- *Corresponding Author:
- Zheng liu
Department of Gastroenterology, Medical Center for Digestive Diseases, The Second Affiliated Hospital of Nanjing Medical University, Nanjing, Jiangsu Province 210011,
China
E-mail: liuzheng117@126.com
| Date of Received | 27 November 2021 |
| Date of Revision | 26 August 2022 |
| Date of Acceptance | 14 January 2023 |
| Indian J Pharm Sci 2023;85(1):37-43 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To look into how naringenin affects mice's intestinal flora and alleviation from constipation. The four groups control, model, positive drug and naringenin, each with ten mice were formed from the forty mice at random. Lactulose aqueous solution (12 mg/kg) was administered intravenously to the positive drug group whereas naringenin aqueous solution (50 mg/kg) was administered intravenously to the naringenin group once day for 37 d. The model and control groups received 0.2 ml of sterile, double-distilled water as a control. The 31st d marked the beginning of the constipation model. A sterile, once-daily aqueous solution containing 10 mg of loperamide hydrochloride was administered to each of the other 3 groups, except the control group, in amounts of 0.2 ml. After modeling, 0.2 ml of active carbon suspension was gavaged to each mouse and the interval between the first black feces passing and that time was noted. The mice were administered loperamide hydrochloride or sterile double distilled water after a 12 h fast and after fasting for 1 h, they were given 0.2 ml activated carbon suspension. After 30 min, all mice were killed. On the 30th d and 36th d, the mice's fresh stools that were voided within 4 h were collected and, their moisture level and short-chain fatty acid content was assessed. Tumor necrosis factor-alpha, serotonin (5-hydroxytryptamine), 16S ribosomal ribonucleic acid and interleukin-1 beta high-throughput sequencing were measured using enzyme-linked immunosorbent assay and the relative contents of intestinal flora were measured using 16S ribosomal ribonucleic acid high-throughput sequencing. There were differences in the levels of various genera and the model group's Firmicutes/Bacteroidetes ratio and Shannon index were smaller than those of the control group (p<0.05). Contrary to the model group, the positive drug and naringenin group had greater Shannon indices and Firmicutes/Bacteroidetes ratios (p<0.05). The sample points from the naringenin, positive drug and control groups had larger overlap ratios than those from the other groups. As opposed to the naringenin, positive drug and control groups, the sample points of the naringenin, positive drug and control groups could be differentiated. Naringenin could regulate intestinal flora, improve the efficiency of intestinal functioning and protect the intestinal mucus barrier in constipated mice.
Keywords
Naringenin, constipation, intestinal flora, intestinal morphology
One of the most prevalent functional gastrointestinal diseases in clinical practice is constipation, which may show as insufficient feces, difficult feces, reduced frequency of feces, dry and hard stools, etc.,[1]. Relevant studies have shown that about 14 % of people in the world suffer from constipation, mostly in women and the elderly, which has a negative influence on patient’s life quality[2,3]. Gender, age, living habits and drug use are risk factors for constipation[4]. Recent clinical studies have shown that intestinal dysbacteriosis can also lead to constipation[5]. Bacteria attached to the intestinal mucosa can affect intestinal motility, defecation habits and stools weight through metabolic activities[6]. Research by Pimentel et al.[7] depicted that the number of bacteria that can produce methane in the intestine of patients with chronic constipation was increased, while methane can slow down bowel motility, which can lead to the development of constipation. Probiotics can reduce colonic transit time, regulate the balance of flora and achieve the effect of relieving constipation[8]. Bifidobacterium is a common probiotic, accounting for about 3 % of the intestinal flora. It has various physiological functions such as affecting human immune regulation and promoting development. It is often used in the treatment of constipation[9]. Naringenin is a bioflavonoid with several functions including anti-inflammatory and antioxidant. Studies have found that naringenin has protective effects against colon damage by reducing the accumulation of Tumor Necrosis Factor-Alpha (TNF-α) and inhibiting apoptosis, but it has been less studied on constipation. In this study, by constructing a constipation mouse model, the effects of naringenin on the small intestine propulsion rate, fecal moisture content and intestinal flora of constipated mice were observed and compared, in order to investigate how naringenin treats mice with constipation, which are reported below.
Materials and Methods
Tools, reagents and animals used in experiments:
Laboratory animals: The animal use license number is SYXK (Beijing) 2020-0028 and Beijing Amersays Biotechnology Co., Ltd. supplied 40 8 w old Specific- Pathogen-Free (SPF) grade male Bagg and Albino (BALB/c) mice having weights from 18-22 g. The studies were conducted in the SPF home after 1 w of adaptive feeding. During the feeding period, the animals were fed and watered freely. The Code of ethical guidelines for animal experiments was followed in all aspects of the experimentation.
Cells and reagents: Human colon adenocarcinoma Cancer coli-2 (Caco-2) cells and naringenin were bought from cell bioscience Inc., Shanghai and Sigma Corporation, respectively; fetal bovine serums (Item No. 1614231), Trizol reagents (Item No. 223245), Dulbecco's Modified Eagle Medium (DMEM) highsugar culture mediums (Item No. 453231), Gram staining kits (Item No. R40345), were purchased from Thermo Fisher, United States of America (USA); lactulose (Item No. L0223478), purchased from Shenzhen Chem-Strong Biotechnology Co., Ltd.; loperamide hydrochloride (Item No. B4567), was purchased from Shanghai Yuanye Biotechnology Co., Ltd.; mouse 5-Hydroxytryptamine (5-HT), Item No. ml001891), mouse Interleukin-1beta (IL-1β) (Item No. ml301814), mouse (TNF-α, Item No. ml002095), from Shanghai Enzyme Linked Biotechnology Co., Ltd., Enzyme-Linked Immunosorbent Assay (ELISA) detection kits were acquired; bacterial Deoxyribonucleic Acid (DNA) extraction kits (Item No. 51037), were purchased from Wuhan Eproll Biotechnology Co., Ltd.; Shanghai Kemin Biotechnology Co., Ltd. provided the TruSeq® DNA Polymerase Chain Reaction (PCR)-Free HT Sample Preparation Kits (Item No. FC-121-3001), while Shanghai Hengfei Biotechnology Co., Ltd. provided the Phusion high-fidelity PCR master mixes (Item No. F-532S), QIAEX II Gel extraction kits (Item No. DXT-20051 and Suzhou Renold Biotechnology Co., Ltd.
Instrument: Thermo Fisher Company of the US Owl D2 wide gel electrophoresis system, Leica, Germany's optical microscope and laser confocal microscope, Qingdao Juchuang Environmental Protection Group Co., Ltd.'s HN series electro thermal incubator, Beijing Jinda Sunshine Technology Co., Ltd.’s CP153 electronic balance, Qingdao Juchuang Environmental Protection Group Co., Ltd.’s Trace 1300-ISQ 7000 gas (Illumina, USA).
Grouping, administration and modeling:
The positive drug naringenin, control and model groups were created using a randomization process with forty mice each. The model and control groups were given 0.2 ml of sterile double distilled water by gavage, the positive drug group was given 12 mg/kg of lactulose aqueous solution by gavage and the naringenin group was given 50 mg/kg of naringenin aqueous solution by gavage once a day for 37 d. The constipation model was established on the 31st d. An extra 0.2 ml of sterile double-distilled water was given to the control group, while the other three groups received an additional 0.2 ml of 10 mg/kg (body weight) loperamide hydrochloride sterile aqueous solution, once a day for 7 d.
Estimating the moment the first black stools leave the body:
On the 37th d, each group received either sterile double steamed water or loperamide hydrochloride by gavage for 1 h and then each mouse received 0.2 ml of active carbon suspension by gavage and the time of completion of gavage was recorded. The time when the first black stools were excreted was observed and recorded, and the interval between the two recording times was the time when the first black stools were excreted.
Calculating the pace at which the small intestine moves:
After 12 h of fasting, each group discontinued the treatment and received 0.2 ml of an activated carbon solution by gavage 1 h later. The other options were sterile double distilled water or loperamide hydrochloride. After 30 min, all the mice were put to death. The remainder of the intestine, from the pylorus to the end of the rectum, was then totally removed. Then, to assess the small intestine propulsion rate, the difference between the intestinal length and the advance length of the activated carbon suspension in the gut was determined. The ratio of the length of the suspension divided by the ratio of the distance from the pylorus to its front end is known as the small intestine propulsion rate.
Collection of sample:
Fresh stools were collected from mice on 30th d and 36th d within 4 h. The remaining tissues were frozen at -80° while the small intestinal tissues were washed and fixed in 4 % paraformaldehyde for 24 h. The technique was carried out three times. The cecal contents, which had a wet weight of around 1 g, were obtained and the supernatant was obtained by centrifugation at 1000 rpm for 5 min after complete re-suspension with 1 ml of sterile phosphate-buffered saline. For 10 min, the obtained supernatant was centrifuged at 5000 rpm. 1 ml of sterile, doubledistilled water was added to the precipitate after it had been collected and centrifuged for 5 min at 12 000 rpm. For future usage, just the precipitate was kept at -70°.
Determination of moisture content of stools:
Using an electronic balance, weigh and note the moist weight of fresh stools. The stool sample should be placed in a vacuum freeze drier. After drying, weigh the stools, note their dry weight and determine their moisture content. The wet weight of stool divided by the dry weight of stool multiplied by 100 % gives the moisture content of stools.
Determination of cytokine levels in intestinal tissues:
A part of the small intestinal tissue that had been cryopreserved was made into homogenate and the pretreatment was finished in accordance with the guidelines for the ELISA test kit. The experiment to measure IL-1β, 5-HT and TNF-α level was conducted strictly in line with the operational instructions.
High-throughput 16S ribosomal Ribonucleic Acid (16S rRNA) sequencing to measure the diversity of gut flora:
Each group's cecum contents were precipitated, defrosted at room temperature and spun at 12 000 rpm for 10 min. Using a DNA extraction kit, the whole DNA of a microbe was retrieved after the supernatant was discarded. Utilizing the primers 338F (5'-ACTCCTAACGGGGAGGCAG-3') and 806R (5'-GGACTACHVGGGTWTCTAAT-3'), the V3 and V4 regions of the 16S rRNA gene were amplified. After the 94° for 5 min, 50 l reaction system, 30 cycles of 94° for 30 s, 50° for 30 s, 72° for 30 s, 72° for 10 min and 12° for 10 min are carried out. Following the purification of the PCR products by electrophoresis on 1.5 % agarose gel, the 250 base pairs (bp) bands were collected and recovered using a DNA gel recovery kit. Utilizing the TruSeq® DNA PCR-free HT preparative kit, sequencing libraries were created and 250 bp end-to-end sequences were obtained.
To create the final legitimate sequences from the raw sequencing data, quality assurance sequences were employed and the tags were grouped at a 97 % similarity level. Finally, Operational Taxonomic Units (OTUs) were created by combining the tags. Using the Silva database built on the mothur method, taxonomic information about the OTUs was annotated. Multi-sequence comparison was done using the Multiple Sequence Comparison by Log- Expectation (MUSCLE) program. The abundance data for OTUs were normalized using the sample's shortest sequence number as the criteria and the diversity index and diversity analysis were then performed. The Kruskal-Wallis Rank Sum test technique along with the multiple test method FDR was used to screen for the different bacteria, with p value of 0.05 indicating the significant difference. The Wilcoxon Rank-Sum test diversity index was utilized to test the difference between groups.
Statistical analysis:
Data processing and analysis were carried out using Statistical Package for the Social Sciences (SPSS) 22.0 statistical software. The measurement data were represented using the mean (average) and Standard Deviation (SD). While independent sample t-tests were used to compare two groups, one-way Analysis of Variance (ANOVA) was used to examine many groups. p≤0.05 is regarded as statistically significant.
Results and Discussion
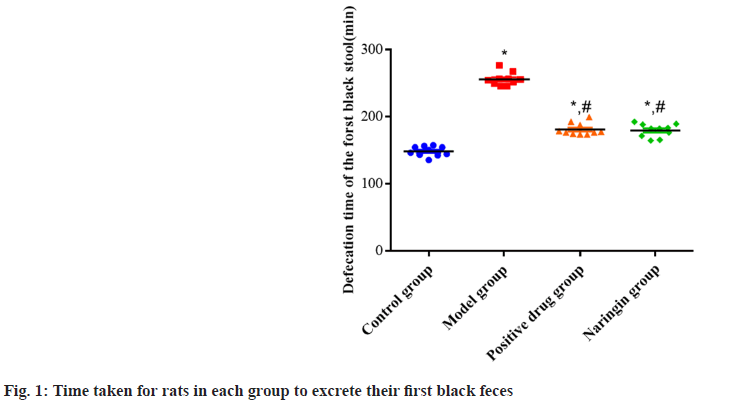
The naringenin, positive drug and model all groups had longer excretion times for the initial black stools than the control group as the p<0.05. However, no major difference has been observed between the groups of naringenin and positive drugs in respect of this measure (p>0.05). The excretion durations of the first black stools in the positive drug and naringenin groups were slowed down compared to the model group as shown in fig. 1.
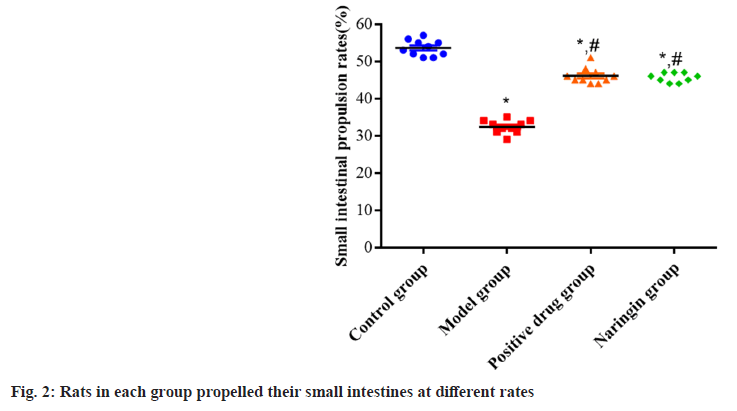
Small intestine propulsion rates were reduced (p<0.05) relative to the control group in the naringenin, positive drug and model groups. Although there was no substantial difference between the groups was found as the p<0.05, the positive drug and naringenin groups both showed higher small intestine propulsion rates when compared to the model group as the p value was found to be more than 0.05 as shown in fig. 2.
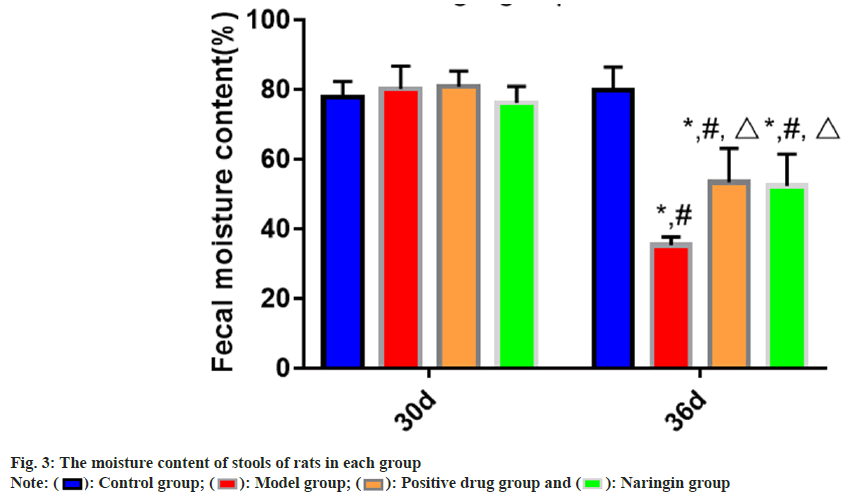
The findings of the intra-group comparison revealed that on 36th d, compared to 30th d, the fecal moisture contents of the naringenin, positive medicine and model groups all reduced (p<0.05). The measurements of fecal moisture content on the 36th d revealed that, in comparison to the control group, fecal moisture contents of the naringenin, positive drug and model groups were decreased (p<0.05); and in comparison, to that of the model group, the stools moisture contents were lower. The comparison of the group’s data showed that on d 30, the quantity of moisture in the feces did not vary between the groups as the p value was found to be more than 0.05 as shown in fig. 3.
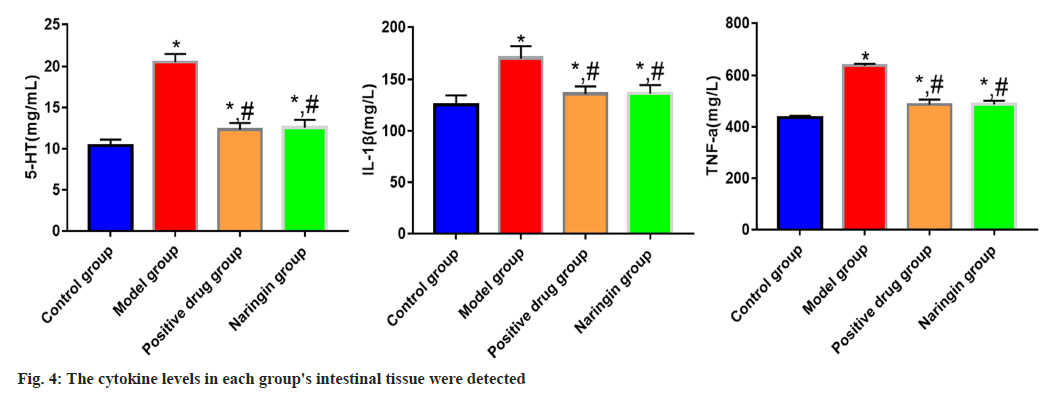
The naringenin, positive drug and model groups all had higher levels of TNF-α, 5-HT and IL-1β expression than the control group (p<0.05); however, the model group's levels of TNF-α, 5-HT and IL-1β expression were lower than those of the positive drug and naringenin groups (p<0.05). TNF-α, IL-1β and 5-HT expression levels between the naringenin group and the positive medication group did not vary significantly (p>0.05) as shown in fig. 4.
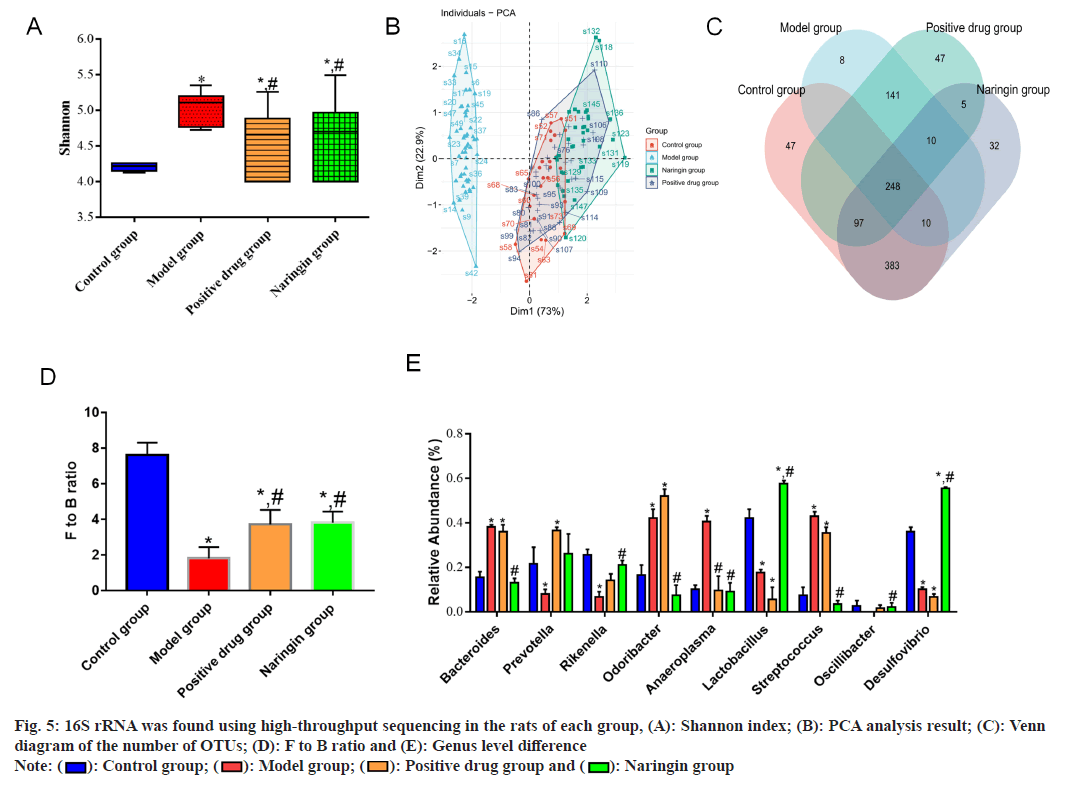
High-throughput 16S rRNA sequencing findings revealed that 40 samples yielded a total of 1256 OTUs. Because the p<0.05, the α-diversity analysis showed that the Shannon index was higher in the positive drug and naringenin groups compared to the model group and lower in the model group compared to the control group. To evaluate the β-diversity, Principal Coordinates Analysis (PCA) was used. Despite the significant overlap ratios of the naringenin, control and positive drug groups' sample points, the findings demonstrated the ability to distinguish between sample points from the model group and those from the naringin, positive drug and control groups. The overall number of OTUs in the naringenin, positive drug, model and control groups was 786, according to the Venn diagram analysis. The gut flora of each group showed that the two most common species were Bacteroidetes and Firmicutes. Firmicutes/Bacteroidetes (F to B) ratios in the positive drug and naringenin groups were greater than those in the model group (p value 0.05), which had a lower F to B ratio than the control group. At the genus level, Bacteroides, Odoribacter, Anaeroplasma and Streptococcus abundances in the model group were greater (p value 0.05), but those of Rikenella, Lactobacillus and Desulfovibrio were lower (p value 0.05) than those of the control group. In the positive drug group, Lactobacillus and Desulfovibrio abundances were reduced, but Bacteroides, Prevotella, Odoribacter and Streptococcus abundances were elevated (p<0.05). As compared to the model group, the abundance of Anaeroplasma the positive drug group was decreased (p<0.05), as were the abundance of Bacteroides, Odoribacter, Anaeroplasma, Streptococcus and Oscillospira. However, the abundances of Rikenella, Lactobacillus and Desulfovibrio were increased (p<0.05) and the naringenin group's abundances of these bacteria were decreased as shown in fig. 5.
Fig. 5: 16S rRNA was found using high-throughput sequencing in the rats of each group, (A): Shannon index; (B): PCA analysis result; (C): Venn diagram of the number of OTUs; (D): F to B ratio and (E): Genus level difference
Note:  : Control group;
: Control group;  : Model group;
: Model group;  : Positive drug group and
: Positive drug group and  : Naringin group
: Naringin group
A prevalent functional gastrointestinal disorder in children is functional constipation. At present, there are few therapeutic drugs for childhood constipation and some of them have unsatisfactory long-term efficacy and are prone to drug dependence[10]. In this study, lactulose was used as the control of drug treatment. The findings demonstrated that the model group's initial black feces were excreted more slowly than those of the positive drug and naringenin groups, the small intestine propulsion rates were increased and the stools moisture contents were increased, suggesting that both lactulose and naringenin can promote the intestinal movement and improve the stools status of constipated mice.
Small intestinal villi are composed of absorbent cells and goblet cells. Goblet cells are dispersed among absorbent cells and secrete mucin to build the intestinal mucus barrier[11]. The intestinal mucus barrier has functions such as lubricating the intestine, protecting the intestinal tissue and isolating pathogenic microorganisms[12]. In this research, it was shown that the levels of the inflammatory proteins TNF-α and IL- 1β were elevated in the model group, suggesting that the intestinal mucus barrier had been disrupted and was inflamed, with aberrant secretion function and challenging fecal excretion. Naringenin and the positive medication group both showed lower levels of TNF-α and IL-1β, indicating that both lactulose and naringenin can defend intestinal cells and restore intestinal mucus barrier function.
5-HT is synthesized and stored in intestinal chromaffin cells and released into the blood can stimulate intestinal smooth muscle contraction. 5-Hydroxytryptamine 4 (5-HT4) receptor is an important receptor for 5-HT in regulating intestinal peristalsis. Tryptophan Hydroxylase 1 (TPH1) is a rate-limiting enzyme in 5-HT synthesis[13-15]. The findings of this study demonstrated that naringenin and the positive drug groups had lower levels of 5-HT in intestinal tissue than the model group and that there were differences in the levels of 5-HT in the positive drug group and naringenin group, indicating that both lactulose and naringin can regulate the 5-HT signaling pathway and enhance intestinal peristalsis effectiveness. Evidence suggested that hostmicrobiota interactions are central to the development of intestinal constipation, in which microbial deregulation is accompanied by loss of intestinal mucosal barrier integrity, inflammation and necrosis. Studies have shown that traditional Chinese medicines have a prebiotic-like effect, which may control intestinal flora structure and the intestinal flora can also encourage the transformation of the active ingredients in traditional Chinese medicines[16-18]. In this research, the alteration in intestinal flora architecture in constipated mice after naringenin intervention was examined using the 16S rDNA gene sequencing approach. In this research, the model group's α diversity declined, the model group's β diversity differed considerably from the control group's diversity and the F to B ratios fell, the abundance of Rikenella, Lactobacillus, Desulfovibrio decreased and the abundance of Bacteroides, Odoribacter, Anaeroplasma, and Streptococcus were increased, indicating that the composition of intestinal flora and microbial metabolism in constipated mice changed significantly. Both lactulose and naringenin may be able to regulate the intestinal flora of constipated mice and restore normal microbial metabolism, as evidenced by the higher α diversity of the positive drug and the naringenin groups compared to the model group, higher overlap ratio between the positive drug and the control groups, increased F to B ratio, and different genus levels of each group from the model group.
In conclusion, naringenin may increase the effectiveness of intestinal functioning, preserve the intestinal mucus barrier and control the intestinal flora of mice that are constipated.
Conflict of interests:
The authors declared no conflict of interests.
References
- Shapiro A, Bradshaw B, Landes S, Kammann P, Bois de Fer B, Lee WN, et al. A novel digital approach to describe real world outcomes among patients with constipation. NPJ Digit Med 2021;4(1):27-35.
[Crossref] [Google Scholar] [PubMed]
- Yurtda? G, Acar-Tek N, Akbulut G, Cemali Ö, Arslan N, Beyaz Co?kun A, et al. Risk factors for constipation in adults: A cross-sectional study. J Am Coll Nutr 2020;39(8):713-9.
[Crossref] [Google Scholar] [PubMed]
- Arco S, Saldaña E, Serra-Prat M, Palomera E, Ribas Y, Font S, et al. Functional constipation in older adults: Prevalence, clinical symptoms and subtypes, association with frailty and impact on quality of life. Gerontology 2022;68(4):397-406.
[Crossref] [Google Scholar] [PubMed]
- Guo X, Shi X, Kang X, Luo H, Wang X, Jia H, et al. Risk factors associated with inadequate bowel preparation in patients with functional constipation. Dig Dis Sci 2020;65(4):1082-91.
[Crossref] [Google Scholar] [PubMed]
- Fu R, Li Z, Zhou R, Li C, Shao S, Li J. The mechanism of intestinal flora dysregulation mediated by intestinal bacterial biofilm to induce constipation. Bioengineered 2021;12(1):6484-98.
[Crossref] [Google Scholar] [PubMed]
- Li XJ, You XY, Wang CY, Li XL, Sheng YY, Zhuang PW, et al. Bidirectional brain-gut-microbiota axis in increased intestinal permeability induced by central nervous system injury. CNS Neurosci Ther 2020;26(8):783-90.
[Crossref] [Google Scholar] [PubMed]
- Pimentel M, Lembo A. Microbiome and its role in irritable bowel syndrome. Dig Dis Sci 2020;65(3):829-39.
[Crossref] [Google Scholar] [PubMed]
- He Y, Zhu L, Chen J, Tang X, Pan M, Yuan W, et al. Efficacy of probiotic compounds in relieving constipation and their colonization in gut micro biota. Molecules 2022;27(3):666.
- Zhang X, Zhuang We, Chen D. The relationship between bifidobacteria and digestive system diseases. Chin J Microecol 2021;33(1):104-15.
- Li XQ, Zhang XM, Wu X, Lan Y, Xu L, Meng XC, et al. Beneficial effects of lactitol on the composition of gut microbiota in constipated patients. J Dig Dis 2020;21(8):445-53.
[Crossref] [Google Scholar] [PubMed]
- Chai M, Wang L, Li X, Zhao J, Zhang H, Wang G, et al. Different Bifidobacterium bifidum strains change the intestinal flora composition of mice via different mechanisms to alleviate loperamide-induced constipation. Food Function 2021;12(13):6058-69.
- Wang T, Sun H, Chen J, Luo L, Gu Y, Wang X, et al. Anti-adhesion effects of Lactobacillus strains on Caco-2 cells against Escherichia coli and their application in ameliorating the symptoms of dextran sulfate sodium-induced colitis in mice. Probiotics Antimicrob Proteins 2021;13(6):1632-43.
[Crossref] [Google Scholar] [PubMed]
- Xia P, Liu X, Hou T, Zhan F, Geng F, Zhang Z, et al. Evaluation of the effect of prebiotic sesame candies on loperamide-induced constipation in mice. Food Function 2022;13(10):5690-700.
- Farré R, Fiorani M, Abdu Rahiman S, Matteoli G. Intestinal permeability, inflammation and the role of nutrients. Nutrients 2020;12(4):1185.
[Crossref] [Google Scholar] [PubMed]
- Foong D, Zhou J, Zarrouk A, Ho V, O’Connor MD. Understanding the biology of human interstitial cells of Cajal in gastrointestinal motility. Int J Mol Sci 2020;21(12):4540.
- Zheng H, Liu YJ, Chen ZC, Fan GQ. miR-222 regulates cell growth, apoptosis and autophagy of interstitial cells of Cajal isolated from slow transit constipation rats by targeting c-kit. Indian J Gastroenterol 2021;40(2):198-208.
[Crossref] [Google Scholar] [PubMed]
- Banskota S, Khan WI. Gut-derived serotonin and its emerging roles in immune function, inflammation, metabolism and the gut–brain axis. Curr Opin Endocrinol Diabetes Obesity 2022;29(2):177-82.
[Crossref] [Google Scholar] [PubMed]
- Zhu Y, Cheng J, Yin J, Yang Y, Guo J, Zhang W, et al. Effects of sacral nerve electrical stimulation on 5-HT and 5-HT3AR/5-HT4R levels in the colon and sacral cord of acute spinal cord injury rat models. Mol Med Rep 2020;22(2):763-73.
[Crossref] [Google Scholar] [PubMed]