| Control | 0.97±0.18 |
| L-Rem | 0.72±0.16* |
| M-Rem | 0.43±0.13*# |
| H-Rem | 0.12±0.04*#& |
| Bindarit | 0.13±0.03*#& |
| H-Rem+GW0742 | 0.81±0.17@ |
Note: *p<0.05 vs. control; #p<0.05 vs. L-Rem; &p<0.05 vs. M-Rem and @p<0.05 vs. H-Rem
Table 1: Proliferative Capacity of SW480 Cells was Inhibited by Rem Through Inhibition of the CCL2/CCR2 Signaling
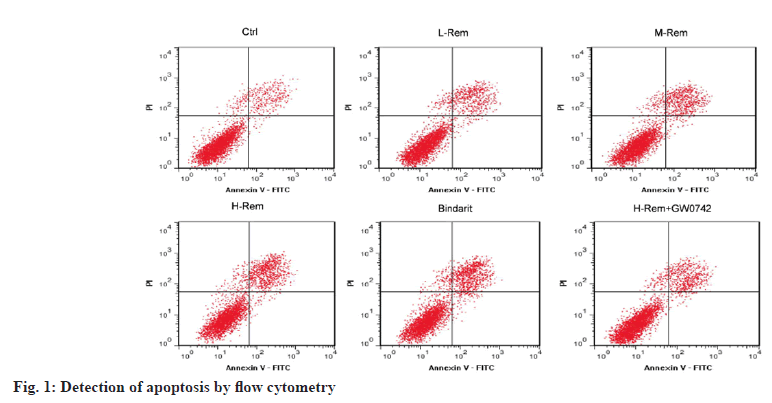
In contrast to the control group, L-Rem, M-Rem, and H-Rem resulted in a gradual increase in SW480 cell apoptosis with growing doses of Rem (p<0.05). Bindarit also led to an improvement in SW480 cell apoptosis in comparison to the control group (p<0.05). It was noteworthy that the usage of GW0742 significantly declined H-Rem treated SW480 cell apoptosis vs. the H-Rem group (p<0.05) (fig. 1 and Table 2).
| Group | Apoptosis (%) |
|---|---|
| Control | 12.37±1.76 |
| L-Rem | 19.52±2.34* |
| M-Rem | 26.51±2.81*# |
| H-Rem | 32.67±3.42*#& |
| Bindarit | 33.05±3.67*#& |
| H-Rem+GW0742 | 18.26±1.98@ |
Note: *p<0.05 vs. control; #p<0.05 vs. L-Rem; &p<0.05 vs. M-Rem and @p<0.05 vs. H-Rem
Table 2: Rem Facilitated SW480 Cell Apoptosis
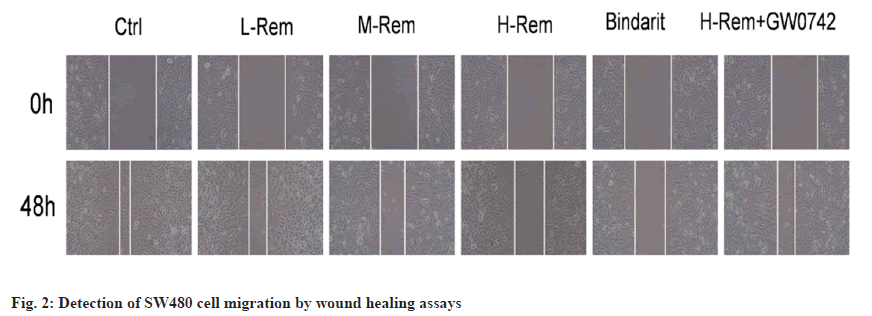
The migratory ability of SW480 cells was gradually inhibited by Rem with the increase of dose (p<0.05). Bindarit also inhibited SW480 cell migration vs. the control group (p<0.05). Comparing with the H-Rem group, GW0742 promoted the migration of H-Rem treated SW480 cells (p<0.05) (fig. 2 and Table 3).
| Group | Wound healing rate (%) |
|---|---|
| Control | 76.28±8.35 |
| L-Rem | 63.17±7.16* |
| M-Rem | 50.16±6.98*# |
| H-Rem | 36.59±4.71*#& |
| Bindarit | 37.61±4.74*#& |
| H-Rem+GW0742 | 63.69±7.26@ |
Note: *p<0.05 vs. control; #p<0.05 vs. L-Rem; &p<0.05 vs. M-Rem and @p<0.05 vs. H-Rem
Table 3: Rem Repressed SW480 Cell Migration
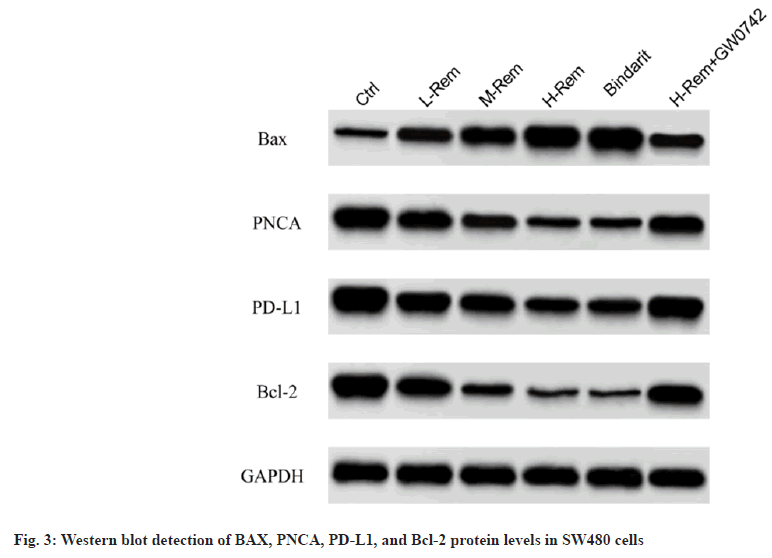
By comparison with the control group, Rem caused a prominent improvement in BAX protein levels as well as a reduction in PNCA, PD-L1, and Bcl-2 protein levels in SW480 cells in a concentrationdependent model (p<0.05). The effects of bindarit on BAX, PNCA, PD-L1, and Bcl-2 proteins were consistent with Rem (p<0.05). However, GW0742 impaired the effects of H-Rem on the above protein alterations (p<0.05) (fig. 3 and Table 4).
| Group | BAX | PNCA | PD-L1 | Bcl-2 |
|---|---|---|---|---|
| Control | 0.37±0.07 | 1.46±0.16 | 1.79±0.18 | 1.46±0.29 |
| L-Rem | 0.75±0.13* | 1.16±0.13* | 1.36±0.14* | 1.05±0.25* |
| M-Rem | 1.06±0.18*# | 0.81±0.11*# | 1.02±0.11*# | 0.56±0.15*# |
| H-Rem | 1.52±0.21*#& | 0.46±0.06*#& | 0.65±0.07*#& | 0.19±0.05*#& |
| Bindarit | 1.59±0.23*#& | 0.49±0.08*#& | 0.68±0.09*#& | 0.18±0.04*#& |
| H-Rem+GW0742 | 0.70±0.09@ | 1.08±0.12@ | 1.32±0.14@ | 1.19±0.27@ |
Note: *p<0.05 vs. control; #p<0.05 vs. L-Rem; &p<0.05 vs. M-Rem and @p<0.05 vs. H-Rem
Table 4: Effects Of Rem On Apoptosis-Related Proteins In Sw480 Cells
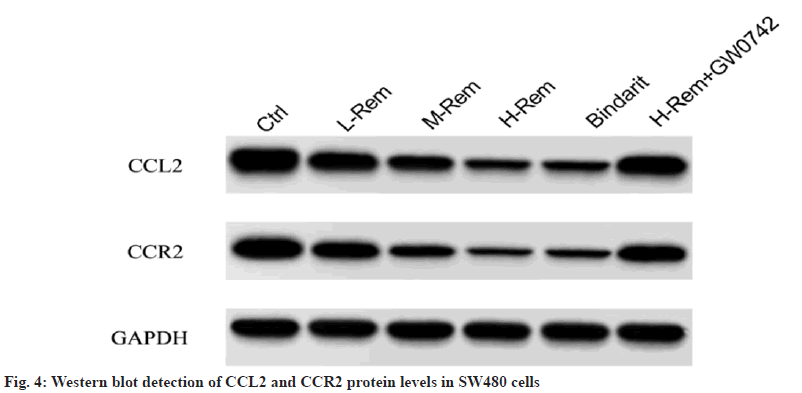
In contrast to the control group, Rem reduced CCL2 and CCR2 protein levels in a concentrationdependent model (p<0.05). Consistently, bindarit also reduced CCL2 and CCR2 protein levels (p<0.05). However, GW0742 attenuated the effect of H-Rem on the above-mentioned protein changes (p<0.05) (fig. 4 and Table 5).
| Group | CCL2 | CCR2 |
|---|---|---|
| Control | 1.57±0.29 | 1.32±0.25 |
| L-Rem | 1.15±0.23* | 0.91±0.16* |
| M-Rem | 0.76±0.17*# | 0.57±0.15*# |
| H-Rem | 0.33±0.10*#& | 0.26±0.07*#& |
| Bindarit | 0.37±0.12*#& | 0.30±0.09*#& |
| H-Rem+GW0742 | 1.25±0.26@ | 0.95±0.21@ |
Note: *p<0.05 vs. control; #p<0.05 vs. L-Rem; &p<0.05 vs. M-Rem and @p<0.05 vs. H-Rem
Table 5: Effects of Rem on the CCL2 and CCR2 Signaling in SW480 Cells
CRC is the 4th and 5th causes of malignancy-related deaths in women and males in our country[18]. There are many factors contributing to CRC, which are generally believed to be related to heredity and lifestyle habits. Surgical resection is the main treatment modality used in clinical practice, with targeted therapy as well as chemotherapy as adjuvant treatments[19]. Owing to the improvement of surgical techniques and advances in adjuvant treatments such as chemotherapy, the 5 y survival of CRC patients has also increased from 50 % to 65 %, but it still fails to achieve the expected results[20]. The molecular mechanisms involved in CRC development are still unclear; therefore, in-depth studies on the underlying mechanisms associated with its development are needed.
Rem, as an opioid, can be hydrolyzed by nonspecific esterases[21]. Previously, Rem has been used as an anesthetic. Recently, it has been found to play a role in tumors. A study reported that Rem exhibits an anti-tumor effect in gastric cancer[22]. In HepG2 cells, Rem restrains cell proliferation and accelerates cell apoptosis[23]. Here, Rem decreased OD values, scratch healing rates, and the levels of PNCA and Bcl-2 proteins, but increased apoptosis rates and BAX protein levels, indicating that Rem can promote SW480 cell apoptosis and restrain SW480 cell proliferation and migration. PD-L1, the ligand of PD-1, is a member of tumor immunosuppression[24]. PD-1 participates in the immune check, and overexpression of PD-1 in tumor cells increases cell immune tolerance, resulting in a favorable immune escape for tumor cells[25]. In this study, Rem was found to reduce PD-L1 protein levels in a dose-dependent pattern, suggesting that Rem can downregulate PD-L1 expression and restrain SW480 cells from immune escape.
PD-1 can play a role in the CCL2/CCR2 signaling axis, thus contributing to the immune escape of cancer cells[13]. CCL2 is an important factor associated with the immune system, and the CCL2/ CCR2 signaling is thought to be closely related to tumor progression, and this signal is frequently used in clinical practice, such as for early detection and postoperative prediction[26]. Liu et al.[2] discovered that the CCL2/CCR2 axis facilitates breast cancer development. Ding et al.[27] found that the CCL2/ CCR2 axis takes part in lung cancer development. A previous study exposed that the upregulation of CCL2 has a promoting effect on CCR2 expression, resulting in the activation of the CCL2/CCR2 signaling, facilitating melanoma cell proliferation and migration[28]. Our results showed that Rem caused a significant decrease in CCL2 and CCR2 protein levels in SW480 cells, suggesting that Rem may play a role by modulating the CCL2/CCR2 axis. Bindarit (CCL2/CCR2 signaling inhibitor) had a similar effect with H-Rem, whereas H-Rem combined with GW0742 significantly increased CCL2 and CCR2 protein levels compared to H-Rem alone. The addition of GW0742 abolished Rem-mediated inhibitory effects on SW480 cell proliferation and migration as well as promoting effects on SW480 cell apoptosis.
In summary, Rem might suppress CRC cell proliferation and migration, as well as promote CRC cell apoptosis by repressing the CCL2/CCR2 signaling. However, this study was only conducted on CRC cells in vitro, and in vivo experiments will be carried out in the future to verify the above results.
Conflict of interests:
The authors declared no conflict of interests.
References
- Xi Y, Xu P. Global colorectal cancer burden in 2020 and projections to 2040. Transl Oncol 2021;14(10):101174.
[Crossref] [Google Scholar] [PubMed]
- Liu Y, Cheng L, Li C, Zhang C, Wang L, Zhang J. Identification of tumor microenvironment-related prognostic genes in colorectal cancer based on bioinformatic methods. Sci Rep 2021;11(1):15040.
[Crossref] [Google Scholar] [PubMed]
- Jain S, Maque J, Galoosian A, Osuna-Garcia A, May FP. Optimal strategies for colorectal cancer screening. Curr Treat Options Oncol 2022;23(4):474-93.
[Crossref] [Google Scholar] [PubMed]
- Boardman LA, Vilar E, You YN, Samadder J. AGA clinical practice update on young adult-onset colorectal cancer diagnosis and management: Expert review. Clin Gastroenterol Hepatol 2020;18(11):2415-24.
[Crossref] [Google Scholar] [PubMed]
- Gong Y, Liu Z, Yuan Y, Yang Z, Zhang J, Lu Q, et al. PUMILIO proteins promote colorectal cancer growth via suppressing p21. Nat Commun 2022;13(1):1627.
[Crossref] [Google Scholar] [PubMed]
- Fang Y, Shen ZY, Zhan YZ, Feng XC, Chen KL, Li YS, et al. CD36 inhibits β-catenin/c-myc-mediated glycolysis through ubiquitination of GPC4 to repress colorectal tumorigenesis. Nat Commun 2019;10(1):3981.
[Crossref] [Google Scholar] [PubMed]
- Hughes LM, Irwin MG, Nestor CC. Alternatives to remifentanil for the analgesic component of total intravenous anaesthesia: A narrative review. Anaesthesia 2023;78(5):620-5.
- Grape S, Kirkham KR, Frauenknecht J, Albrecht E. Intra-operative analgesia with remifentanil vs. dexmedetomidine: A systematic review and meta-analysis with trial sequential analysis. Anaesthesia 2019;74(6):793-800.
[Crossref] [Google Scholar] [PubMed]
- Zhang Y, Jiang W, Luo X. Remifentanil combined with dexmedetomidine on the analgesic effect of breast cancer patients undergoing modified radical mastectomy and the influence of perioperative T lymphocyte subsets. Front Surg 2022;9:1016690.
[Crossref] [Google Scholar] [PubMed]
- Liang W, Ke J. Remifentanil reduces the proliferation, migration and invasion of HCC cells via lncRNA NBR2/miR-650/TIMP3 axis. Int J Exp Pathol 2022;103(2):44-53.
[Crossref] [Google Scholar] [PubMed]
- Chang J, Zhang W. Remifentanil modulates the TLR4-mediated MMP-9/TIMP1 balance and NF-κB/STAT3 signaling in LPS-induced A549 cells. Exp Ther Med 2023;25(2):79.
[Crossref] [Google Scholar] [PubMed]
- Hao Q, Vadgama JV, Wang P. CCL2/CCR2 signaling in cancer pathogenesis. Cell Commun Signal 2020;18(1):82.
[Crossref] [Google Scholar] [PubMed]
- Yang H, Zhang Q, Xu M, Wang L, Chen X, Feng Y, et al. CCL2-CCR2 axis recruits tumor associated macrophages to induce immune evasion through PD-1 signaling in esophageal carcinogenesis. Mol Cancer 2020;19(1):41.
[Crossref] [Google Scholar] [PubMed]
- Kang SU, Cho SY, Jeong H, Han J, Chae HY, Yang H, et al. Matrix metalloproteinase 11 (MMP11) in macrophages promotes the migration of HER2-positive breast cancer cells and monocyte recruitment through CCL2-CCR2 signaling. Lab Investig 2022;102(4):376-90.
[Crossref] [Google Scholar] [PubMed]
- Li X, Yao W, Yuan Y, Chen P, Li B, Li J, et al. Targeting of tumour-infiltrating macrophages via CCL2/CCR2 signalling as a therapeutic strategy against hepatocellular carcinoma. Gut 2017;66(1):157-67.
[Crossref] [Google Scholar] [PubMed]
- Miyamoto T, Murakami R, Hamanishi J, Tanigaki K, Hosoe Y, Mise N, et al. B7-H3 suppresses antitumor immunity via the CCL2–CCR2–M2 macrophage axis and contributes to ovarian cancer progression. Cancer Immunol Res 2022;10(1):56-69.
[Crossref] [Google Scholar] [PubMed]
- Yang Z, Li H, Wang W, Zhang J, Jia S, Wang J, et al. CCL2/CCR2 axis promotes the progression of salivary adenoid cystic carcinoma via recruiting and reprogramming the tumor-associated macrophages. Front Oncol 2019;9:231.
[Crossref] [Google Scholar] [PubMed]
- Xia C, Dong X, Li H, Cao M, Sun D, He S, et al. Cancer statistics in China and United States, 2022: Profiles, trends, and determinants. Chin Med J 2022;135(5):584-90.
[Crossref] [Google Scholar] [PubMed]
- Shinji S, Yamada T, Matsuda A, Sonoda H, Ohta R, Iwai T, et al. Recent advances in the treatment of colorectal cancer: A review. J Nippon Med School 2022;89(3):246-54.
[Crossref] [Google Scholar] [PubMed]
- Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin 2023;73(3):233-54.
[Crossref] [Google Scholar] [PubMed]
- Jeon HO, Choi IS, Yoon JY, Kim EJ, Yoon JU, Cho AR, et al. Effect of remifentanil on pre-osteoclast cell differentiation in vitro. J Dent Anesth Pain Med 2018;18(1):9-17.
[Crossref] [Google Scholar] [PubMed]
- Sun CC, Liu Y, Hu X. Remifentanil inhibits proliferation and promotes apoptosis of gastric cancer cells by regulating miR-519d-3p/STAT3 expression. World Chin J Digestol 2019.
- Deguo XI, Keshi YA, Dahao LU, Chi ZH, Ju GA. Study on remifentanilin inhibiting Notch signal pathway and cell growth of human hepatocarcinoma cells. J Clin Med Pract 2022;26(8):32-6.
- Zhou YJ, Li G, Wang J, Liu M, Wang Z, Song Y, et al. PD-L1: Expression regulation. Blood Sci 2023;5(2):77.
[Crossref] [Google Scholar] [PubMed]
- Jiang X, Wang J, Deng X, Xiong F, Ge J, Xiang B, et al. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol Cancer 2019;18(1):1-7.
[Crossref] [Google Scholar] [PubMed]
- Xu M, Wang Y, Xia R, Wei Y, Wei X. Role of the CCL2/CCR2 signalling axis in cancer: Mechanisms and therapeutic targeting. Cell Prolif 2021;54(10):e13115.
[Crossref] [Google Scholar] [PubMed]
- Ding M, He SJ, Yang J. MCP-1/CCL2 mediated by autocrine loop of PDGF-BB promotes invasion of lung cancer cell by recruitment of macrophages via CCL2–CCR2 axis. J Interferon Cytokine Res 2019;39(4):224-32.
[Crossref] [Google Scholar] [PubMed]
- Pozzi S, Scomparin A, Ben-Shushan D, Yeini E, Ofek P, Nahmad AD, et al. MCP-1/CCR2 axis inhibition sensitizes the brain microenvironment against melanoma brain metastasis progression. JCI Insight 2022;7(17):e154804.
[Crossref] [Google Scholar] [PubMed]